Construction of IsoVoc Database for the Authentication of Natural Flavours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sample Preparation
2.3. Elemental Analysis-Isotope Ratio Mass Spectrometry (EA-IRMS)
2.4. Gas Chromatography-Mass Spectrometry (GC-MS)
2.5. Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS)
2.6. Data Analysis
3. Results and Discussion
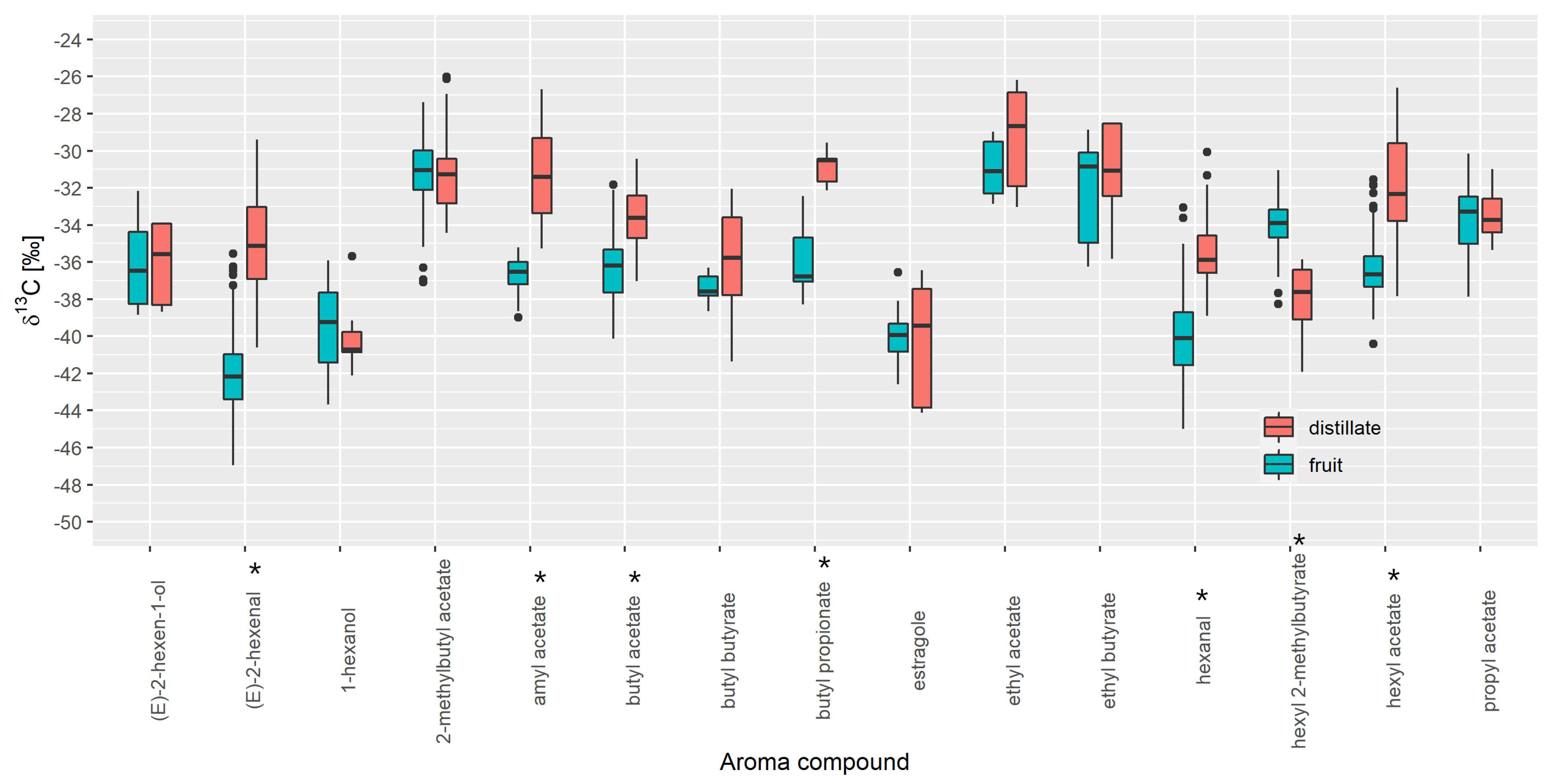
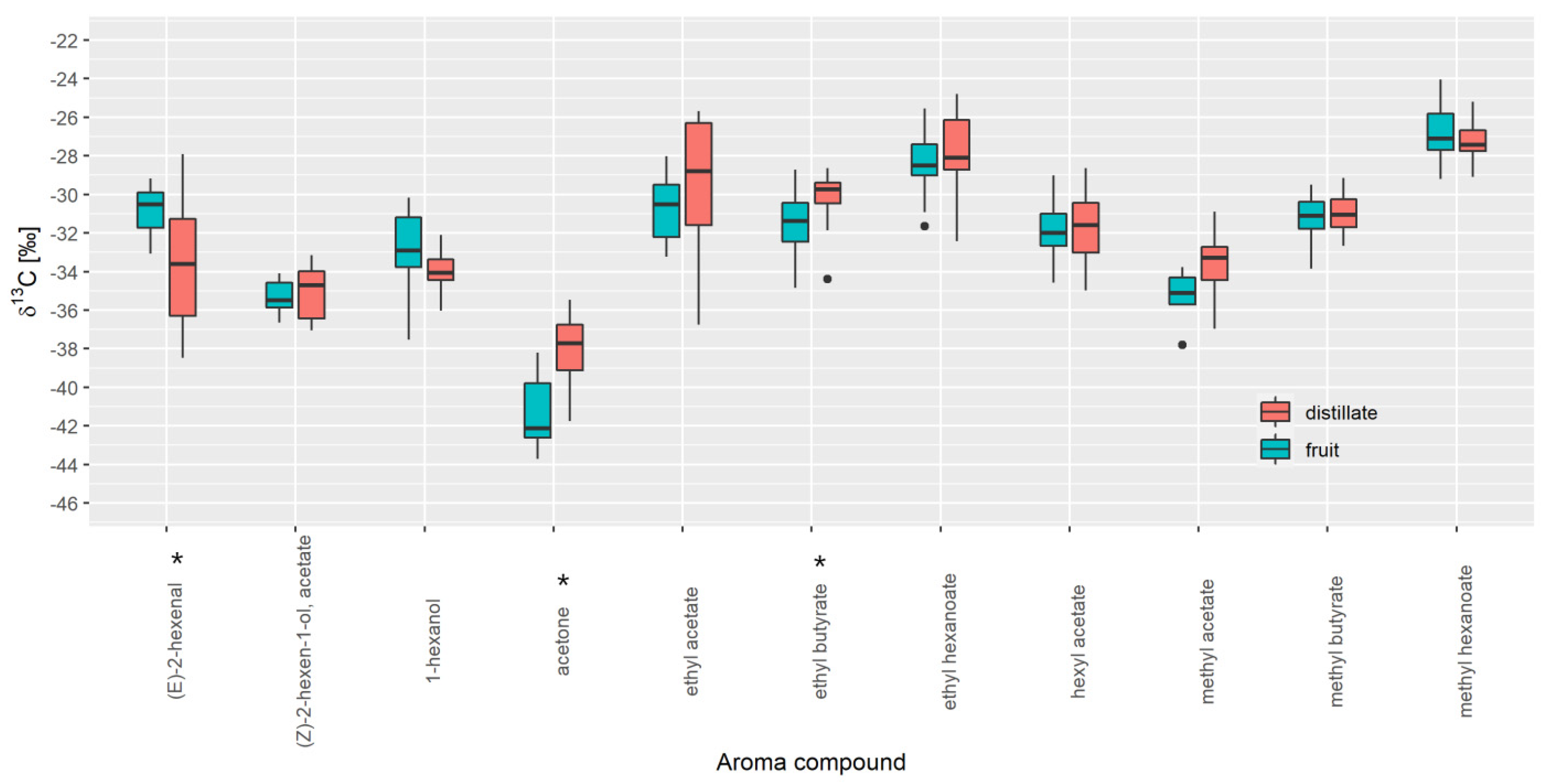
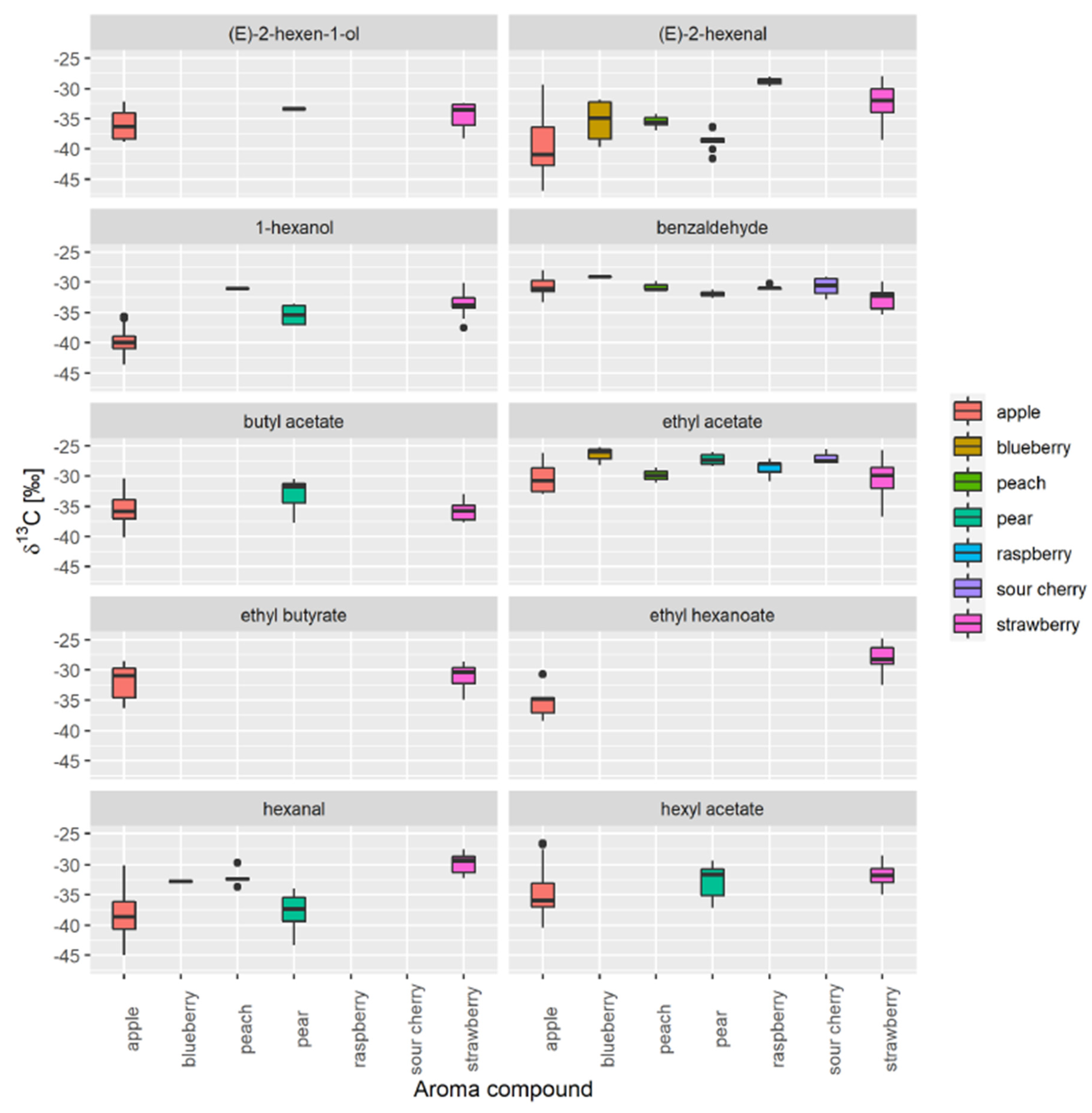
3.1. Selection of Authentic Reference Sample
3.2. Database Creation
3.3. Authenticity Assessment of Commercial Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fruit Type | Method of Sample Preparation a | Stable Isotopic Value | Samples with Known Origin (No. Samples) | Aroma Compound (No. of Samples Where VOC Was Detected) | Reference |
|---|---|---|---|---|---|
| strawberry, peach, plum, apricot | SDE | δ13C | fruit (NS); synthetic (NS); microbial (NS) | γ-decalactone (7; 3; 2) | [8] |
| raspberries | NA | δ13C | fruit (NA); synthetic (NS);biotechnological (NS) | (E)-α-ionone (2; NS; NS), (E)-β-ionone (2; NS; NS) | [18] |
| bergamot oil | SCF extraction | δ13C | essential oil (2) | linalyl acetate (NS), limonene (NS), linalool (NS), β-pinene (NS), γ-terpinene (NS), β-mycrene (NS), neryl acetate (NS), geranyl acetate (NS) | [5] |
| raspberries | NA | δ13C | fruit (NS) | α-ionone (NS), β-ionone (NS), δ-decalactone (NS), cis-3-hexen-1-ol (NS) | [19] |
| strawberry | SDE | δ13C | fruit (NS) | methyl hexanoate (NS), pentyl valerate (NS), butanoic acid (NS), 2-methylbutanoic acid (NS), 4-methylvaleric acid (NS), hexanoic acid (NS), γ-decalactone (NS), γ-uncalactone (NS), γ-dodecalactone (NS) | [20] |
| raspberry | SCF extraction | δ13C | fruit (NS); synthetic (NS) | (E)-α-ionone (NS), (E)-β-ionone (NS) | [27] |
| banana | LLE | δ13C | fruit (7) | pentan-2-one (NS), isobutyl acetate (NS), isoamyl acetate (NS), pentan-2-ol (NS), isobutyl butyrate (NS), isoamyl butyrate (NS), isoamyl isovalerate (NS), isobutyl acetate (NS), isoamyl acetate (NS), isoamyl alcohol (NS), isoamyl butyrate (NS) | [22] |
