Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Heat Resistance Determinations
2.3. Mathematical Models of Microbial Inactivation
2.4. Data Analysis
3. Results and Discussion
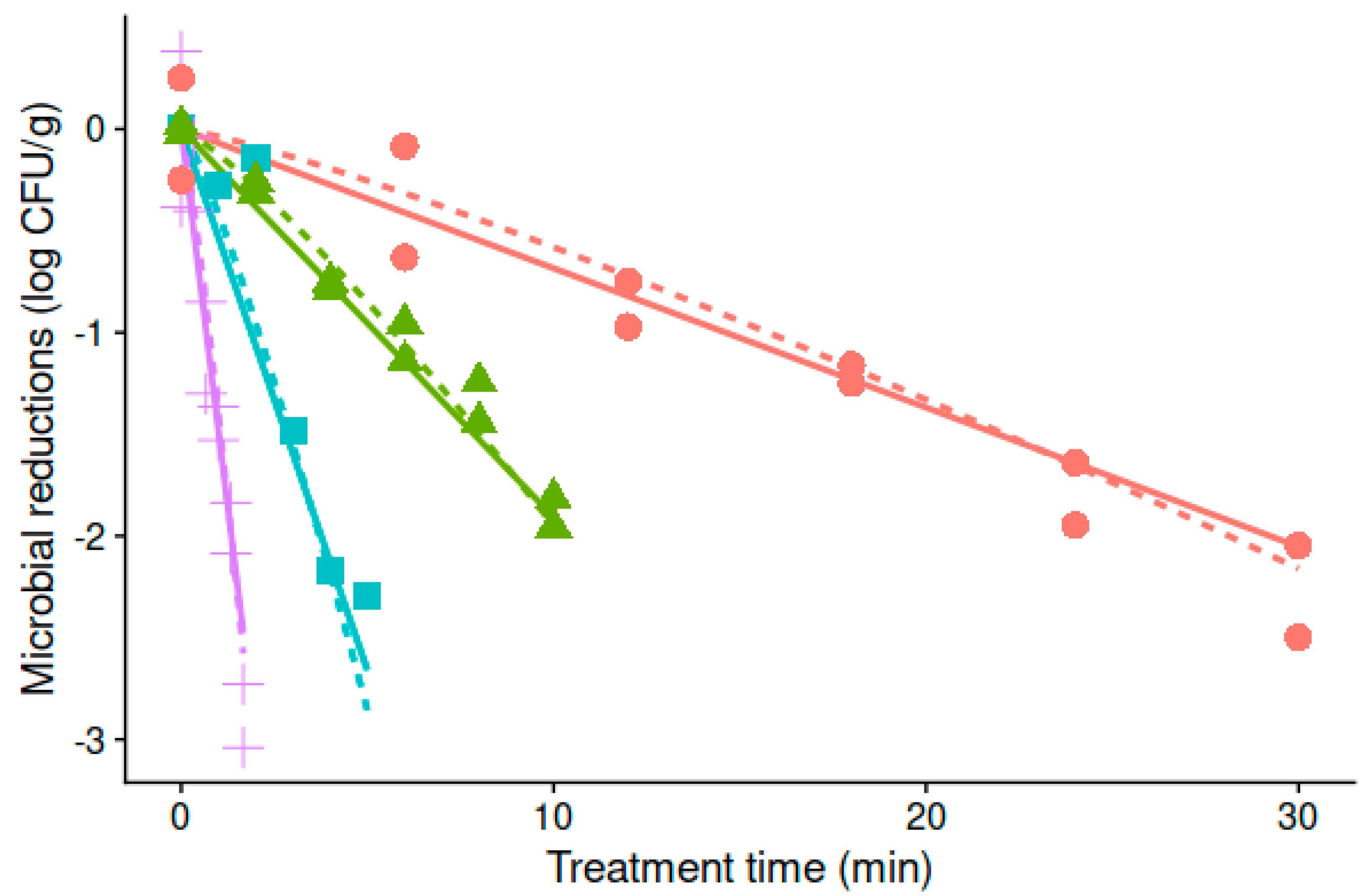
3.1. Thermal Resistance of Alicyclobacillus Acidoterrestris under Isothermal Treatment Conditions
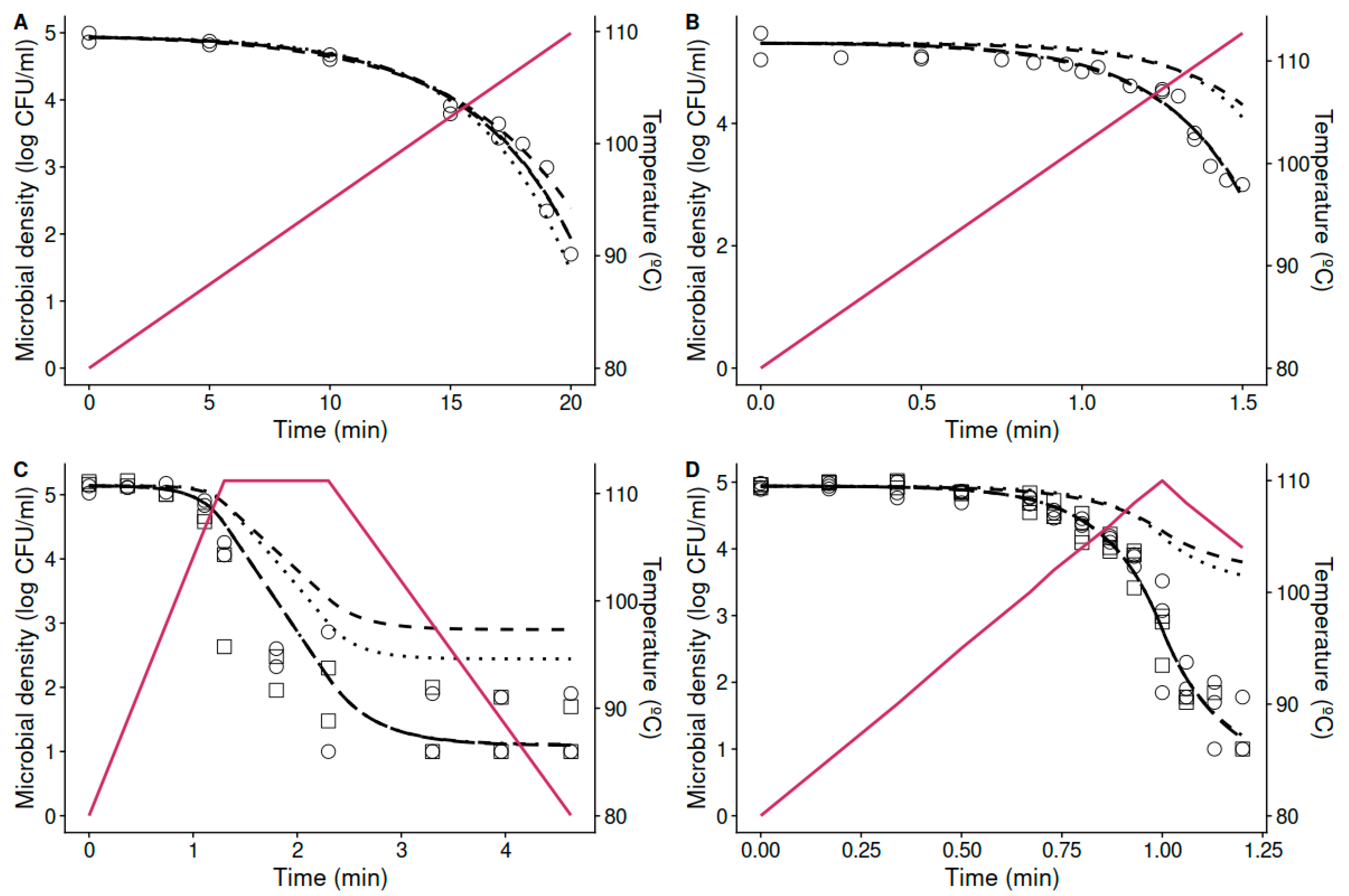
3.2. Prediction of Non-Isothermal Inactivation Based on Isothermal Data
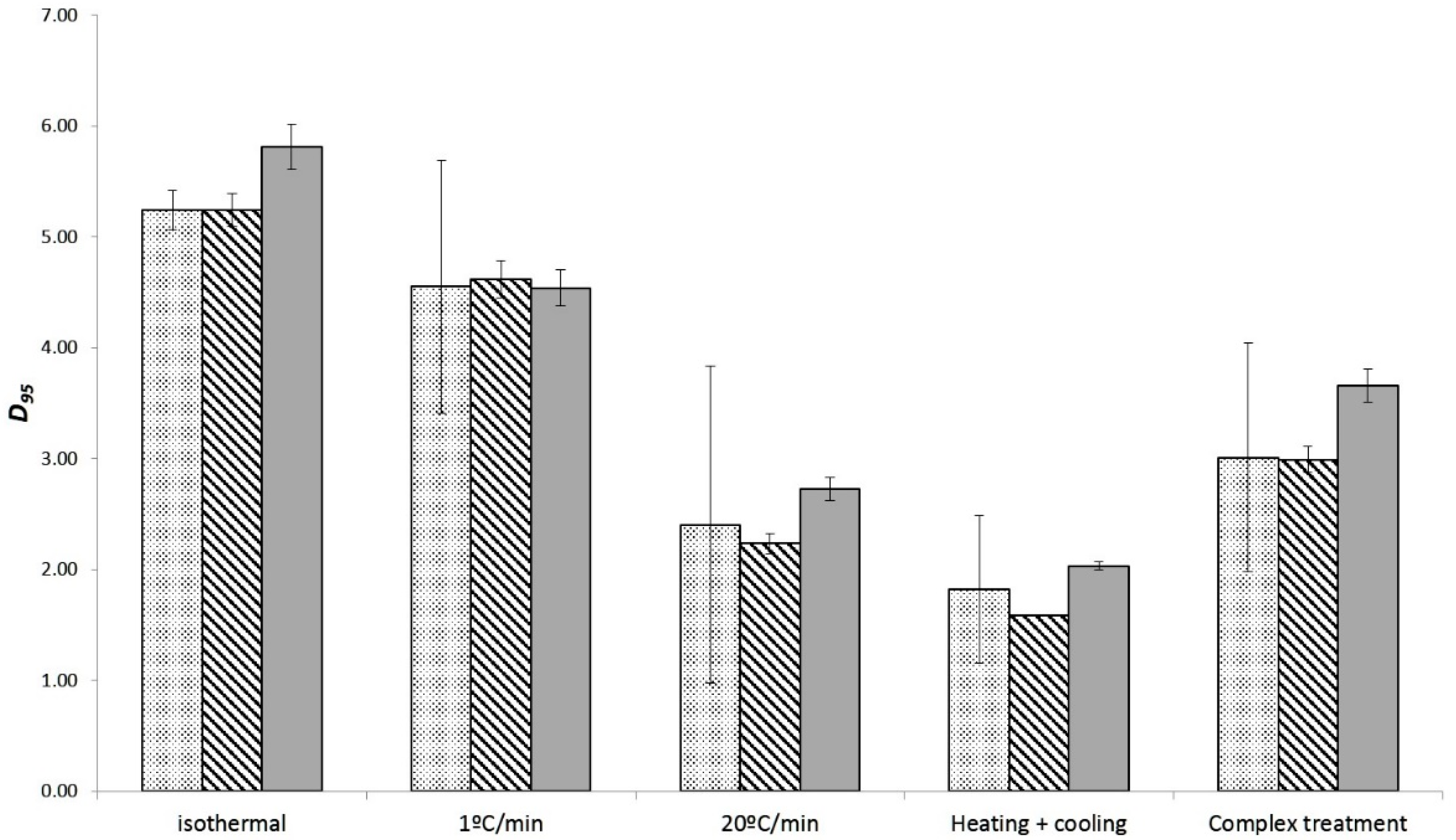
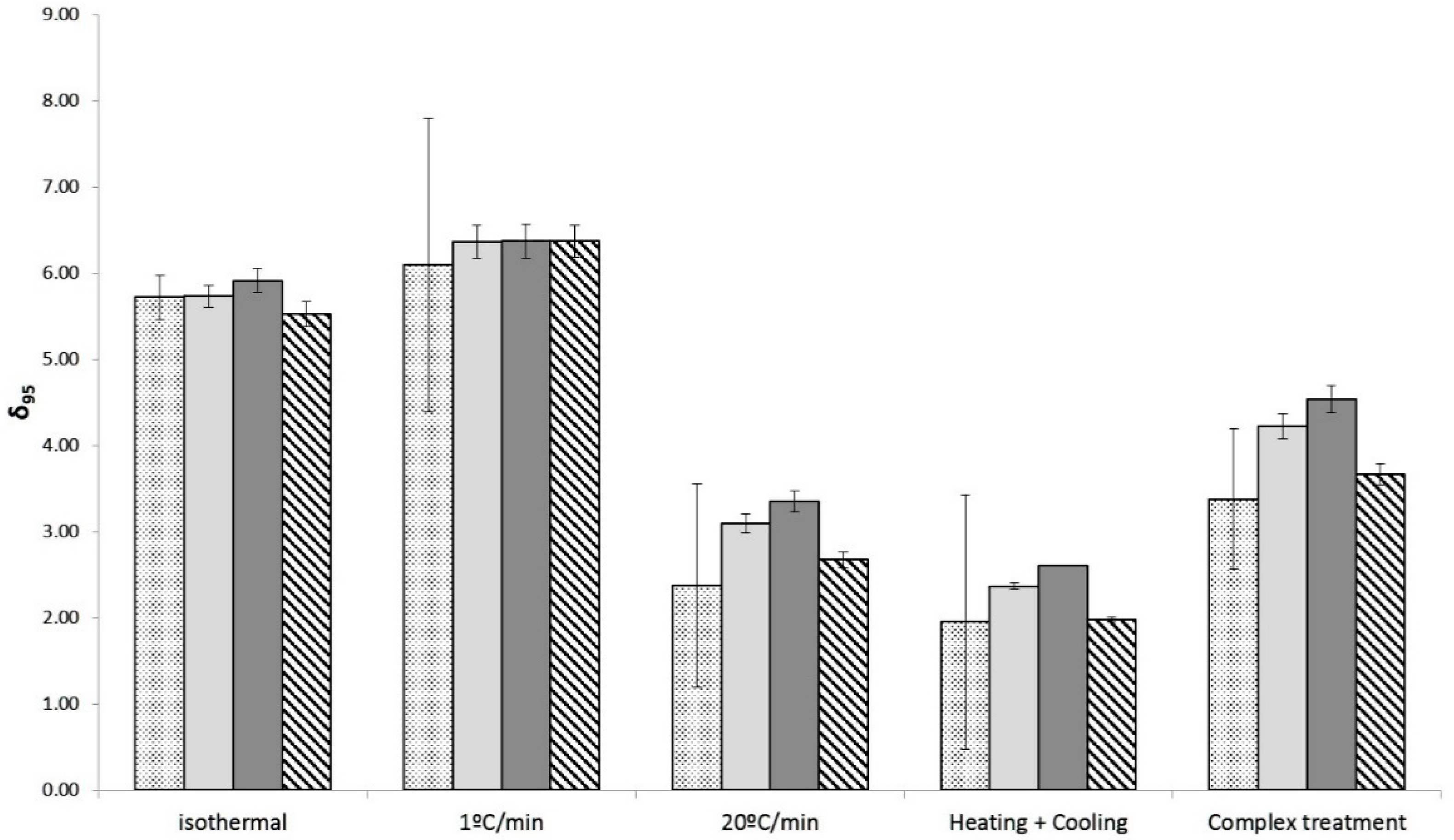
3.3. Estimation of the Thermal Resistance and the Effect of the Heating Rates from Non-Isothermal Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, S.E.; Miller, A.J. Microbiological safety of fresh citrus and apple juices. In Microbiology of Fruits and Vegetables; Sapers, J.R.G.G.M., Yousef, A.E., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 211–230. [Google Scholar]
- Walker, M.; Phillips, C. Alicyclobacillus acidoterrestris: An increasing threat to the fruit juice industry? Int. J. Food Sci. Technol. 2008, 43, 250–260. [Google Scholar] [CrossRef]
- Parish, M.E. Food safety issues and the microbiology of fruit beverages and bottled water. In Microbiologically Safe Foods; Hereida, I.W.S.G.N., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 279–297. [Google Scholar]
- Bevilacqua, A.; Sinigaglia, M.; Corbo, M. Effects of pH, cinnamaldehyde and heat-treatment time on spore viability of Alicyclobacillus acidoterrestris. Int. J. Food Sci. Technol. 2009, 44, 340–385. [Google Scholar] [CrossRef]
