1. Introduction
Listeria monocytogenes, a ubiquitous intracellular pathogen [
1], has the ability to proliferate at refrigeration temperature and can often contaminate products post-pasteurization, after which there is no further kill step to prevent ingestion of the pathogen by consumers.
L. monocytogenes is of particular concern for certain dairy processors due to its ubiquitous presence in the environment and the physical properties of some ready-to-eat (RTE) dairy products such as fresh cheese [
2].
The consumption of Hispanic-style cheeses is increasing in the United States [
3]. This upsurge in popularity is likely due to the growth of the Hispanic population in the U.S., which reached 18.4% of the total population in 2019 [
4]. Queso fresco, the most popular Hispanic-style fresh cheese [
5], is a rennet coagulated cheese with intrinsic properties such as high moisture content, low salt content, and near-neutral pH [
3], which make it susceptible to
L. monocytogenes outgrowth when contaminated. According to the Centers for Disease Control and Prevention (CDC), there were eight foodborne outbreaks associated with
L. monocytogenes contamination in cheese products between 2014 and 2018. The outbreaks included products such as quesarito (fresh cheese) curd [
6], soft raw milk cheese [
7], and most recently, queso fresco [
8]. According to the National Outbreak Reporting System (NORS), there have been 5 outbreaks, 46 illnesses, 34 hospitalizations, and 2 deaths associated with
L. monocytogenes contamination in queso fresco in the United States between 1971 and 2018 [
9], which demonstrates that an effective control strategy for this pathogen is necessary.
There have been studies dedicated to controlling
L. monocytogenes in queso fresco using a range of processing and antimicrobial treatments. High-hydrostatic-pressure processing (HPP) was found to immediately reduce
L. monocytogenes contamination when queso fresco was treated at 600 MPa and held at a temperature of 20 °C for 3 min. However, this method is currently not cost-effective, significant wheying-off was observed, and the queso fresco was found to have different textural and rheological properties when compared to a control at 20 °C [
10]. The efficacy of antimicrobial combinations of nisin, lauric arginate, and ɛ-polylysine [
11], protective cultures [
12], and phage endolysin PlyP100 [
13] have all been explored to inhibit
L. monocytogenes in queso fresco, demonstrating that finding an effective control method for this pathogen is a priority for the dairy industry. Consumers are currently moving toward a trend of desiring products with “clean labels” [
14]. Therefore, finding effective antimicrobial agents that fall into the clean label category and are effective at preventing or reducing
L. monocytogenes contamination in queso fresco would be beneficial to both consumers and producers by preventing further foodborne outbreaks and their associated human and financial costs.
Lactose oxidase (LO) is a naturally derived enzyme produced by a strain of mold called
Microdochium nivale [
15] that consumers would likely categorize under the clean-label category. Some cheeses are coagulated enzymatically with rennet [
16], and therefore, consumers may be primed to view enzymes as a normal occurrence in dairy products. LO oxidizes lactose into lactobionic acid (LBA) [
17] with the concurrent reduction of oxygen into hydrogen peroxide (H
2O
2) [
15]. The structure of LBA consists of a polyhydroxy gluconic acid that is bonded to a glucose sugar (C
12H
22O
12) [
18]. It has been shown to inhibit counts of
L. monocytogenes in milk when combined with other antimicrobial agents such as nisin and thymol [
19]. Its effect on
L. monocytogenes in a complex food matrix, such as cheese, when combined with hydrogen peroxide may yield promising results. H
2O
2 has been used in the dairy industry to preserve raw milk and breaks down into nontoxic compounds in solution [
20]. It is approved for use at a concentration of 0.05% in solution for the milk used to produce several varieties of cheese, such as Colby, Cheddar, Swiss, and Emmentaler (21 Code of Federal Regulations (CFR) 184.1366). Treatment of fresh cheese with H
2O
2 was found to reduce
L. monocytogenes counts by 3 log CFU/g when the cheese was exposed to a 10% solution for 5 s [
21], and concentrations of 400 and 800 mg/L were bactericidal on
L. monocytogenes within 24 h in a previous study [
22].