| bitter almond oils, sweet cherry, sour cherry, peach, nectarine | SDE | δ2H | fruit (NS); essential oil (NS); synthetic (NS); “natural” (NS) | benzaldehyde (NS) | [23] |
| cactus pear | SDE | δ13C | fruit (NS) | 1-hexanol (NS), E-2-hexenol (NS), E-2-nonenol (NS), E,Z-2,6-nonadienol (NS) | [24] |
| different essential oils, orange, apple, nectarine/peach | LLE, SDE | δ2H | fruit/juice/aroma (NS); essential oil (NS); synthetic (NS); “natural” (NS) | linalool (5; 50; 0; 1), linalyl acetate (0; 19; 5; 2), E-2-hexenal (23; 0; 5; 4), E-2-hexenol (35; 0; 5; 0) | [25] |
| pineapple | LLE | δ13C, δ2H | fruit/recovery aroma (16);synthetic (NS); “natural” (NS) | methyl 2-methylbutanate (6; 5; 2), ethyl 2-methylbutanoate (9; 5; 6), methyl hexanoate (17; 5; NA), ethyl hexanoate (14; 0; 0), 2,5-dimethyl-4-methoxy-3-(2H)-furanone (12; 1; 0) | [9] |
| raspberries | SDE | δ13C, δ2H | fruit (NS); nature-identical (NS); “natural” (NS) | (E)-α-ionone (10; 4; 1), (E)-β-ionone (10; 4; 1) | [10] |
| peach, apricot, nectarine | SDE | δ13C, δ2H | fruit (18); nature-identical (NS); “natural” (NS) | γ-decalactone (16; 2; 5), δ-decalactone (12; 1; 3) | [11] |
| pear | SDE, LLE | δ13C, δ2H | fruit (20); synthetic (NS); “natural” (NS) | butyl acetate (14; 3; 3), 1-butanol (18; 3; 2), hexyl acetate (16; 2; 7), 1-hexanol (3; 11; 8), methyl E, Z-2,4-decadienaoate (6; 2; 0), ethyl E,Z-2,4-decadienoate (10; 5; 3), ethyl E,E-2,4-decadienoate (2; 0; 0) | [12] |
| apple | SDE | δ13C, δ2H | juice/recovery aroma (62) | E-2-hexenal (NS), 1-hexanol (NS), E-2-hexenol (NS) | [28] |
| raspberry, litsea cubeba, lemongrass | SDE | δ13C, δ2H | fruit (8); essential oil (NS); synthetic (NS); “natural” (NS) | acetone (NS), citral (NS), α-ionone (NS), β-ionone (NS) | [13] |
| blackberry | SDE | δ13C, δ2H, δ18O | fruit (10); synthetic (3) | 2-heptanol (10; 1), trans-linalool oxide (10; 1), cis-linalool oxide (10; 1) | [14] |
