Identification of Erythromycin and Clarithromycin Metabolites Formed in Chicken Liver Microsomes Using Liquid Chromatography–High-Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Standard Stock and Working Solutions
2.3. Microsomal Incubations
2.4. LC/ESI-HR-MS Analysis
2.5. Data Analysis
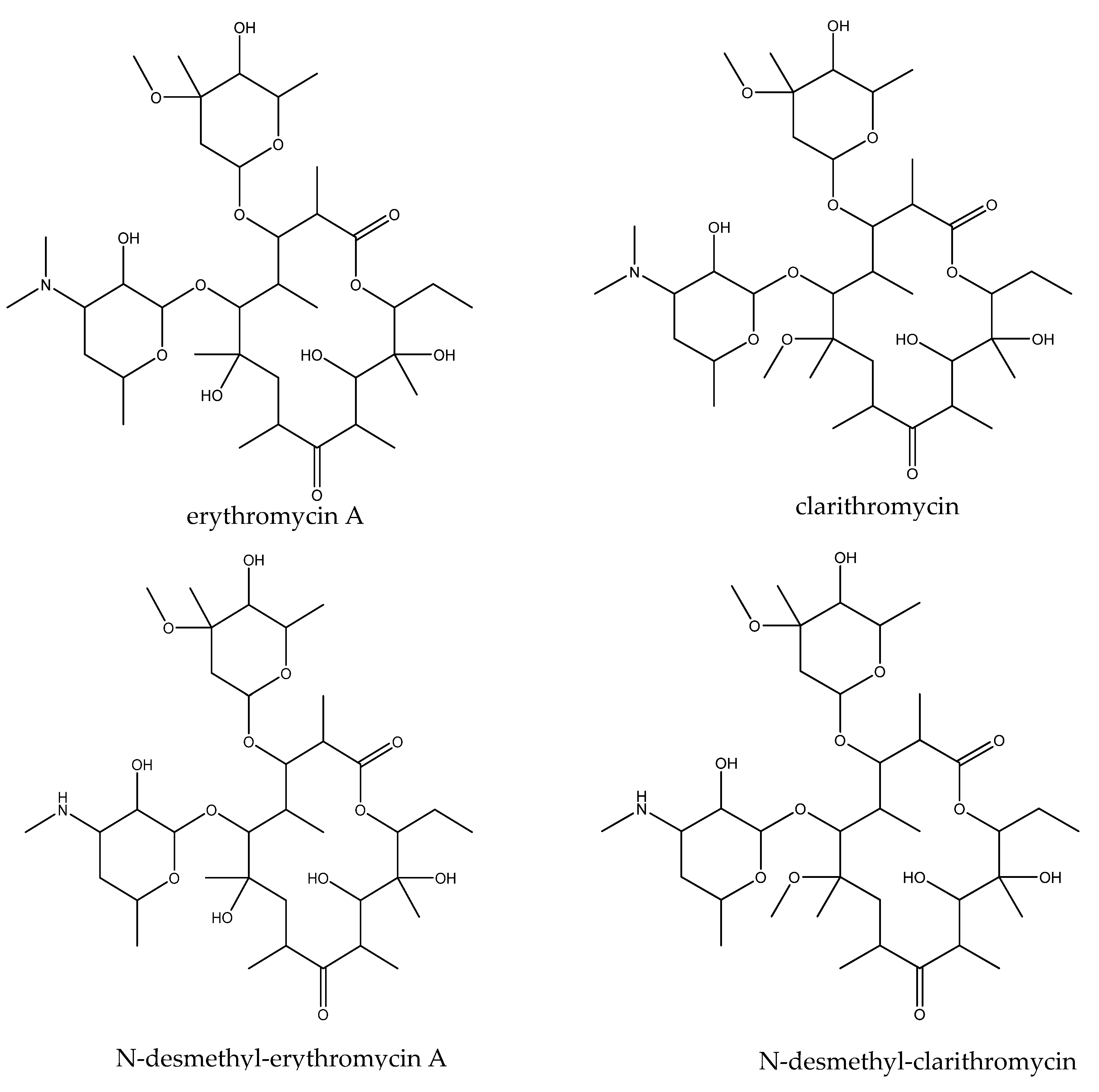
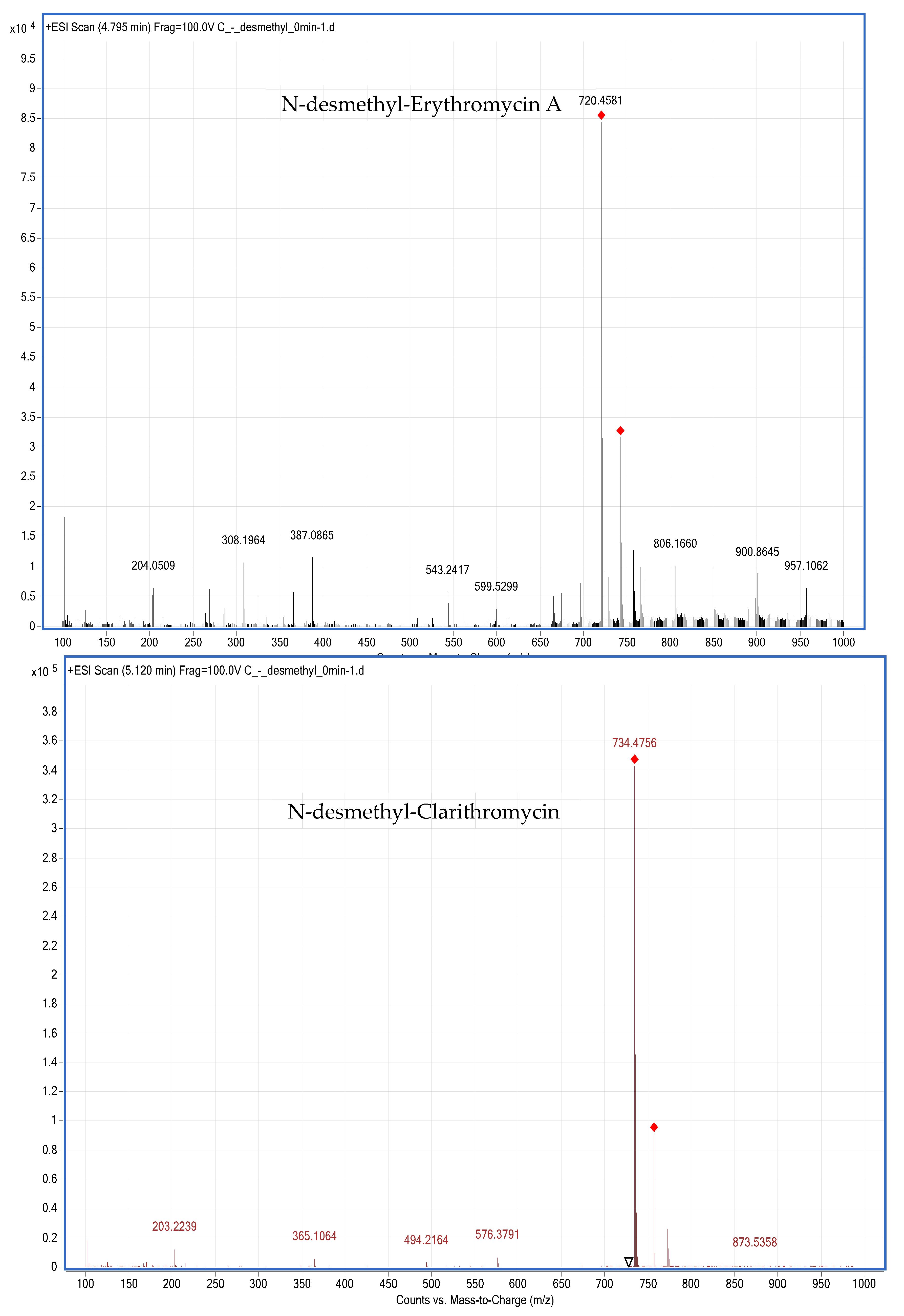
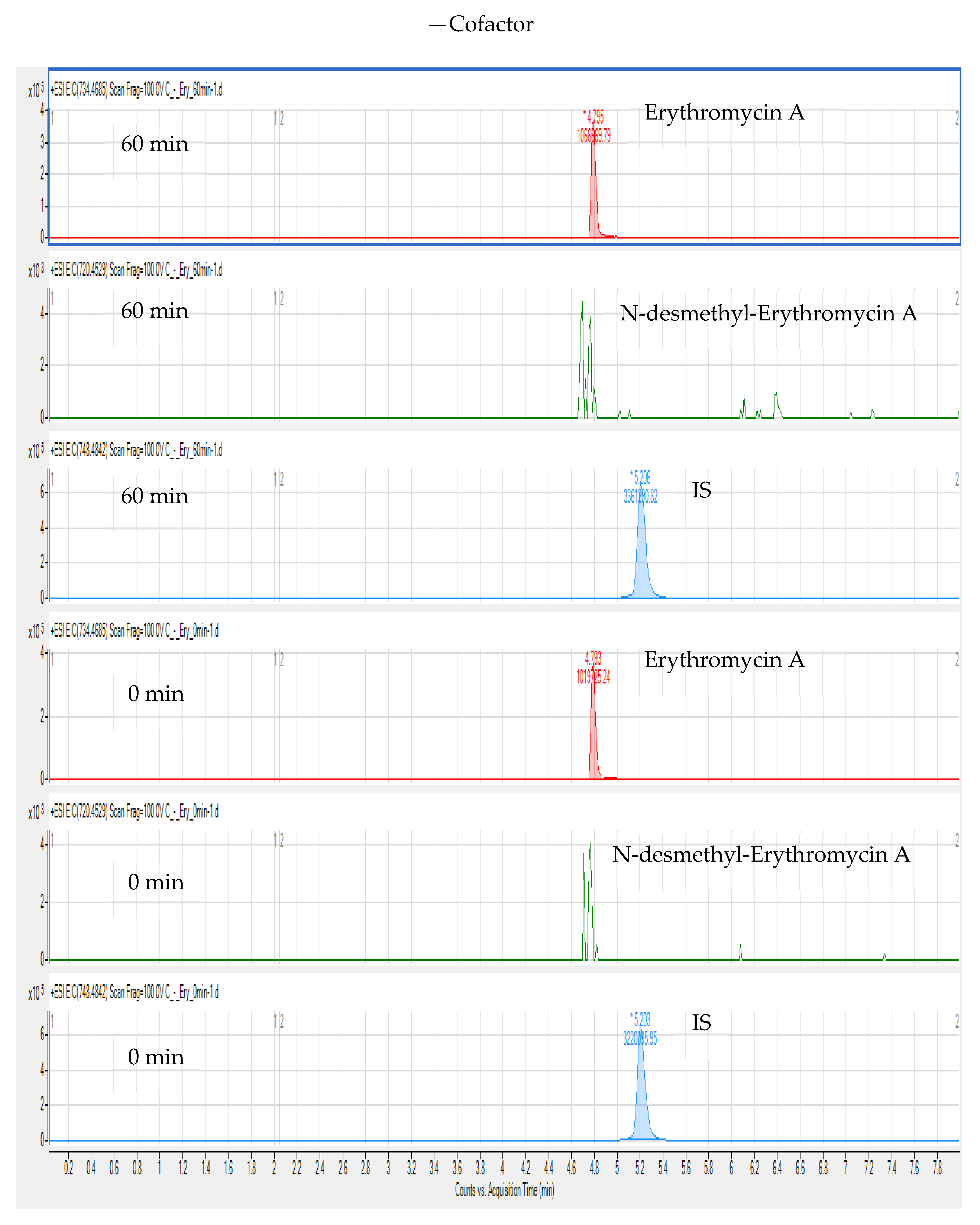
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vannuffel, P.; Cocito, C. Mechanism of action of streptogramins and macrolides. Drugs 1996, 51, 20–30. [Google Scholar] [CrossRef]
- Schănfeld, W.; Kirst, H.A. Macrolide Antibiotics; Birkhauser Verlag: Basel, Switzerland, 2002. [Google Scholar]
- Martin, S.J.; Garvin, C.G.; McBurney, C.R.; Sahloff, E.G. The activity of 14-hydroxy clarithromycin, alone and in combination with clarithromycin, against penicillin- and erythromycin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 2001, 47, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Yin, D.; Yang, Z.; Zhao, W.; Chen, Y.; Zhang, W.; Zhang, S. Determination of six macrolide antibiotics in chicken sample by liquid chromatography-tandem mass spectrometry based on solid phase extraction. J. Anal. Methods Chem. 2019, 2019, 6849457. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. National Food Safety Standard-Maximum Residue Limits for Veterinary Drugs in Foods; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2019.