- Tianli, Y.; Jiangbo, Z.; Yahong, Y. Spoilage by Alicyclobacillus bacteria in juice and beverage products: Chemical, physical, and combined control methods. Comprenhensive Rev. Food Sci. Food Saf. 2014, 13, 771–797. [Google Scholar] [CrossRef]
- Merle, J.; Montville, T.J. Alicyclobacillus acidoterrestris: The organism, the challenge, potential interventions. J. Food Proces. Preserv. 2014, 38, 153–158. [Google Scholar] [CrossRef]
- Smit, Y.; Cameron, M.; Venter, P.; Witthuhn, R.C. Alicyclobacillus spoilage and isolation—A review. Food Microbiol. 2011, 28, 331–349. [Google Scholar] [CrossRef]
- McKnight, I.C.; Eiroa, M.N.U.; Sant’Ana, A.S.; Massaguer, P.R. Alicyclobacillus acidoterrestris in pasteurized exotic Brazilian fruit juices: Isolation, genotypic characterization and heat resistance. Food Microbiol. 2010, 27, 1016–1022. [Google Scholar] [CrossRef]
- Steyn, C.E.; Cameron, M.; Witthuhn, R.C. Ocurrence of Alicyclobacillus in the fruit processing environment—A review. Int. J. Food Microbiol. 2011, 147, 1–11. [Google Scholar] [CrossRef]
- Silva, F.; Gibbs, P. Alicyclobacillus acidoterrestris spores in fruit products and design of pasteurization processes. Trends Food Sci. Technol. 2001, 12, 68–74. [Google Scholar] [CrossRef]
- Ceviz, G.; Tulek, Y.; Hilmi Con, A. Thermal resistance of Alicyclobacillus acidoterrestris spores in different heating media. Int. J. Food Sci. Technol. 2009, 44, 1770–1777. [Google Scholar] [CrossRef]
- Peng, J.; Tang, J.; Barrett, D.M.; Sablani, S.S.; Anderson, N.; Powers, J.R. Thermal pasteurization of ready-to-eat foods and vegetables: Critical factors for process design and effects on quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 2970–2995. [Google Scholar] [CrossRef]
