Meat and Human Health—Current Knowledge and Research Gaps
Abstract
:1. Introduction
2. Meat as a Source of Nutrients
2.1. Amino Acids
2.2. Vitamins and Minerals
2.3. Fatty Acids
2.4. The Nutrient Contribution from Meat
3. What Is Fresh and Processed Meat?
3.1. Industrial Processing of Meat
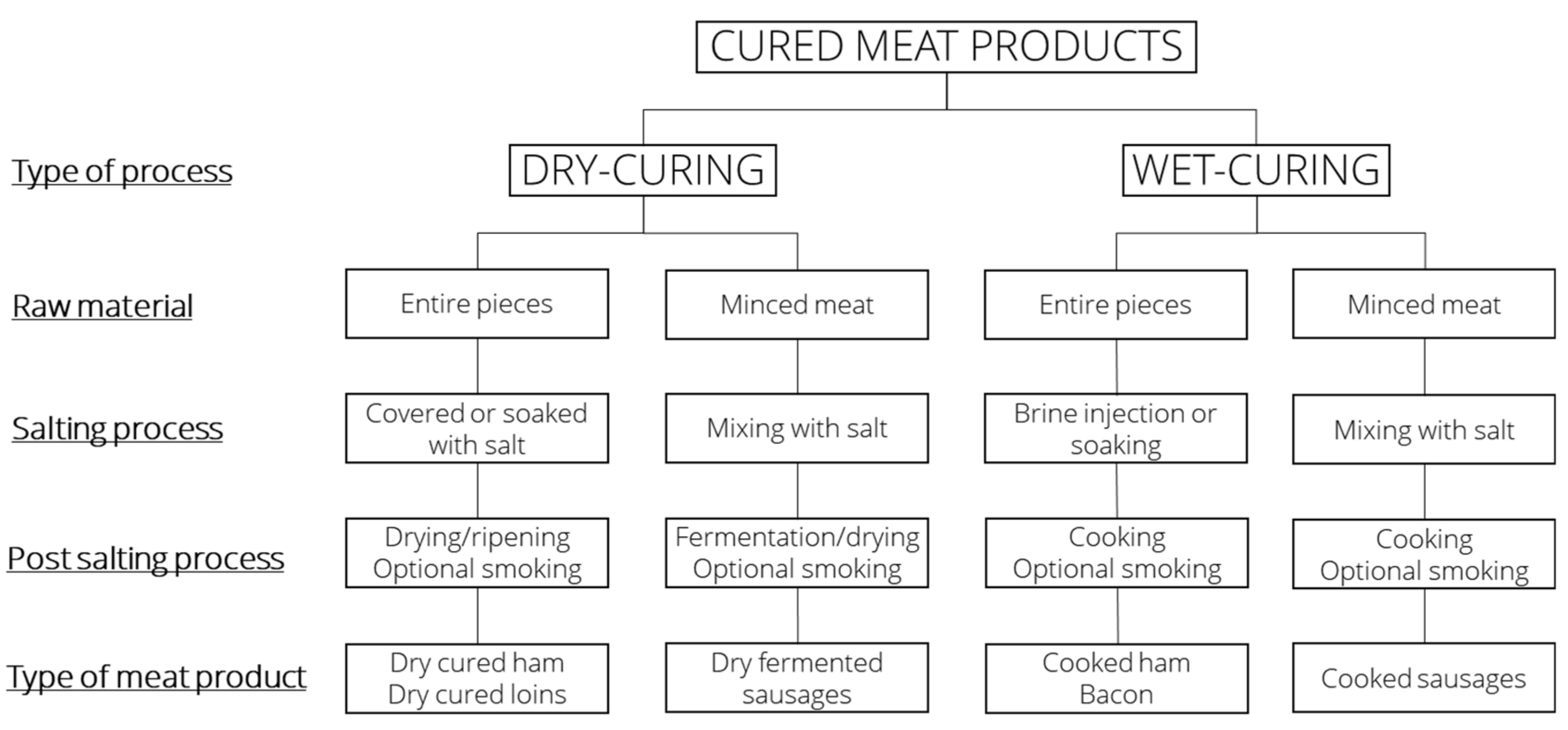
3.2. Dry Curing
3.3. Wet Curing
4. Maturation and Fermentation
5. Fortification of Meat Products
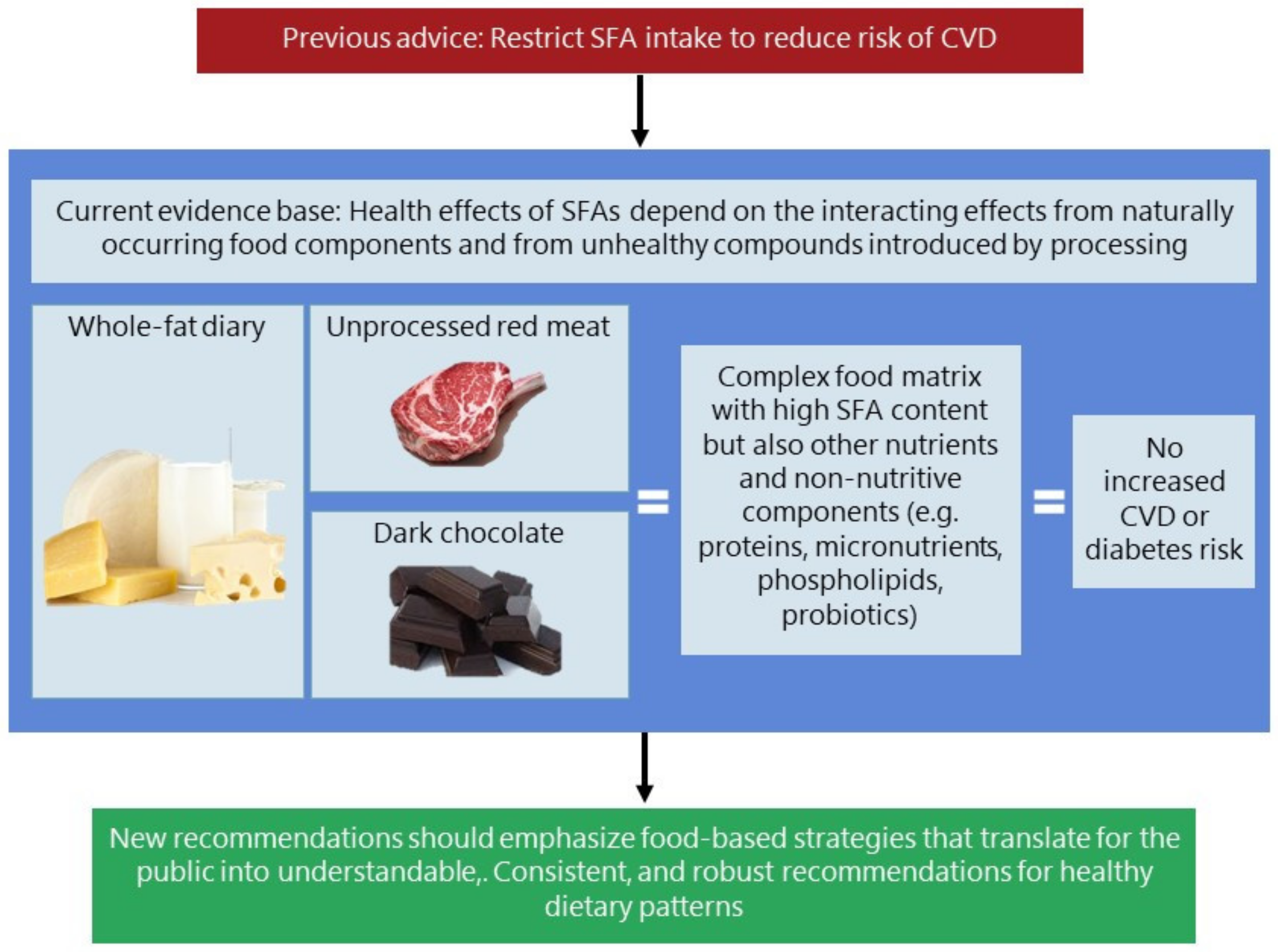
6. What Do We Know and Not Know on the Food Matrix
7. Meat and Chronic Disease—How Good Is the Evidence?
7.1. Meat and Cancer
7.2. Meat, Cardiovascular and Chronic Disease
7.3. Interpretation of Observational Studies
8. The Importance of Confounders and Co-Factors
9. Research Gaps and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Tieland, M.; Borgonjen-Van den Berg, K.J.; van Loon, L.J.; de Groot, L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: Scope for improvement. Eur. J. Nutr. 2012, 51, 173–179. [Google Scholar] [CrossRef]
- Council, N. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordic Co-Operation: Copenhagen, Denmark, 2014. [Google Scholar]
- Wyness, L.; Weichselbaum, E.; O’Connor, A.; Williams, E.B.; Benelam, B.; Riley, H.; Stanner, S. Red meat in the diet: An update. Nutr. Bull. 2011, 36, 34–77. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch. Intern. Med. 2012, 172, 555–563. [Google Scholar]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, E.J.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose–response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science—Implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations. Fats and fatty acids in human nutrition: Report of an expert consultation. In FAO Food and Nutrition Paper; FAO: Rome, Italy, 2010. [Google Scholar]
- Astrup, A.; Bertram, H.C.; Bonjour, J.-P.; De Groot, L.C.; Otto, M.C.D.O.; Feeney, E.L.; Garg, M.L.; Givens, I.; Kok, F.J.; Krauss, R.M.; et al. WHO draft guidelines on dietary saturated and trans fatty acids: Time for a new approach? BMJ 2019, 366, l4137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S. Saturated fats and health: A reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, C.K. Time for a New Approach to Reducing Cardiovascular Disease: Is Limitation on Saturated Fat and Meat Consumption Still Justified? Am. J. Med. 2020, 133, 1009–1010. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, Q.; Yin, Z.; Shuang, J.; Bai, B.; Yu, P.; Guo, M.; Zhao, Q. Red and processed meat consumption and colorectal cancer risk: A systematic review and meta-analysis. Oncotarget 2017, 8, 83306–83314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, C.S.C.; Lam, W.; Fielding, R. A summary of meat intakes and health burdens. Eur. J. Clin. Nutr. 2018, 72, 18–29. [Google Scholar] [CrossRef]
- Händel, M.N.; Cardoso, I.; Rasmussen, K.M.; Rohde, J.F.; Jacobsen, R.; Nielsen, S.M.; Christensen, R.; Heitmann, B.L. Processed meat intake and chronic disease morbidity and mortality: An overview of systematic reviews and meta-analyses. PLoS ONE 2019, 14, e0223883. [Google Scholar] [CrossRef] [Green Version]
- Han, M.A.; Zeraatkar, D.; Guyatt, G.H.; Vernooij, R.W.; El Dib, R.; Zhang, Y.; Algarni, A.; Leung, G.; Storman, D.; Valli, C. Reduction of red and processed meat intake and cancer mortality and incidence: A systematic review and meta-analysis of cohort studies. Ann. Intern. Med. 2019, 171, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Händel, M.N.; Rohde, J.F.; Jacobsen, R.; Nielsen, S.M.; Christensen, R.; Alexander, D.D.; Frederiksen, P.; Heitmann, B.L. Processed meat intake and incidence of colorectal cancer: A systematic review and meta-analysis of prospective observational studies. Eur. J. Clin. Nutr. 2020, 74, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.A.; Norat, T.; Overvad, K.; Dahm, C.C.; Bueno-de-Mesquita, H.B.; Jenab, M.; Fedirko, V.; Van Duijnhoven, F.J.; Skeie, G.; Romaguera-Bosch, D. Pre-diagnostic meat and fibre intakes in relation to colorectal cancer survival in the European Prospective Investigation into Cancer and Nutrition. Br. J. Nutr. 2016, 116, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, P.J.T.; Albero, J.S.; Rodríguez-Montes, J.A. Primary and Secondary Prevention of Colorectal Cancer. Clin. Med. Insights Gastroenterol. 2014, 7, 33–46. [Google Scholar] [CrossRef]