- US Food and Drug Administration. CFR-Code of Federal Regulations Title 21 Part 556 Tolerances for Residue of New Animal Drugs in Food; US Food and Drug Administration: Rockville, MD, USA, 2014.
- The European Medicines Agency. Commission Regulation (EU) No. 37/2010 of 22 December 2009 on Pharmacologically Active Sub-stances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; The European Medicines Agency: Amsterdam, The Netherlands, 2010. [Google Scholar]
- JECFA. Residue Evaluation of Certain Veterinary Drugs (Seventy-Fifth Report of the Joint FAO/WHO Expert Committee on Food Additives); Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Lam, J.L.; Okochi, H.; Huang, Y.; Benet, L.Z. In vitro and in vivo correlation of hepatic transporter effects on erythromycin metabolism: Characterizing the importance of transporter-enzyme interplay. Drug Metab. Dispos. 2006, 34, 1336–1344. [Google Scholar] [CrossRef] [Green Version]
- Sartori, E.; Delaforge, M.; Mansuy, D. In vitro interaction of rat liver cytochromes P-450 with erythromycin, oleandomycin and erythralosamine derivatives. Importance of structural factors. Biochem. Pharmacol. 1989, 38, 2061–2068. [Google Scholar] [CrossRef]
- Echizen, H.; Kawasaki, H.; Chiba, K.; Tani, M.; Ishizaki, T. A potent inhibitory effect of erythromycin and other macrolide antibiotics on the mono-N-dealkylation metabolism of disopyramide with human liver microsomes. J. Pharmacol. Exp. Ther. 1993, 264, 1425–1431. [Google Scholar]
- Nduka, S.O.; Okonta, M.J.; Ajaghaku, D.L.; Ukwe, C.V. In vitro and in vivo cytochrome P450 3A enzyme inhibition by Aframomum melegueta and Denniettia tripetala extracts. Asian Pac. J. Trop. Med. 2017, 10, 576–581. [Google Scholar] [CrossRef]
- Nduka, S.O.; Okonta, M.J.; Ajaghaku, D.L.; Amorha, K.C.; Ukwe, C.V. Inhibition of cytochrome P450 3A enzyme by Millettia aboensis: Its effect on the pharmacokinetic properties of efavirenz and nevirapine. Rev. Bras. Farmacogn. 2017, 27, 228–235. [Google Scholar] [CrossRef]
- Murcia, H.W.; Díaz, G.J.; Cepeda, S.M. Enzymatic activity in Turkey, duck, quail and chicken liver microsomes against four human cytochrome P450 prototype substrates and aflatoxin B1. J. Xenobiot. 2011, 1, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.P.; Kawai, Y.K.; Ikenaka, Y.; Kawata, M.; Ikushiro, S.; Sakaki, T.; Ishizuka, M. Avian cytochrome P450 (CYP) 1-3 family genes: Isoforms, evolutionary relationships, and mRNA expression in chicken liver. PLoS ONE 2013, 8, e75689. [Google Scholar] [CrossRef] [Green Version]
- Palócz, O.; Szita, G.; Csikó, G. Alteration of avian hepatic cytochrome P450 gene expression and activity by certain feed additives. Acta Vet. Hung. 2019, 67, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M.; Boermans, H.J. The role of selected cytochrome P450 enzymes on the bioactivation of aflatoxin B1 by duck liver microsomes. Avian Pathol. 2010, 39, 279–285. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Chen, B.; Zhang, J.P.; Chen, N.; Liu, C.Z.; Hu, C.Q. Liver toxicity of macrolide antibiotics in zebrafish. Toxicology 2020, 441, 152501. [Google Scholar] [CrossRef]
- Wang, B.; Xie, K.; Lee, K. Veterinary drug residues in animal-derived foods: Sample preparation and analytical methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef]
- Horie, M.; Takegami, H.; Toya, K.; Nakazawa, H. Determination of macrolide antibiotics in meat and fish by liquid chromatography-electrospray mass spectrometry. Anal. Chim. Acta 2003, 492, 187–197. [Google Scholar] [CrossRef]