- McMeekin, T.A.; Ross, T. Predictive microbiology: Providing a knowledge-based framework for change management. Int. J. Food Microbiol. 2002, 78, 133–153. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, V. Predictive Microbiology in Foods; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zwietering, M.H.; Garre, A.; den Besten, H.M.W. Incorporating strain variability in the design of heat treatments: A stochastic approach and a kinetic approach. Food Res. Int. 2021, 139, 109973. [Google Scholar] [CrossRef]
- Clemente-Carazo, M.; Cebrián, G.; Garre, A.; Palop, A. Variability in the heat resistance of Listeria monocytogenes under dynamic conditions can be more relevant than that evidenced by isothermal treatments. Food Res. Int. 2020, 137, 109538. [Google Scholar] [CrossRef]
- Hassani, M.; Condon, S.; Pagán, R. Predicting microbial heat inactivation under nonisothermal treatments. J. Food Prot. 2007, 70, 1457–1467. [Google Scholar] [CrossRef]
- Valdramidis, V.P.; Geeraerd, A.H.; Bernaerts, K.; Van Impe, J.F. Microbial dynamics versus mathematical model dynamics: The case of microbial heat resistance induction. Innov. Food Sci. Emerg. Technol. 2006, 7, 80–87. [Google Scholar] [CrossRef]
- Garre, A.; Egea, J.A.; Iguaz, A.; Palop, A.; Fernandez, P.S. Relevance of the induced stress resistance when identifying the critical microorganism for microbial risk assessment. Front. Microbiol. 2018, 9, 01663. [Google Scholar] [CrossRef] [Green Version]