| strawberry, pineapple, peach | HS-SPME | δ13C | fruit (NS) | methyl butanoate (NS), ethyl butanoate (NS), E-2-hexenal (NS), methyl hexanoate (NS), butyl butyrate (NS), ethyl hexanoate (NS), hexyl acetate (NS), linalool (NS), hexyl butyrate (NS), octyl isovalerate (NA), γ-decalactone (NS), octyl hexanoate (NS) | [7] |
| sweet orange | HS-SPME | δ13C | essential oil (20) | myrcene (20), limonene (20), nonanal (20), decanal (20), linalool (20), α-terpineol (20), geranial (20) | [15] |
| wolfberry | HS-SPME | δ13C | fruit (52) | limonene (NS), tetramethylpyrazine (NS), safranal (NS), geranylacetone (NA), β-ionone (NS) | [32] |
| apple | HS-SPME | δ13C | recovery aroma (18); synthetic (16) | ethyl acetate (1; 1), ethyl butyrate (1; 1), ethyl-2-methyll butyrate (1; 1), butyl acetate (6; 1), 1-hexanal (12; 1), 2-methylbutyl acetate (10; 1), 1-butanol (15; 1), amyl acetate (8; 1), butyl butyrate (8; 1), trans-2-hexenal (12; 1), hexyl acetate (8; 1), 2-hexen-1-ol, acetate (1, 1), 1-hexanol (15; 1), trans-2-hexenol (2; 1), benzaldehyde (15; 1), 1-octanol (8; 1) | [16] |
References
- Jiang, Y.; Song, J. Fruits and fruit flavor: Classification and biological characterization. In Handbook of Fruit and Vegetable Flavors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–23. [Google Scholar]
- A Helpful Guide to Fruit Flavor Combinations. Available online: https://www.thespruceeats.com/fruit-flavor-combinations-for-cocktails-760298 (accessed on 19 January 2021).
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol Biotechnol Production of Food Aroma Compounds: Microbial and Enzymatic Methodologies. Food Technol. Biotechnol. 2006, 3, 335–353. [Google Scholar]
- Economic Adulteration and the Need for Carbon-14 Testing in the Natural Products Industry, Nutritional Outlook. Available online: https://www.nutritionaloutlook.com/view/economic-adulteration-and-need-carbon-14-testing-natural-products-industry (accessed on 20 January 2021).