- Wang, J.; Leung, D.; Butterworth, F. Determination of five macrolide antibiotic residues in eggs using liquid chromatography/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Moltó, J.C.; Mañes, J.; Font, G. Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control 2010, 21, 1703–1709. [Google Scholar] [CrossRef]
- Tao, Y.; Yu, G.; Chen, D.; Pan, Y.; Liu, Z.; Wei, H.; Peng, D.; Huang, L.; Wang, Y.; Yuan, Z. Determination of 17 macrolide antibiotics and avermectins residues in meat with accelerated solvent extraction by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 897, 64–71. [Google Scholar] [CrossRef]
- Zhou, W.; Ling, Y.; Liu, T.; Zhang, Y.; Li, J.; Li, H.; Wu, W.; Jiang, S.; Feng, F.; Yuan, F.; et al. Simultaneous determination of 16 macrolide antibiotics and 4 metabolites in milk by using Quick, Easy, Cheap, Effective, Rugged, and Safe extraction (QuEChERS) and high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1061–1062, 411–420. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, C.; Lu, X.; Xu, G. Nontargeted screening of chemical contaminants and illegal additives in food based on liquid chromatography-high resolution mass spectrometry. Trends Anal. Chem. 2017, 96, 89–98. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Chen, J.; Liu, H.; Lu, X.; Wu, J.; Zhang, Y.; Lin, Y.; Liu, Q.; Wang, H.; et al. Sensitive untargeted screening of nerve agents and their degradation products using liquid chromatography-high resolution mass spectrometry. Anal. Chem. 2020, 92, 10578–10587. [Google Scholar] [CrossRef] [PubMed]
- Knolhoff, A.M.; Zweigenbaum, J.A.; Croley, T.R. Nontargeted screening of food matrices: Development of a chemometric software strategy to identify unknowns in liquid chromatography-mass spectrometry data. Anal. Chem. 2016, 88, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, M.; Winter, M.; Åberg, M.; Hellenäs, K.E.; Rosén, J. Non-targeted analysis of unexpected food contaminants using LC-HRMS. Anal. Bioanal. Chem. 2018, 410, 5593–5602. [Google Scholar] [CrossRef] [Green Version]
- Anumol, T.; Lehotay, S.J.; Stevens, J.; Zweigenbaum, J. Comparison of veterinary drug residue results in animal tissues by ultrahigh-performance liquid chromatography coupled to triple quadrupole or quadrupole-time-of-flight tandem mass spectrometry after different sample preparation methods, including use of a commercial lipid removal product. Anal. Bioanal. Chem. 2017, 409, 2639–2653. [Google Scholar] [CrossRef]
- Boix, C.; Ibáñez, M.; Sancho, J.V.; León, N.; Yusá, V.; Hernández, F. Qualitative screening of 116 veterinary drugs in feed by liquid chromatography-high resolution mass spectrometry: Potential application to quantitative analysis. Food Chem. 2014, 160, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Knolhoff, A.M.; Croley, T.R. Non-targeted screening approaches for contaminants and adulterants in food using liquid chromatography hyphenated to high resolution mass spectrometry. J. Chromatogr. A 2016, 1428, 86–96. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Widmer, M. Quantitative multiresidue method for about 100 veterinary drugs in different meat matrices by sub 2-microm particulate high-performance liquid chromatography coupled to time of flight mass spectrometry. J. Chromatogr. A 2008, 1194, 66–79. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Zhou, Z.; Lu, X.; Lin, X.; Zhao, C.; Xu, G. Screening and determination of potential risk substances based on liquid chromatography-high-resolution mass spectrometry. Anal. Chem. 2018, 90, 8454–8461. [Google Scholar] [CrossRef]