- Huertas, J.P.; Aznar, A.; Esnoz, A.; Fernández, P.S.; Iguaz, A.; Periago, P.M.; Palop, A. High heating rates affect greatly the inactivation rate of Escherichia coli. Front. Microbiol. 2016, 7, 01256. [Google Scholar] [CrossRef]
- Silva, L.P.; Gonzales-Barron, U.; Cadavez, V.; Sant’Ana, A. Modelling the effects of temperature and pH on the resistance of Alicyclobacillus acidoterrestris in conventional heat-treated fruit beverages through a meta-analysis approach. Food Microbiol. 2015, 46, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Leguerinel, I.; Maucotel, M.; Arnoux, T.; Gaspari, M.; Desriac, N.; Chatzitzika, C.; Valdramidis, V.P. Effects of heating and recovery media pH on the heat resistance of Alicyclobacillus acidoterrestris Ad746 spores. J. Appl. Microbiol. 2020, 129, 1674–1683. [Google Scholar] [CrossRef]
- López, M.D.; García, P.; Muñoz-Cuevas, M.; Fernández, P.S.; Palop, A. Thermal inactivation of Alicyclobacillus acidoterrestris spores under conditions simulating industrial heating processes of tangerine vesicles and its use in time temperature integrators. Eur. Food Res. Technol. 2011, 232, 821–827. [Google Scholar] [CrossRef]
- Conesa, R.; Andreu, S.; Fernández, P.S.; Esnoz, A.; Palop, A. Non-isothermal heat resistance determinations with the thermoresistometer Mastia. J. Appl. Microbiol. 2009, 107, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.M.C.; Elliot, D.C.; Elliot, W.H.; Jones, K.M. Data for Biochemical Research; Oxford at the Clarendon Press: Oxford, UK, 1974. [Google Scholar]
- Bigelow, W.D. The logarithmic nature of thermal death time curves. J. Infect. Dis. 1921, 29, 528–536. [Google Scholar] [CrossRef]