- Martin, G.J. Multisite and multicomponent approach for the stable isotope analysis of aromas and essential oils. Fruit Flavor Biog. Charact. Authentication (ACS 596) 1993, 596, 94–113. [Google Scholar]
- Richling, E.; Appel, M.; Heckel, F.; Kahle, K.; Kraus, M.; Preston, C.; Hümmer, W.; Schreier, P. Flavor authenticity studies by isotope ratio mass spectrometry: Perspectives and limits. In Authentication of Food and Wine; American Chemical Society: Washington, DC, USA, 2006; pp. 75–86. [Google Scholar]
- Schipilliti, L.; Dugo, P.; Bonaccorsi, I.; Mondello, L. Headspace-solid phase microextraction coupled to gas chromatography–combustion-isotope ratio mass spectrometer and to enantioselective gas chromatography for strawberry flavoured food quality control. J. Chromatogr. A 2011, 1218, 7481–7486. [Google Scholar] [CrossRef] [PubMed]
- Bernreuther, A.; Koziet, J.; Brunerie, P.; Krammer, G.; Christoph, N.; Schreier, P. Chirospecific capillary gaschromatographic (HRGC) and on-line HRGC-isotope ratio mass spectrometry of -decalactone from various sources. Z. Leb. Unters. Forsch. 1990, 191, 299–301. [Google Scholar] [CrossRef]
- Preston, C.; Richling, E.; Elss, S.; Appel, M.; Heckel, F.; Hartlieb, A.; Schreier, P. On-line gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (HRGC-C/P-IRMS) of pineapple (Ananas comosus L. Merr.) volatiles. J. Agric. Food Chem. 2003, 51, 8027–8031. [Google Scholar] [CrossRef]
- Sewenig, S.; Bullinger, D.; Hener, U.; Mosandl, A. Comprehensive authentication of (E)-α(β)-ionone from raspberries, using constant flow MDGC-C/P-IRMS and enantio-MDGC-MS. J. Agric. Food Chem. 2005, 53, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Appel, M.; Richling, E.; Schreier, P. Authenticity assessment of γ- and δ-decalactone from Prunus fruits by gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (GC-C/P-IRMS). J. Agric. Food Chem. 2005, 53, 5397–5401. [Google Scholar] [CrossRef]
- Kahle, K.; Preston, C.; Richling, E.; Heckel, F.; Schreier, P. On-line gas chromatography combustion/pyrolysis isotope ratio mass spectrometry (HRGC-C/P-IRMS) of major volatiles from pear fruit (Pyrus communis) and pear products. Food Chem. 2005, 91, 449–455. [Google Scholar] [CrossRef]
- Caja, D.M.; Preston, C.; Kempf, M.; Schreier, P. Flavor authentication studies of alpha-ionone, beta-ionone, and alpha-ionol from various sources. J. Agric. Food Chem. 2007, 55, 6700–6704. [Google Scholar] [CrossRef]
- Greule, M.; Mosandl, A. Heptan-2-ol and trans-linalool oxide (fur.) as inherent indicators of natural blackberry flavour using enantioselective and multielement-MDGC-IRMS analysis. Eur. Food Res. Technol. 2008, 226, 1001–1006. [Google Scholar] [CrossRef]
- Schipilliti, L.; Bonaccorsi, I.; Cotroneo, A.; Dugo, P.; Mondello, L. Carbon isotope ratios of selected volatiles in Citrus sinensis and in orange-flavoured food. J. Sci. Food Agric. 2015, 95, 2944–2950. [Google Scholar] [CrossRef]
- Strojnik, L.; Stopar, M.; Zlatič, E.; Kokalj, D.; Gril, M.N.; Ženko, B.; Žnidaršič, M.; Bohanec, M.; Boshkovska, B.M.; Luštrek, M.; et al. Authentication of key aroma compounds in apple using stable isotope approach. Food Chem. 2019, 277, 766–773. [Google Scholar] [CrossRef]
- van Leeuwen, K.A.; Prenzler, P.D.; Ryan, D.; Camin, F. Gas chromatography-combustion-isotope ratio mass spectrometry for traceability and authenticity in foods and beverages. Compr. Rev. Food Sci. Food Saf. 2014, 13, 814–837. [Google Scholar] [CrossRef] [Green Version]