- Jia, W.; Shi, L.; Chu, X.; Chang, J.; Chen, Y.; Zhang, F. A strategy for untargeted screening of macrolides and metabolites in bass by liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Food Chem. 2018, 262, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.M.; Watkins, P.B.; Saenger, P.; Stave, G.M.; Barlascini, N.; Watlington, C.O.; Wright, J.T., Jr.; Guzelian, P.S. Heterogeneity of CYP3A isoforms metabolizing erythromycin and cortisol. Clin. Pharmacol. Ther. 1992, 51, 18–23. [Google Scholar] [CrossRef]
- Ferrero, J.L.; Bopp, B.A.; Marsh, K.C.; Quigley, S.C.; Johnson, M.J.; Anderson, D.J.; Lamm, J.E.; Tolman, K.G.; Sanders, S.W.; Cavanaugh, J.H.; et al. Metabolism and disposition of clarithromycin in man. Drug Metab. Dispos. 1990, 18, 441–446. [Google Scholar]
- Zhou, Y.; Oh, M.H.; Kim, Y.J.; Kim, E.Y.; Kang, J.; Chung, S.; Ju, C.; Kim, W.K.; Lee, K. Metabolism and pharmacokinetics of SP-8356, a novel (1S)-(−)-verbenone derivative, in rats and dogs and its implications in humans. Molecules 2020, 25, 1775. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jang, H.; Lee, J.Y.; Kwon, K.I.; Oh, S.J.; Kim, S.K. Inhibition of cytochrome P450 by ethambutol in human liver microsomes. Toxicol. Lett. 2014, 229, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, W.; Jimenez, H.; Zhang, D.; Yeola, S.; Dai, R.; Vachharajani, N.; Mitroka, J. Cytochrome P450 3A-mediated metabolism of buspirone in human liver microsomes. Drug Metab. Dispos. 2005, 33, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drugs.com. Buspirone Monograph. Available online: https://www.drugs.com/pro/buspirone.html (accessed on 27 August 2011).




| Analyte | Matrix | +Cofactor | Mean ± SD (%) | −Cofactor | Mean ± SD (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Remaining Content (%) | Remaining Content (%) | ||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| BUS | CLM | 46.4 | 46.2 | 54.1 | 48.9 ± 4.5 | 87.5 | 65.7 | 91.2 | 81.5 ± 13.8 |
| ERY | CLM | 82.6 | 83.1 | 94.1 | 86.6 ± 6.5 | 99.0 | 97.9 | 87.6 | 94.8 ± 6.3 |
| CLA | CLM | 77.5 | 77.3 | 114.7 | 89.8 ± 21.5 | 100.7 | 78.7 | 80.3 | 86.6 ± 12.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Nam, S.; Kim, E.; Jeon, H.; Lee, K.; Xie, K. Identification of Erythromycin and Clarithromycin Metabolites Formed in Chicken Liver Microsomes Using Liquid Chromatography–High-Resolution Mass Spectrometry. Foods 2021, 10, 1504. https://doi.org/10.3390/foods10071504
Wang B, Nam S, Kim E, Jeon H, Lee K, Xie K. Identification of Erythromycin and Clarithromycin Metabolites Formed in Chicken Liver Microsomes Using Liquid Chromatography–High-Resolution Mass Spectrometry. Foods. 2021; 10(7):1504. https://doi.org/10.3390/foods10071504
Chicago/Turabian StyleWang, Bo, Soyeon Nam, Eunyeong Kim, Hayoung Jeon, Kiho Lee, and Kaizhou Xie. 2021. "Identification of Erythromycin and Clarithromycin Metabolites Formed in Chicken Liver Microsomes Using Liquid Chromatography–High-Resolution Mass Spectrometry" Foods 10, no. 7: 1504. https://doi.org/10.3390/foods10071504
APA StyleWang, B., Nam, S., Kim, E., Jeon, H., Lee, K., & Xie, K. (2021). Identification of Erythromycin and Clarithromycin Metabolites Formed in Chicken Liver Microsomes Using Liquid Chromatography–High-Resolution Mass Spectrometry. Foods, 10(7), 1504. https://doi.org/10.3390/foods10071504