- Peñalver-Soto, J.L.; Garre, A.; Esnoz, A.; Fernández, P.S.; Egea, J.A. Guidelines for the design of (optimal) isothermal inactivation experiments. Food Res. Int. 2019, 126, 108714. [Google Scholar] [CrossRef] [PubMed]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Van Zuijlen, A.; Periago, P.M.; Amézquita, A.; Palop, A.; Brul, S.; Fernández, P.S. Characterization of Bacillus sporothermodurans IC4 spores; putative indicator microorganism for optimisation of thermal processes in food sterilisation. Food Res. Int. 2010, 43, 1895–1901. [Google Scholar] [CrossRef]
- Garre, A.; Fernández, P.S.; Lindqvist, R.; Egea, J.A. Bioinactivation: Software for modelling dynamic microbial inactivation. Food Res. Int. 2017, 93, 66–74. [Google Scholar] [CrossRef]
- Garre, A.; Clemente-Carazo, M.; Fernández, P.S.; Lindqvist, R.; Egea, J.A. Bioinactivation FE: A free web application for modelling isothermal and dynamic microbial inactivation. Food Res. Int. 2018, 112, 353–360. [Google Scholar] [CrossRef]
- Haario, H.; Laine, M.; Mira, A.; Saksman, E. DRAM: Efficient adaptive MCMC. Stat. Comput. 2006, 16, 339–354. [Google Scholar] [CrossRef]
- Brooks, S.; Gelman, A.; Jones, G.; Meng, X.-L. Handbook of Markov Chain Monte Carlo; Chapman and Hall/CRC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hindmarsh, A.; Odepack, A. Systematized Collection of ODE Solvers; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1992. [Google Scholar]
- Baranyi, J.; Pin, C.; Ross, T. Validating and comparing predictive models. Int. J. Food Microbiol. 1999, 48, 159–166. [Google Scholar] [CrossRef]