LBA and H
2O
2 have been shown to be effective inhibitors of
L. monocytogenes in dairy products, demonstrating that the production of these antimicrobial agents by LO itself may be an effective strategy to control
L. monocytogenes in cheese. In a previous inhibition assay study, LO combined with sodium thiocyanate (TCN) was shown to inhibit the growth of
L. monocytogenes [
23]. TCN combined with hydrogen peroxide has been shown to be an effective activator of the lactoperoxidase system, which is a natural antimicrobial system present in raw milk [
24,
25]. In our previous study, LO and LO-TCN combinations were shown to inhibit
L. monocytogenes in UHT skim milk and raw milk [
26] (in review).
The efficacy of LO at inhibiting
L. monocytogenes in a complex food matrix such as fresh cheese has not yet been investigated. The purpose of this study was to test multiple methods in which LO could be utilized, and the use of a laboratory-scale cheese model allowed various
L. monocytogenes contamination scenarios and applications of LO and LO-TCN combinations to be explored. The first part of this study looks at the scenario in which the milk was contaminated with
L. monocytogenes and looked at the efficacy of adding either LO or LO-TCN to the milk prior to making the cheese on the subsequent outgrowth of
L. monocytogenes in the final cheese. The second part of this study investigates scenarios in which the surface of the queso fresco is contaminated by
L. monocytogenes from the environment as well as the subsequent effect of LO and LO-TCN combinations application to the surface to prevent outgrowth.
L. monocytogenes was inoculated onto the surface of the cheese at concentrations of either 200 CFU/g or 2 CFU/g. Then, LO and LO-TCN combinations were topically applied to the cheese surface. Optimal LO and LO-TCN concentrations for inhibiting
L. monocytogenes growth in milk products were determined in our previous study [
26] and tested during the cheese-making process. The addition of LO into the milk used for cheese making or topical application both represent potential control strategies that could be implemented by cheese makers to reduce the risk of
L. monocytogenes outbreaks attributed to queso fresco and similar style cheeses.
2. Materials and Methods
2.1. Preparation of Listeria monocytogenes Inoculum
A cocktail of
L. monocytogenes was prepared using five isolates of
L. monocytogenes (
Table 1). The strains, four from fresh cheese outbreaks and one a laboratory strain, were obtained from Dr. Martin Wiedmann’s Food Safety Laboratory at Cornell University (Ithaca, NY, USA). Each strain was streaked onto Brain Heart Infusion (BHI) agar (Beckton, Dickinson and Co., Franklin Lakes, NJ, USA) and incubated at 37 °C for 24 h. An individual colony of each strain from each streak plate was used to separately inoculate 5 mL of BHI broth. Broth cultures were incubated at 37 °C to obtain a concentration of OD = 1.00 (9 log CFU/mL). One mL of each culture was combined to produce a cocktail for the inoculation of milk or cheese samples. The
L. monocytogenes cocktail was serially diluted in phosphate buffer saline (PBS) solution to the appropriate concentration for each experiment. The appropriate serial dilutions of the cocktail were enumerated on BHI agar for each experiment to confirm target inoculum concentrations.
2.2. Cheese Making
Laboratory-scale queso fresco was produced following previous methods with modifications dependent on the goal of each experiment that will be discussed as follows with modifications from a previously established method [
12]. Pasteurized, homogenized milk was collected from the Cornell Dairy. Milk (600 mL) was aseptically added to 1 L sterile bottles (VWR International, Solon, OH, USA) and brought to 35 °C over the course of one hour in a water bath. The following were added to each bottle: 1875 μL of a 32% calcium chloride (CaCl
2) solution (Dairy Connection Inc., Madison, WI, USA) and 78 μL of double-strength rennet (Chy-Max Extra; Chr. Hansen, Milwaukee, WI, USA). Each bottle was swirled to mix and poured into individual plastic cheese vats. The vats were incubated in a water bath at 35 °C for 65 min to promote curd formation. After 65 min, the curd was cut and allowed to heal for 10 min at 35 °C. Then, the temperature of the water bath was slowly brought to 43 °C over the course of 30 min, and the vats were incubated for 30 min at this temperature. Then, 60 mL of whey were removed from each cheese vat and replaced with 60 mL of a 0.16 g/mL NaCl solution. Each vat was returned to the water bath and incubated at a temperature set at 43 °C for 20 min. Then, the whey was drained using a sterile cheesecloth for 1 h. After drainage, 6 (±0.2) grams of cheese curd were aseptically transferred into 12-well plates (Corning, Inc., Corning, NY, USA). Then, the curd was pressed for 16 h overnight using cheese weights provided from the Cornell Dairy to produce a final curd weight of approximately 5 (±0.2) grams.