- Góngora, V.M.; Matthes, K.L.; Castaño, P.R.; Linseisen, J.; Rohrmann, S. Dietary Heterocyclic Amine Intake and Colorectal Adenoma Risk: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval-Insausti, H.; Pérez-Tasigchana, R.F.; López-García, E.; García-Esquinas, E.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Macronutrients Intake and Incident Frailty in Older Adults: A Prospective Cohort Study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2016, 71, 1329–1334. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Mata, F.; Morales, J.S.; Castillo-García, A.; Lucia, A. Does Beef Protein Supplementation Improve Body Composition and Exercise Performance? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 1429. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, S.M.; Montoya, C.A.; Scholten, P.T.; Rutherfurd, S.M.; Moughan, P.J. Cooking Conditions Affect the True Ileal Digestible Amino Acid Content and Digestible Indispensable Amino Acid Score (DIAAS) of Bovine Meat as Determined in Pigs. J. Nutr. 2018, 148, 1564–1569. [Google Scholar] [CrossRef]
- Buffière, C.; Gaudichon, C.; Hafnaoui, N.; Migné, C.; Scislowsky, V.; Khodorova, N.; Mosoni, L.; Blot, A.; Boirie, Y.; Dardevet, D.; et al. In the elderly, meat protein assimilation from rare meat is lower than that from meat that is well done. Am. J. Clin. Nutr. 2017, 106, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food, D. Frida Food Data. Available online: https://frida.fooddata.dk/ (accessed on 2 September 2020).
- Kalpalathika, P.V.M.; Clark, E.M.; Mahoney, A.W. Heme iron content in selected ready-to-serve beef products. J. Agric. Food Chem. 1991, 39, 1091–1093. [Google Scholar] [CrossRef]
- Czerwonka, M.; Tokarz, A. Iron in red meat–friend or foe. Meat Sci. 2017, 123, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.; Cook, J.D. Meat Protein Fractions Enhance Nonheme Iron Absorption in Humans. J. Nutr. 2006, 136, 2808–2812. [Google Scholar] [CrossRef]
- Kristensen, M.B.; Hels, O.; Morberg, C.; Marving, J.; Bügel, S.; Tetens, I. Pork meat increases iron absorption from a 5-day fully controlled diet when compared to a vegetarian diet with similar vitamin C and phytic acid content. Br. J. Nutr. 2005, 94, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Burnett, D.D.; Legako, J.F.; Phelps, K.J.; Gonzalez, J.M. Biology, strategies, and fresh meat consequences of manipulating the fatty acid composition of meat. J. Anim. Sci. 2020, 98, 033. [Google Scholar] [CrossRef]
- Vahmani, P.; Ponnampalam, E.N.; Kraft, J.; Mapiye, C.; Bermingham, E.N.; Watkins, P.J.; Proctor, S.D.; Dugan, M.E. Bioactivity and health effects of ruminant meat lipids. Invited Review. Meat Sci. 2020, 165, 108114. [Google Scholar] [CrossRef]