- Braunsdorf, R.; Hener, U.; Lehmann, D.; Mosandl, A. Analytische Differenzierung zwischen natürlich gewachsenen, fermentativ erzeugten und synthetischen (naturidentischen) Aromastoffen. I, Herkunftsspezifische Analyse des (E)-α(β)-Ionons. Dtsch. Leb. 1991, 87, 227–280. [Google Scholar]
- Casabianca, H.; Graff, J.B. Enantiomeric and isotopic analysis of flavour compounds of some raspberry cultivars. J. Chromatogr. A 1994, 684, 360–365. [Google Scholar] [CrossRef]
- Schumacher, K.; Turgeon, H.; Mosandl, A. Sample preparation for gas chromatography isotope ratio mass spectrometry-an investigation with volatile components from strawberries. Phytochem. Anal. 1995, 6, 258–261. [Google Scholar] [CrossRef]
- Zhang, B.L.; Lees, M.; Martin, G.J. Stable isotope fractionation in fruit juice concentrates: Application to the authentication of grape and orange products. J. Agric. Food Chem. 1995, 43, 2411–2417. [Google Scholar] [CrossRef]
- Salmon, B.; Martin, G.J.; Remaud, G.; Fourel, F. Compositional and isotopic studies of fruit flavours. Part I. The banana aroma. Flavour Fragr. J. 1996, 11, 353–359. [Google Scholar] [CrossRef]
- Ruff, C.; Hör, K.; Weckerle, B.; Schreier, P.; König, T. 2H/1H Ratio analysis of flavor compounds by on-line gas chromatography pyrolysis isotope ratio mass spectrometry (HRGC-P-IRMS): Benzaldehyde. J. High Resolut. Chromatogr. 2000, 23, 357–359. [Google Scholar] [CrossRef]
- Weckerle, B.; Bastl-Borrmann, R.; Richling, E.; Hör, K.; Ruff, C.; Schreier, P. Cactus pear (Opuntia ficus indica) flavour constituents-Chiral evaluation (MDGC-MS) and isotope ratio (HRGC-IRMS) analysis. Flavour Fragr. J. 2001, 16, 360–363. [Google Scholar] [CrossRef]
- Hör, K.; Ruff, C.; Weckerle, B.; König, T.; Schreier, P. Flavor Authenticity Studies by 2H/1H Ratio Determination Using On-line Gas Chromatography Pyrolysis Isotope Ratio Mass Spectrometry. J. Agric. Food Chem. 2001, 49, 21–25. [Google Scholar] [CrossRef]
- Strojnik, L.; Camin, F.; Ogrinc, N. Compound-specific carbon and hydrogen isotope analysis of volatile organic compounds using headspace solid-phase microextraction. Talanta 2020, 219, 121264. [Google Scholar] [CrossRef]
- Mosandl, A. Enantioselective capillary gas chromatography and stable isotope ratio mass spectrometry in the authenticity control of flavors and essential oils. Food Rev. Int. 1995, 11, 597–664. [Google Scholar] [CrossRef]
- Elss, S.; Preston, C.; Appel, M.; Heckel, F.; Schreier, P. Influence of technological processing on apple aroma analysed by high resolution gas chromatography-mass spectrometry and on-line gas chromatography-combustion/pyrolysis-isotope ratio mass spectrometry. Food Chem. 2006, 98, 269–276. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Gonçalves, B.; Oliveira, I.; Bacelar, E.; Morais, M.C.; Aires, A.; Cosme, F.; Ventura-Cardoso, J.; Anjos, R.; Pinto, T. Aromas and flavours of fruits. In Generation of Aromas and Flavours; InTech: London, UK, 2018. [Google Scholar]
- Perini, M.; Pianezze, S.; Strojnik, L.; Camin, F. C and H stable isotope ratio analysis using solid-phase microextraction and gas chromatography-isotope ratio mass spectrometry for vanillin authentication. J. Chromatogr. A 2019, 1595, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Liu, Z.; Gou, C.L.; Rogers, K.M.; Yu, W.J.; Zhang, S.S.; Yuan, Y.W.; Zhang, L. Geographical origin of Chinese wolfberry (goji) determined by carbon isotope analysis of specific volatile compounds. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1105, 104–112. [Google Scholar] [CrossRef]