- Palop, A.; Alvarez, I.; Raso, J.; Condon, S. Heat resistance of Alicyclobacillus acidoterrestris in water, various buffers and orange juice. J. Food Prot. 2000, 63, 353–380. [Google Scholar] [CrossRef]
- Huertas, J.P.; Esteban, M.D.; Antolinos, V.; Palop, A. Combined effect of natural antimicrobials and thermal treatments on Alicyclobacillus acidoterrestris spores. Food Control 2014, 35, 73–78. [Google Scholar] [CrossRef]
- Garre, A.; González-Tejedor, G.A.; Aznar, A.; Fernández, P.S.; Egea, J.A. Mathematical modelling of the stress resistance induced in Listeria monocytogenes during dynamic, mild heat treatments. Food Microbiol. 2019, 84, 103238. [Google Scholar] [CrossRef]
- Kohler, L.J.; Quir, A.V.; Welkos, S.L.; Cote, C.K. Incorporating germination-induction into decontamination strategies for bacterial spores. J. Appl. Microbiol. 2018, 124, 2–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ran, J.; Jiao, L.; Liang, X.; Zhao, R. Label free quantitative analysis of Alicyclobacillus acidoterrestris spore germination subjected to low ambient pH. Food Res. Int. 2019, 115, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Terano, H.; Takahashi, K.; Sakakibara, Y. Characterization of spore germination of a thermoacidophilic spore-forming bacterium, Alicyclobacilus acidoterrestris. Biosci. Biotechnol. Biochem. 2005, 69, 1217–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban, M.D.; Huertas, J.P.; Fernández, P.S.; Palop, A. Effect of the medium characteristics and the heating and cooling rates on the nonisothermal heat resistance of Bacillus sporothermodurans IC4 Spores. Food Microbiol. 