- Derakhshandeh-Rishehri, S.-M.; Rahbar, A.R.; Ostovar, A. Effects of Conjugated Linoleic Acid Intake in the Form of Dietary Supplement or Enriched Food on C-Reactive Protein and Lipoprotein (a) Levels in Humans: A Literature Review and Meta-Analysis. Iran. J. Med Sci. 2019, 44, 359–373. [Google Scholar]
- Rahbar, A.R.; Ostovar, A.; Derakhshandeh-Rishehri, S.-M.; Janani, L.; Rahbar, A. Effect of Conjugated Linoleic Acid as a Supplement or Enrichment in Foods on Blood Glucose and Waist Circumference in Humans: A Metaanalysis. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 1. [Google Scholar] [CrossRef]
- Wharton, S.; Bonder, R.; Jeffery, A.; Christensen, R.A. The safety and effectiveness of commonly-marketed natural sup-plements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016. Crit. Rev. Food Sci. Nutr. 2020, 60, 1614–1630. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.-P.; Zhou, L.-M.; Zhou, L.; Chen, T.; Qin, L.-Q. Effect of conjugated linoleic acid on blood pressure: A meta-analysis of randomized, double-blind placebo-controlled trials. Lipids Health Dis. 2015, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, A.N.; Christensen, T.; Matthiessen, J.; Knudsen, V.K.; Sørensen, M.R.; Biltoft-Jensen, A.P.; Hinsch, H.-J.; Ygil, K.H.; Kørup, K.; Saxholt, E. Danskernes Kostvaner 2011–2013; DTU Fødevareinstituttet, Danmarks Tekniske Universitet: Copenhagen, Denmark, 2015. [Google Scholar]
- Biltoft-Jensen, A.P.; Kørup, K.; Christensen, T.; Eriksen, K.; Ygil, K.H.; Fagt, S. Køds Rolle i Kosten; DTU Library: Copenhagen, Denmark, 2016. [Google Scholar]
- Knudsen, V.K.; Fagt, S.; Trolle, E.; Matthiessen, J.; Groth, M.V.; Biltoft-Jensen, A.P.; Sørensen, M.R.; Pedersen, A.N. Evaluation of dietary intake in Danish adults by means of an index based on food-based dietary guidelines. Food Nutr. Res. 2012, 56, 17129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, A.D.; Christensen, L.M.; Trolle, E. Development of a Danish Adapted Healthy Reference Diet. Nutrients 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUR-Lex. Consolidated Text: Regulation (EC) No. 852/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02004R0852-20090420 (accessed on 25 June 2021).