- Schipilliti, L.; Bonaccorsi, I.L.; Mondello, L. Characterization of natural vanilla flavour in foodstuff by HS-SPME and GC-C-IRMS. Flavour Fragr. J. 2017, 32, 85–91. [Google Scholar] [CrossRef]
- Hansen, A.-M.S.; Fromberg, A.; Frandsen, H.L. Authenticity and traceability of vanilla flavors by analysis of stable isotopes of carbon and hydrogen. J. Agric. Food Chem. 2014, 62, 10326–10331. [Google Scholar] [CrossRef] [PubMed]
- Schipilliti, L.; Bonaccorsi, I.L.; Occhiuto, C.; Dugo, P.; Mondello, L. Authentication of citrus volatiles based on carbon isotope ratios. J. Essent. Oil Res. 2018, 30, 1–15. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Vogiatzaki, M.; Zufferey, V. Gas chromatography and isotope ratio mass spectrometry of Pinot Noir wine volatile compounds (δ13C) and solid residues (δ13C, δ15N) for the reassessment of vineyard water-status. J. Chromatogr. A 2017, 1517, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Xiaobo, Z.; Jiewen, Z. Comparative analyses of apple aroma by a tin-oxide gas sensor array device and GC/MS. Food Chem. 2008, 107, 120–128. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L.; Wu, S.; Huang, M.; Yu, W.; Zhang, S. Developing an authentication approach using SPME-GC-IRMS based on compound-specific δ13C analysis of six typical volatiles in wine. Food Qual. Saf. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Wernig, F.; Buegger, F.; Pritsch, K.; Splivallo, R. Composition and authentication of commercial and home-made white truffle-flavored oils. Food Control 2018, 87, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Donarski, J.; Camin, F.; Fauhl-Hassek, C.; Posey, R.; Sudnik, M. Sampling guidelines for building and curating food authenticity databases. Trends Food Sci. Technol. 2019, 90, 187–193. [Google Scholar] [CrossRef]
- Webinar Review: Use of Stable Isotope Analysis in Commercial Food Authenticity Testing-New Food Magazine. Available online: https://www.newfoodmagazine.com/article/41431/stable-isotope-analysis-food-authenticity-testing/ (accessed on 19 January 2021).
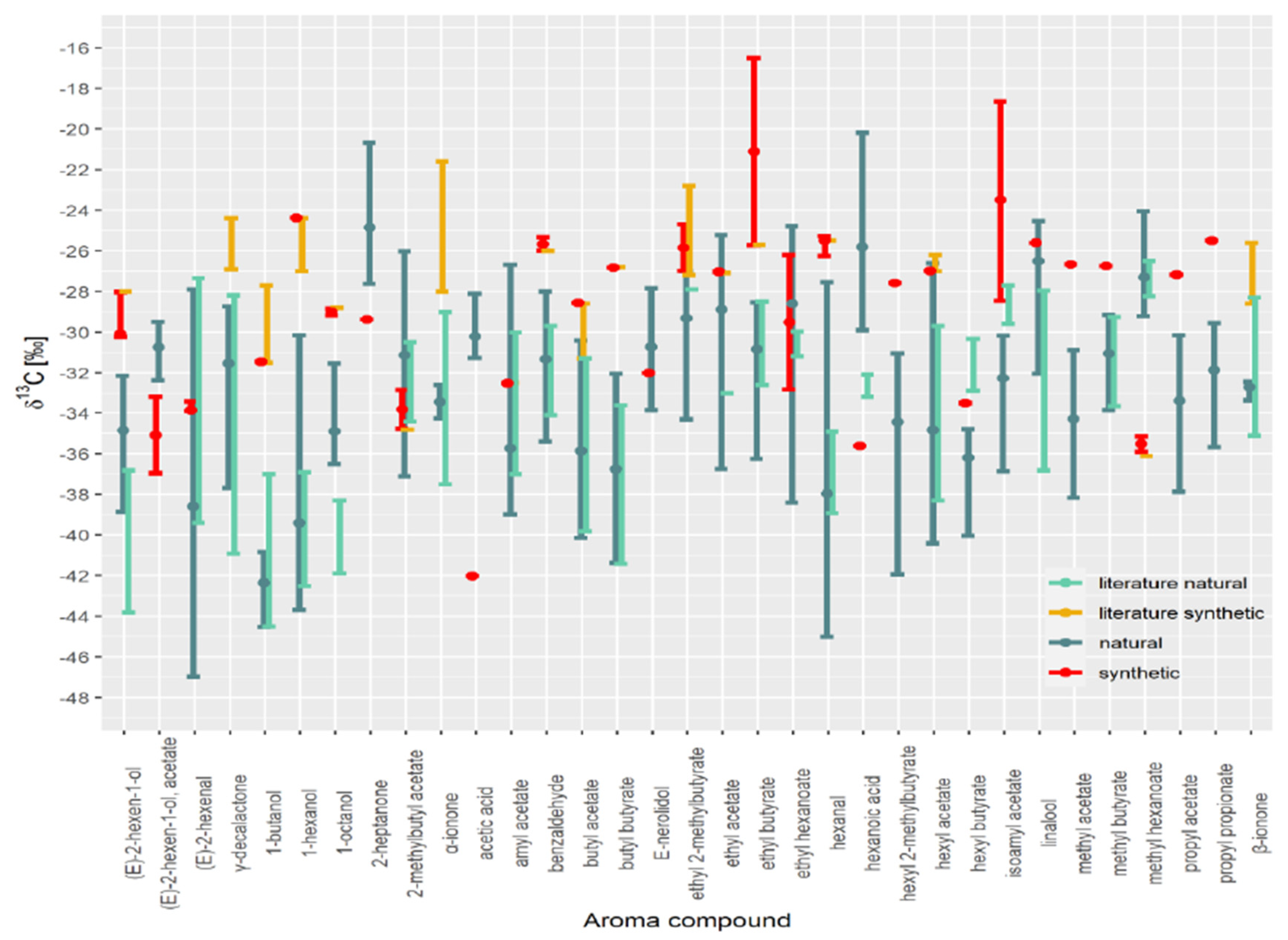
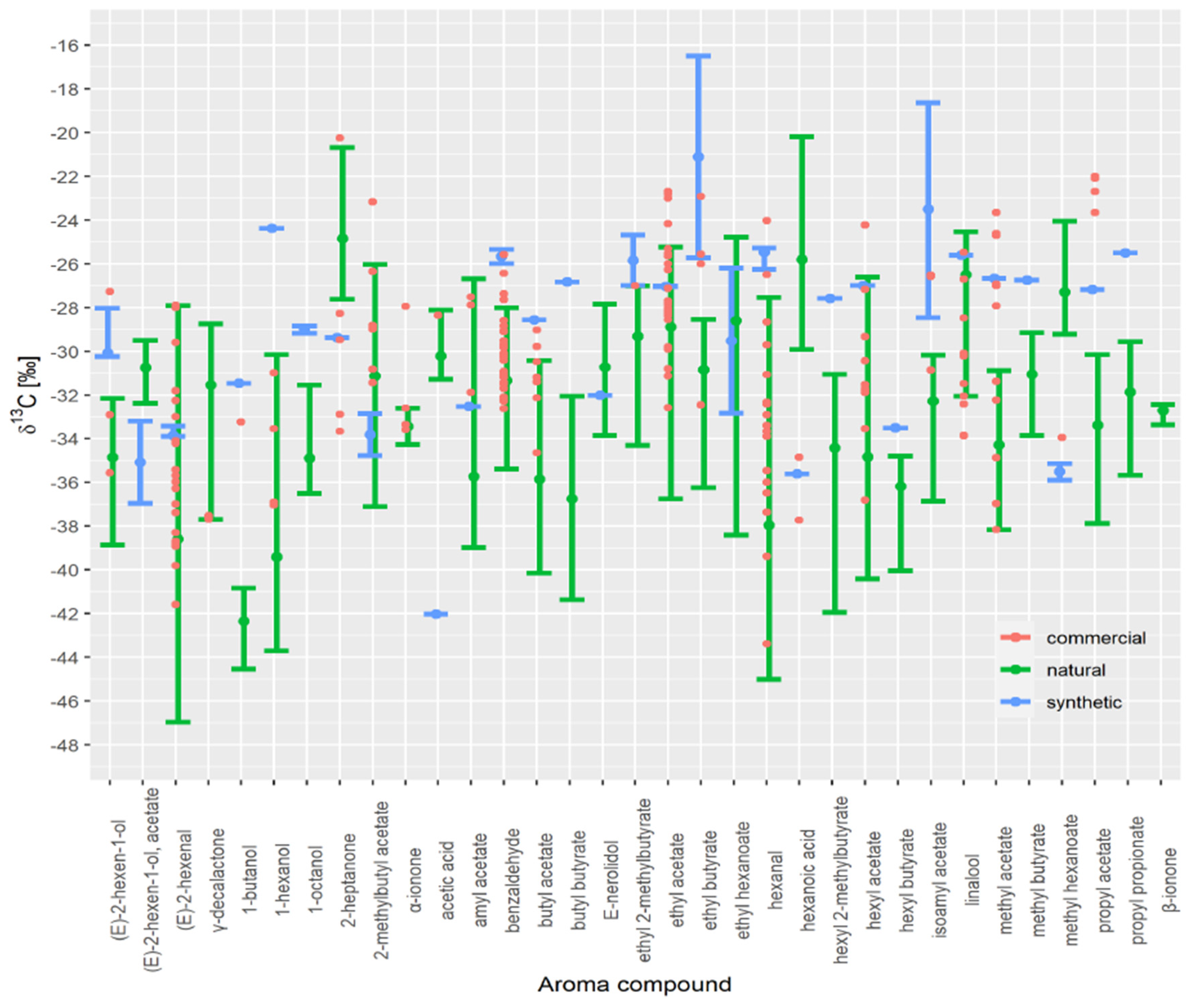
| (E)-2-hexen-1-ol | (E)-2-hexen-1-ol, acetate | (E)-2-hexenal | 1-butanol | 1-hexanol | 1-octanol | 2-heptanone | 2-methylbutyl Acetate | Acetic Acid | Amyl Acetate | Benzaldehyde | Butyl Acetate | Butyl Butyrate | E-nerolidol | ethyl 2-methylbutyrate | Ethyl Acetate | Ethyl Butyrate | Ethyl Hexanoate | Hexanal | Hexanoic Acid | hexyl 2-methylbutyrate | Hexyl Acetate | Hexyl Butyrate | Isoamyl Acetate | Linalool | Methyl Acetate | Methyl Butyrate | Methyl Hexanoate | Propyl Acetate | Propyl Propionate | α-ionone | β-ionone | γ-decalactone | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic | ||||||||||||||||||||||||||||||||||
| No. | 3 | 2 | 3 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 2 | 1 | 1 | ||||
| min | −30.2 | −37.0 | −33.9 | −31.4 | −24.4 | −29.2 | −29.4 | −34.8 | −42.0 | −32.5 | −26.0 | −28.6 | −26.8 | −32.0 | −27.0 | −27.1 | −25.7 | −32.8 | −26.2 | −35.6 | −27.6 | −27.0 | −33.5 | −28.5 | −25.6 | −26.7 | −26.7 | −35.9 | −27.2 | −25.5 | ||||
| max | −28.0 | −33.2 | −33.4 | −31.4 | −24.4 | −28.8 | −29.4 | −32.9 | −42.0 | −32.5 | −25.3 | −28.6 | −26.8 | −32.0 | −24.7 | −27.0 | −16.5 | −26.2 | −25.3 | −35.6 | −27.6 | −27.0 | −33.5 | −18.6 | −25.6 | −26.7 | −26.7 | −35.1 | −27.2 | −25.5 | ||||
| Apple | ||||||||||||||||||||||||||||||||||
| No. | 19 | 1 | 127 | 20 | 59 | 4 | 100 | 60 | 33 | 100 | 33 | 15 | 13 | 18 | 6 | 121 | 35 | 102 | 24 | 2 | 25 | 9 | ||||||||||||
| min | −38.8 | −31.9 | −47.0 | −44.5 | −43.7 | −33.3 | −37.1 | −39.0 | −33.3 | −40.1 | −41.4 | −34.3 | −33.0 | −36.2 | −38.4 | −45.0 | −41.9 | −40.4 | −40.0 | −36.9 | −37.9 | −35.7 | ||||||||||||