2013, 34, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Jódar, I.; Ros-Chumillas, M.; Palop, A. Effect of heating rate on highly heat-resistant spore-forming microorganisms. Food Sci. Technol. Int. 2015, 22, 164–172. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Saltaouras, K.P.; Stoforos, N.G. On optimum dynamic temperature profiles for thermal inactivation kinetics determination. J. Food Sci. 2021, in press. [Google Scholar] [CrossRef]
- Garre, A.; Huertas, J.P.; González-Tejedor, G.A.; Fernández, P.S.; Egea, J.A.; Palop, A.; Esnoz, A. Mathematical quantification of the induced stress resistance of microbial populations during non-isothermal stresses. Int. J. Food Microbiol. 2018, 266, 133–141. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Marks, B.P.; Orta-Ramirez, A.; Smith, D.M. Modeling the effect of prior sublethal thermal history on the thermal inactivation rate of Salmonella in ground turkey. J. Food Prot. 2008, 71, 279–285. [Google Scholar] [CrossRef]
| RMSE | AICc | |||||||
|---|---|---|---|---|---|---|---|---|
| Bigelow | Weibull | Bigelow | Weibull | Bigelow | Weibull | Bigelow | Weibull | |
| 1 °C/min | 0.27 | 0.30 | 1.86 | 1.98 | 1.33 | 0.74 | 28.03 | 30.21 |
| 20 °C/min | 0.71 | 0.67 | 5.18 | 4.68 | 3.72 | 3.59 | 44.66 | 45.64 |
| Heating + Cooling | 1.15 | 1.08 | 14.09 | 12.02 | 5.59 | 5.24 | 212.62 | 204.48 |
| Complex treatment | 1.28 | 1.05 | 19.17 | 11.12 | 10.39 | 6.39 | 129.72 | 111.89 |
| D95 (min) | z (°C) | RMSE | AICc | |||
|---|---|---|---|---|---|---|
| 1 °C/min | 5.77 ± 1.69 | 11.47 ± 2.03 | 0.20 | 1.58 | 1.04 | 27.61 |
| 20 °C/min | 4.11 ± 2.00 | 10.79 ± 1.94 | 0.17 | 1.49 | 1.11 | 36.51 |