- Cenci-Goga, B.T.; Karama, M.; Hadjichralambous, C.; Sechi, P.; Grispoldi, L. Is EU regulation on the use of antioxidants in meat preparation and in meat products still cutting edge? Eur. Food Res. Technol. 2020, 246, 661–668. [Google Scholar] [CrossRef]
- Rixson, D. History of Meat Trading; Nottingham University Press: Nottingham, UK, 2000. [Google Scholar]
- Seman, D.L.; Boler, D.D.; Carr, C.C.; Dikeman, M.E.; Owens, C.M.; Keeton, J.T.; Pringle, T.D.; Sindelar, J.J.; Woerner, D.R.; De Mello, A.S.; et al. Meat Science Lexicon. Meat Muscle Biol. 2018, 2, 127–141. [Google Scholar] [CrossRef]
- Flores, J.; Toldrá, F. Curing: Processes and applications. Encycl. Food Sci. Food Technol. Nutr. 1993, 1277–1282. [Google Scholar]
- Toldrá, F. The Storage and Preservation of Meat: III—Meat Processing. In Lawrie’ s Meat Science; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–296. [Google Scholar]
- Da Cruz, R.M.; Vieira, M.M. Mediterranean Foods: Composition and Processing; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Marušić, N.; Aristoy, M.-C.; Toldrá, F. Nutritional pork meat compounds as affected by ham dry-curing. Meat Sci. 2013, 93, 53–60. [Google Scholar] [CrossRef]
- Ruiz, J.; Garcı́a, C.; del Carmen Dı́az, M.a.; Cava, R.; Tejeda, J.F.; Ventanas, J. Dry cured Iberian ham non-volatile com-ponents as affected by the length of the curing process. Food Res. Int. 1999, 32, 643–651. [Google Scholar] [CrossRef]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.M.; Boirie, Y.; van Loon, L.J.C. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Toldrá, F.; Reig, M.; Aristoy, M.-C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Moughan, P.J.; Fuller, M.F.; Han, K.-S.; Kies, A.K.; Miner-Williams, W. Food-derived bioactive peptides influence gut function. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, S5–S22. [Google Scholar] [CrossRef]
- Mora, L.; Escudero, E.; Arihara, K.; Toldrá, F. Antihypertensive effect of peptides naturally generated during Iberian dry-cured ham processing. Food Res. Int. 2015, 78, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Young, J.F.; Therkildsen, M. Bioactive peptides in beef: Endogenous generation through postmortem aging. Meat Sci. 2017, 123, 134–142. [Google Scholar] [CrossRef]
- Liu, D.; Chen, X.; Huang, J.; Huang, M.; Zhou, G. Generation of bioactive peptides from duck meat during post-mortem aging. Food Chem. 2017, 237, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Escudero, E.; Toldrá, F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microbiol. 2018, 276, 71–78. [Google Scholar] [CrossRef]
- Fernández, M.; Benito, M.J.; Martín, A.; Casquete, R.; Córdoba, J.J.; Córdoba, M.G. Influence of starter culture and a pro-tease on the generation of ACE-inhibitory and antioxidant bioactive nitrogen compounds in Iberian dry-fermented sausage “salchichón”. Heliyon 2016, 2, e00093. [Google Scholar] [CrossRef] [Green Version]
- Liaset, B.; Madsen, L.; Hao, Q.; Criales, G.; Mellgren, G.; Marschall, H.-U.; Hallenborg, P.; Espe, M.; Frøyland, L.; Kristiansen, K. Fish protein hydrolysate elevates plasma bile acids and reduces visceral adipose tissue mass in rats. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2009, 1791, 254–262. [Google Scholar] [CrossRef]