| max | −32.2 | −31.9 | −29.4 | −40.8 | −35.7 | −31.5 | −26.0 | −26.7 | −28.0 | −30.4 | −32.1 | −27.0 | −26.2 | −28.5 | −30.8 | −30.1 | −31.0 | −26.6 | −34.8 | −36.4 | −30.2 | −29.6 | ||||||||||||
| Strawberry | ||||||||||||||||||||||||||||||||||
| No. | 12 | 10 | 22 | 13 | 7 | 13 | 1 | 5 | 16 | 10 | 5 | 3 | 24 | 29 | 26 | 7 | 6 | 27 | 1 | 3 | 8 | 20 | 29 | 28 | 7 | |||||||||
| min | −38.2 | −32.4 | −38.5 | −37.5 | −36.5 | −27.6 | −33.4 | −31.3 | −35.4 | −37.6 | −33.8 | −29.1 | −36.7 | −34.8 | −32.4 | −32.2 | −29.9 | −35.0 | −35.0 | −33.7 | −26.9 | −37.8 | −33.8 | −29.2 | −35.6 | |||||||||
| max | −32.4 | −29.5 | −27.9 | −30.2 | −34.4 | −20.7 | −33.4 | −28.1 | −29.9 | −33.0 | −27.8 | −27.4 | −25.7 | −28.6 | −24.8 | −27.5 | −20.2 | −28.6 | −35.0 | −30.2 | −24.5 | −30.9 | −29.2 | −24.1 | −28.7 | |||||||||
| Blueberry | ||||||||||||||||||||||||||||||||||
| No. | 5 | 1 | 1 | 3 | 1 | 1 | 4 | |||||||||||||||||||||||||||
| min | −39.6 | −28.4 | −29.1 | −28.2 | −32.7 | −28.5 | −38.2 | |||||||||||||||||||||||||||
| max | −31.8 | −28.4 | −29.1 | −25.2 | −32.7 | −28.5 | −32.5 | |||||||||||||||||||||||||||
| Pear | ||||||||||||||||||||||||||||||||||
| No. | 1 | 9 | 4 | 3 | 4 | 5 | 7 | 6 | 9 | 6 | 1 | |||||||||||||||||||||||
| min | −33.4 | −41.6 | −37.0 | −30.8 | −31.9 | −32.6 | −37.7 | −28.4 | −43.4 | −37.2 | −30.9 | |||||||||||||||||||||||
| max | −33.4 | −36.3 | −33.5 | −27.3 | −27.5 | −31.2 | −30.5 | −26.0 | −33.9 | −29.3 | −30.9 | |||||||||||||||||||||||
| Raspberry | ||||||||||||||||||||||||||||||||||
| No. | 2 | 2 | 5 | 6 | 5 | 8 | 6 | |||||||||||||||||||||||||||
| min | −29.6 | −32.7 | −31.2 | −30.9 | −32.0 | −34.2 | −33.4 | |||||||||||||||||||||||||||
| max | −28.0 | −31.4 | −30.2 | −27.1 | −30.1 | −32.6 | −32.4 | |||||||||||||||||||||||||||
| Peach | ||||||||||||||||||||||||||||||||||
| No. | 5 | 1 | 3 | 3 | 5 | 2 | ||||||||||||||||||||||||||||
| min | −37.0 | −31.0 | −31.2 | −31.1 | −33.7 | −37.7 | ||||||||||||||||||||||||||||
| max | −34.2 | −31.0 | −29.8 | −28.6 | −29.7 | −37.5 | ||||||||||||||||||||||||||||
| Sour cherry | ||||||||||||||||||||||||||||||||||
| No. | 4.0 | 3.0 | 1.0 | |||||||||||||||||||||||||||||||
| min | −32.8 | −27.8 | −25.5 | |||||||||||||||||||||||||||||||
| max | −29.1 | −25.5 | −25.5 | |||||||||||||||||||||||||||||||
| Synthetic literature | ||||||||||||||||||||||||||||||||||
| min | −28.0 | −31.5 | −27.0 | −28.8 | −34.8 | −32.5 | −26.0 | −31.3 | −26.8 | −27.2 | −27.1 | −25.7 | −25.5 | −27.0 | −36.1 | |||||||||||||||||||
| max | −28.0 | −27.7 | −24.4 | −28.8 | −34.8 | −32.5 | −26.0 | −28.6 | −26.8 | −22.8 | −27.1 | −25.7 | −25.5 | −26.2 | −36.1 | |||||||||||||||||||
| Natural literature | ||||||||||||||||||||||||||||||||||
| min | −43.8 | −39.4 | −44.5 | −42.5 | −41.9 | −34.4 | −37.0 | −34.1 | −39.8 | −41.4 | −27.9 | −33.0 | −32.6 | −31.2 | −38.9 | −33.2 | −38.3 | −32.9 | −36.8 | −33.7 | −28.2 | |||||||||||||
| max | −36.8 | −27.3 | −37.0 | −36.9 | −38.3 | −30.5 | −30.0 | −29.7 | −31.3 | −33.6 | −27.9 | −33.0 | −28.5 | −30.0 | −34.9 | −32.1 | −29.7 | −30.3 | −28.0 | −29.3 | −26.5 | |||||||||||||
| Aroma Compound | Natural Compound | Synthetic Compound | Detectable Synthetic Fraction | |||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Median | Min | Max | Median | x | x | |
| δ13C (‰) | δ13C (‰) | 50% Threshold | 100% Threshold | |||||
| γ-decalactone | −37.7 | −28.7 | −31.5 | −26.9 | −24.4 | −26.1 | 0.52 | 0.83 |
| 1-butanol | −44.5 | −40.8 | −42.3 | −31.4 | −31.4 | −31.4 | 0.14 | 0.28 |