| Heating + Cooling | 2.39 ± 1.02 | 10.40 ± 1.80 | 0.25 | 1.78 | 1.03 | 129.59 |
| Complete treatment | 3.41 ± 1.16 | 11.18 ± 1.89 | 0.63 | 4.28 | 1.39 | 84.79 |
| z (°C) | p | RMSE | AICc | ||||
|---|---|---|---|---|---|---|---|
| 1 °C/min | 5.06 ± 0.66 | 10.87 ± 2.40 | 1.10 ± 0.07 | 0.20 | 1.58 | 1.01 | 29.62 |
| 20 °C/min | 3.44 ± 1.00 | 12.22 ± 2.03 | 1.24 ± 0.14 | 0.18 | 1.50 | 1.12 | 38.52 |
| Heating + Cooling | 2.29 ± 0.90 | 12.77 ± 2.05 | 1.24 ± 0.14 | 0.25 | 1.78 | 1.02 | 131.64 |
| Complete treatment | 3.89 ± 1.01 | 12.45 ± 1.84 | 1.22 ± 0.14 | 0.63 | 4.28 | 1.39 | 86.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huertas, J.-P.; Ros-Chumillas, M.; Garre, A.; Fernández, P.S.; Aznar, A.; Iguaz, A.; Esnoz, A.; Palop, A. Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing. Foods 2021, 10, 1496. https://doi.org/10.3390/foods10071496
Huertas J-P, Ros-Chumillas M, Garre A, Fernández PS, Aznar A, Iguaz A, Esnoz A, Palop A. Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing. Foods. 2021; 10(7):1496. https://doi.org/10.3390/foods10071496
Chicago/Turabian StyleHuertas, Juan-Pablo, María Ros-Chumillas, Alberto Garre, Pablo S. Fernández, Arantxa Aznar, Asunción Iguaz, Arturo Esnoz, and Alfredo Palop. 2021. "Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing" Foods 10, no. 7: 1496. https://doi.org/10.3390/foods10071496
APA StyleHuertas, J.-P., Ros-Chumillas, M., Garre, A., Fernández, P. S., Aznar, A., Iguaz, A., Esnoz, A., & Palop, A. (2021). Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing. Foods, 10(7), 1496. https://doi.org/10.3390/foods10071496