- Cudennec, B.; Fouchereau-Peron, M.; Ferry, F.; Duclos, E.; Ravallec, R. In vitro and in vivo evidence for a satiating effect of fish protein hydrolysate obtained from blue whiting (Micromesistius poutassou) muscle. J. Funct. Foods 2012, 4, 271–277. [Google Scholar] [CrossRef]
- Woltman, T.; Castellanos, D.; Reidelberger, R. Role of cholecystokinin in the anorexia produced by duodenal delivery of oleic acid in rats. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R1420–R1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talon, R.; Leroy, S. FERMENTED FOODS|Fermented Meat Products and the Role of Starter Cultures. Encycl. Food Microbiol. 2014, 870–874. [Google Scholar]
- Klingberg, T.; Budde, B. The survival and persistence in the human gastrointestinal tract of five potential probiotic lactobacilli consumed as freeze-dried cultures or as probiotic sausage. Int. J. Food Microbiol. 2006, 109, 157–159. [Google Scholar] [CrossRef]
- De Vuyst, L.; Falony, G.; Leroy, F. Probiotics in fermented sausages. Meat Sci. 2008, 80, 75–78. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products–A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Carrascal, J.R. Cured foods: Health effects. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 338–342. [Google Scholar]
- Thøgersen, R.; Castro-Mejía, J.L.; Sundekilde, U.K.; Hansen, L.H.; Hansen, A.K.; Nielsen, D.S.; Bertram, H.C. Ingestion of an Inulin-Enriched Pork Sausage Product Positively Modulates the Gut Microbiome and Metabolome of Healthy Rats. Mol. Nutr. Food Res. 2018, 62, 1800608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Gill, P.A.; Van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Burillo, S.; Pastoriza, S.; Gironés, A.; Avellaneda, A.; Francino, M.P.; Rufián-Henares, J. Potential probiotic salami with dietary fiber modulates metabolism and gut microbiota in a human intervention study. J. Funct. Foods 2020, 66, 103790. [Google Scholar] [CrossRef]
- Le Leu, R.K.; Winter, J.; Christophersen, C.T.; Young, G.; Humphreys, K.J.; Hu, Y.; Gratz, S.W.; Miller, R.B.; Topping, D.L.; Bird, A.R.; et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: A randomised clinical trial. Br. J. Nutr. 2015, 114, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Corpet, E.D.; Pierre, F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol. Biomark. Prev. 2003, 12, 391–400. [Google Scholar]
- Huncharek, M.; Muscat, J.; Kupelnick, B. Colorectal Cancer Risk and Dietary Intake of Calcium, Vitamin D, and Dairy Products: A Meta-Analysis of 26,335 Cases From 60 Observational Studies. Nutr. Cancer 2008, 61, 47–69. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Yu, J.; Wang, C.; Su, J. Dietary Intakes of Calcium, Iron, Magnesium, and Potassium Elements and the Risk of Colorectal Cancer: A Meta-Analysis. Biol. Trace Elem. Res. 2018, 189, 325–335. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. National Agricultural Thesaurus and Glossary. Available online: https://agclass.nal.usda.gov/mtwdk.exe?k=default&l=60&n=1&s=5&t=2&w=17240 (accessed on 2 September 2020).