| 1-hexanol | −43.7 | −30.2 | −39.4 | −24.4 | −24.4 | −24.4 | 0.61 | 0.70 |
| 1-octanol | −36.5 | −31.5 | −34.9 | −29.2 | −28.8 | −29.0 | 0.57 | 0.68 |
| 2-heptanone | −27.6 | −20.7 | −24.8 | −29.4 | −29.4 | −29.4 | 0.61 | 0.80 |
| (E)-2-hexen-1-ol | −38.8 | −32.2 | −34.9 | −30.2 | −28.0 | −30.1 | 0.56 | 0.78 |
| (E)-2-hexen-1-ol, acetate, | −32.4 | −29.5 | −30.8 | −37.0 | −33.2 | −35.1 | 0.37 | 0.78 |
| acetic acid | −31.3 | −28.1 | −30.2 | −42.0 | −42.0 | −42.0 | 0.09 | 0.23 |
| benzaldehyde | −35.4 | −28.0 | −31.3 | −26.0 | −25.3 | −25.7 | 0.59 | 0.79 |
| butyl acetate | −40.1 | −30.4 | −35.8 | −28.6 | −28.6 | −28.6 | 0.75 | 0.84 |
| butyl butyrate | −41.4 | −32.1 | −36.8 | −26.8 | −26.8 | −26.8 | 0.47 | 0.64 |
| ethyl 2-methyl butyrate | −34.3 | −27.0 | −29.3 | −27.0 | −24.7 | −25.8 | 0.66 | 1.00 |
| ethyl butyrate | −36.2 | −28.5 | −30.9 | −25.7 | −16.5 | −21.1 | 0.24 | 0.73 |
| hexanal | −45.0 | −27.5 | −38.0 | −26.2 | −25.3 | −25.5 | 0.83 | 0.93 |
| hexanoic acid | −29.9 | −20.2 | −25.8 | −35.6 | −35.6 | −35.6 | 0.42 | 0.63 |
| hexyl 2-methyl butyrate | −41.9 | −31.0 | −34.4 | −27.6 | −27.6 | −27.6 | 0.49 | 0.76 |
| hexyl butyrate | −40.0 | −34.8 | −36.2 | −33.5 | −33.5 | −33.5 | 0.52 | 0.80 |
| isoamyl acetate | −36.9 | −30.2 | −32.3 | −28.5 | −18.6 | −23.5 | 0.24 | 0.80 |
| methyl acetate | −38.2 | −30.9 | −34.3 | −26.7 | −26.7 | −26.7 | 0.45 | 0.63 |
| methyl butyrate | −33.8 | −29.2 | −31.1 | −26.7 | −26.7 | −26.7 | 0.44 | 0.66 |
| methyl hexanoate | −29.2 | −24.1 | −27.3 | −35.9 | −35.1 | −35.5 | 0.23 | 0.47 |
| propyl acetate | −37.9 | −30.2 | −33.4 | −27.2 | −27.2 | −27.2 | 0.52 | 0.72 |
| propyl propionate | −35.7 | −29.6 | −31.9 | −25.5 | −25.5 | −25.5 | 0.36 | 0.60 |
| α-ionone | −34.2 | −32.6 | −33.4 | −28.0 | −21.6 | −25.9 | 0.11 | 0.26 |
| β-ionone | −33.4 | −32.4 | −32.7 | −28.6 | −25.6 | −27.7 | 0.05 | 0.19 |
| Aroma Compound | Commercial Fruit Distillate Isotope Value | Min Estimated Synthetic Fraction | Median Estimated Synthetic Fraction | Max Estimated Synthetic Fraction |
|---|---|---|---|---|
| δ13C (‰) | x | x | x | |
| hexanal | −26.5 | 0.46 | 0.92 | 0.99 |
| butyl acetate | −29.8 | 0.34 | 0.83 | 0.89 |
| benzaldehyde | −26.4 | 0.59 | 0.86 | 0.95 |
| ethyl butyrate | −26.0 | 0.21 | 0.50 | 0.97 |
| methyl hexanoate | −34.0 | 0.71 | 0.81 | 0.89 |
| methyl acetate | −27.0 | 0.92 | 0.96 | 0.97 |
| 2-heptanone | −28.3 | 0.38 | 0.76 | 0.87 |
| hexanoic acid | −34.8 | 0.86 | 0.92 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strojnik, L.; Hladnik, J.; Weber, N.C.; Koron, D.; Stopar, M.; Zlatić, E.; Kokalj, D.; Strojnik, M.; Ogrinc, N. Construction of IsoVoc Database for the Authentication of Natural Flavours. Foods 2021, 10, 1550. https://doi.org/10.3390/foods10071550
Strojnik L, Hladnik J, Weber NC, Koron D, Stopar M, Zlatić E, Kokalj D, Strojnik M, Ogrinc N. Construction of IsoVoc Database for the Authentication of Natural Flavours. Foods. 2021; 10(7):1550. https://doi.org/10.3390/foods10071550
Chicago/Turabian StyleStrojnik, Lidija, Jože Hladnik, Nika Cvelbar Weber, Darinka Koron, Matej Stopar, Emil Zlatić, Doris Kokalj, Martin Strojnik, and Nives Ogrinc. 2021. "Construction of IsoVoc Database for the Authentication of Natural Flavours" Foods 10, no. 7: 1550. https://doi.org/10.3390/foods10071550
APA StyleStrojnik, L., Hladnik, J., Weber, N. C., Koron, D., Stopar, M., Zlatić, E., Kokalj, D., Strojnik, M., & Ogrinc, N. (2021). Construction of IsoVoc Database for the Authentication of Natural Flavours. Foods, 10(7), 1550. https://doi.org/10.3390/foods10071550