- Crowe, K.M. Designing functional foods with bioactive polyphenols: Highlighting Lessons learned from original plant matrices. J. Hum. Nutr. Food Sci. 2013, 1, 1018–1019. [Google Scholar]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2019, 60, 826–843. [Google Scholar] [CrossRef]
- West, E.C.; Castenmiller, J.J. Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion. Int. J. Vitam. Nutr. Res. 1998, 68, 371–377. [Google Scholar]
- Arimoto-Kobayashi, S.; Harada, N.; Tokunaga, R.; Odo, J.-I.; Hayatsu, H. Adsorption of mutagens to chlorophyllin–chitosan, an insoluble form of chlorophyllin. Mutat. Res. Mol. Mech. Mutagen. 1997, 381, 243–249. [Google Scholar] [CrossRef]
- Egner, P.A.; Wang, J.-B.; Zhu, Y.-R.; Zhang, B.-C.; Wu, Y.; Zhang, Q.-N.; Qian, G.-S.; Kuang, S.-Y.; Gange, S.; Jacobson, L.P.; et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14601–14606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L.E. Effects of macro-nutrient, micro-nutrient composition and cooking con-ditions on in vitro digestibility of meat and aquatic dietary proteins. Food Chem. 2018, 254, 292–301. [Google Scholar] [CrossRef]
- Gerber, N.; Scheeder, M.; Wenk, C. The influence of cooking and fat trimming on the actual nutrient intake from meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef]
- Weinborn, V.; Valenzuela, C.; Olivares, M.; Arredondo, M.; Weill, R.; Pizarro, F. Prebiotics increase heme iron bioavaila-bility and do not affect non-heme iron bioavailability in humans. Food Funct. 2017, 8, 1994–1999. [Google Scholar] [CrossRef]
- World Cancer Research Fund AAfC. CUP III Report, Meat, Fish and Dairy Products and the Risk of Cancer; World Cancer Research Fund International: London, UK, 2018; ISBN 978-1-912259-16-8. [Google Scholar]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Preterre, A.L.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.; Abar, L.; Chan, D.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Cuparencu, C.; Praticó, G.; Hemeryck, L.Y.; Harsha, P.S.C.S.; Noerman, S.; Rombouts, C.; Xi, M.; Vanhaecke, L.; Hanhineva, K.; Brennan, L.; et al. Biomarkers of meat and seafood intake: An extensive literature review. Genes Nutr. 2019, 14, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Cuparencu, C.; Rinnan, Å.; Dragsted, L.O. Combined markers to assess meat intake—Human metabolomic studies of dis-covery and validation. Mol. Nutr. Food Res. 2019, 63, 1900106. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; Andriolo, V.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2017, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Sanchis-Gomar, F. Red meat consumption and ischemic heart disease. A systematic literature review. Meat Sci. 2015, 108, 32–36. [Google Scholar] [CrossRef]
- Cui, K.; Liu, Y.; Zhu, L.; Mei, X.; Jin, P.; Luo, Y. Association between intake of red and processed meat and the risk of heart failure: A meta-analysis. BMC Public Healh 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, M.; Flynn, A.; Kiely, M. Consumption of red meat, white meat and processed meat in Irish adults in relation to dietary quality. Br. J. Nutr. 2005, 93, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuparencu, C.; Rinnan, Å.; Silvestre, M.P.; Poppitt, S.D.; Raben, A.; Dragsted, L.O. The anserine to carnosine ratio: An excellent discriminator between white and red meats consumed by free-living overweight participants of the PREVIEW study. Eur. J. Nutr. 2021, 60, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, R.; Keski-Rahkonen, P.; Robinot, N.; Viallon, V.; Rothwell, J.A.; Boutron-Ruault, M.C.; Aleksandrova, K.; Wittenbecher, C.; Schulze, M.B.; Halkjær, J. Pepper Alkaloids and Processed Meat Intake: Results from a Randomized Trial and the European Prospective Investigation into Cancer and Nutrition (EPIC) Cohort. Mol. Nutr. Food Res. 2021, 65, 2001141. [Google Scholar] [CrossRef] [PubMed]
- Mejborn, H.; Biltoft-Jensen, A.; Hansen, M.; Licht, T.R.; Olesen, P.T.; Sørensen, I.K. Mechanisms Behind Cancer Risks Associated with Consumption of Red and Processed Meat; National Food Institute: Soeborg, Denmark, 2016; ISBN 978-87-93109-82-7. [Google Scholar]
- Mejborn, H.; Kørup, K.; Biltoft-Jensen, A. Food and Nutrient Characteristics of 15–75 Year Old Danes-Healthy or Unhealthy Diets with Different Meat Content; National Food Institute: Kgs Lyngby, Denmark, 2019. [Google Scholar]
- Chao, A.; Thun, M.J.; Connell, C.J.; McCullough, M.L.; Jacobs, E.J.; Flanders, W.D.; Rodriguez, C.; Sinha, R.; Calle, E.E. Meat consumption and risk of colorectal cancer. JAMA 2005, 293, 172–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjerpsted, J.; Leedo, E.; Tholstrup, T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am. J. Clin. Nutr. 2011, 94, 1479–1484. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, N.; Chiu, S.; Williams, P.T.; King, S.M.; Krauss, R.M. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Raziani, F.; Ebrahimi, P.; Engelsen, S.B.; Astrup, A.; Raben, A.; Tholstrup, T. Consumption of regular-fat vs reduced-fat cheese reveals gender-specific changes in LDL particle size-a randomized controlled trial. Nutr. Metab. 2018, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.; Ansorena, D.; Van Hecke, T.; Astiasarán, I.; De Smet, S.; Estévez, M. Meat lipids, NaCl and carnitine: Do they unveil the conundrum of the association between red and processed meat intake and cardiovascular diseases? Invited Review. Meat Sci. 2021, 171, 108278. [Google Scholar] [CrossRef]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake—Critical assessment of candidate biomarkers. Genes Nutr. 2018, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
| Recommendations Standardization of the definition of red, processed and unprocessed meat products Completion of randomized controlled studies with a solid methodological approach to thoroughly examine and identify the pathophysiological effects of: Different types of fresh meats; red and white Fermented meat products (dry cured meats) Other processed meat products To investigate the metabolic effects of consuming meat as part of a healthy diet Improve the identification of metabolic changes in response to meat consumption, including biomarkers of intake and effect. Future strategies Future studies should identify a possible threshold for apparent healthy factors that become unhealthy when consumption increases beyond a certain level—can this level be influenced by intake of other foods/nutrients, e.g., does a high intake of dietary fiber make you more robust and resilient to a high intake of meat? Do processed meat products fortified with, e.g., dietary fiber or calcium exert an effect different from regular processed meat? Does fresh minced meat exert an effect different from regular fresh meat? Assess the effect of different amounts of meat consumption as part of a healthy diet in a healthy population as well as in those with overweight and obesity and thereby at risk of CVD and type 2 diabetes Characterization of nutrients and non-nutritive compounds in processed meat, wet and dry cured How does processing/fermentation affect content and bioavailability of nutrients? Including partly liberation of nutrients from connective tissues. Link to/investigation of expected biological effects Include identification of different lipoprotein particle sizes when analyzing changes in plasma cholesterol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiker, N.R.W.; Bertram, H.C.; Mejborn, H.; Dragsted, L.O.; Kristensen, L.; Carrascal, J.R.; Bügel, S.; Astrup, A. Meat and Human Health—Current Knowledge and Research Gaps. Foods 2021, 10, 1556. https://doi.org/10.3390/foods10071556
Geiker NRW, Bertram HC, Mejborn H, Dragsted LO, Kristensen L, Carrascal JR, Bügel S, Astrup A. Meat and Human Health—Current Knowledge and Research Gaps. Foods. 2021; 10(7):1556. https://doi.org/10.3390/foods10071556
Chicago/Turabian StyleGeiker, Nina Rica Wium, Hanne Christine Bertram, Heddie Mejborn, Lars O. Dragsted, Lars Kristensen, Jorge R. Carrascal, Susanne Bügel, and Arne Astrup. 2021. "Meat and Human Health—Current Knowledge and Research Gaps" Foods 10, no. 7: 1556. https://doi.org/10.3390/foods10071556
APA StyleGeiker, N. R. W., Bertram, H. C., Mejborn, H., Dragsted, L. O., Kristensen, L., Carrascal, J. R., Bügel, S., & Astrup, A. (2021). Meat and Human Health—Current Knowledge and Research Gaps. Foods, 10(7), 1556. https://doi.org/10.3390/foods10071556