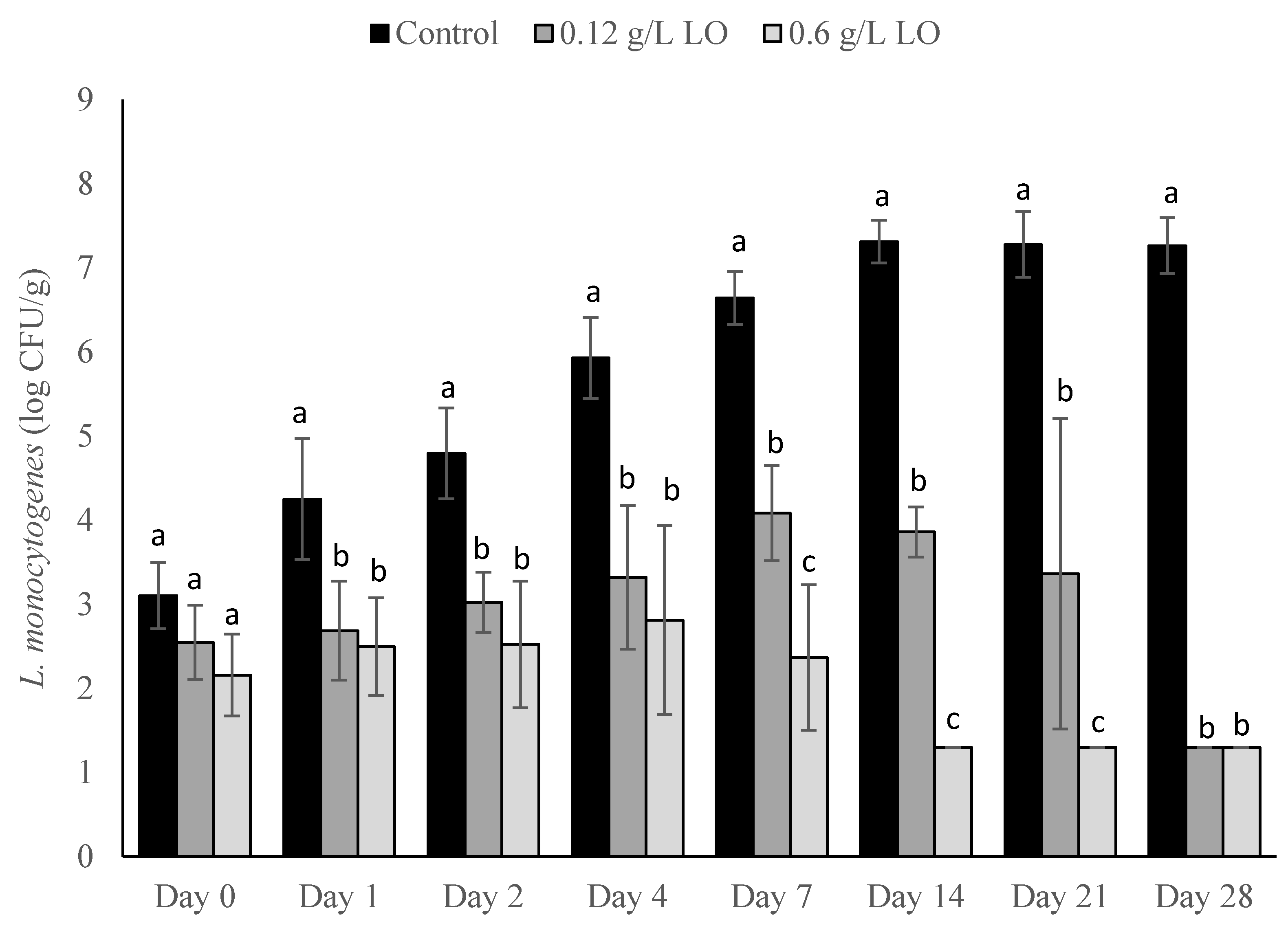
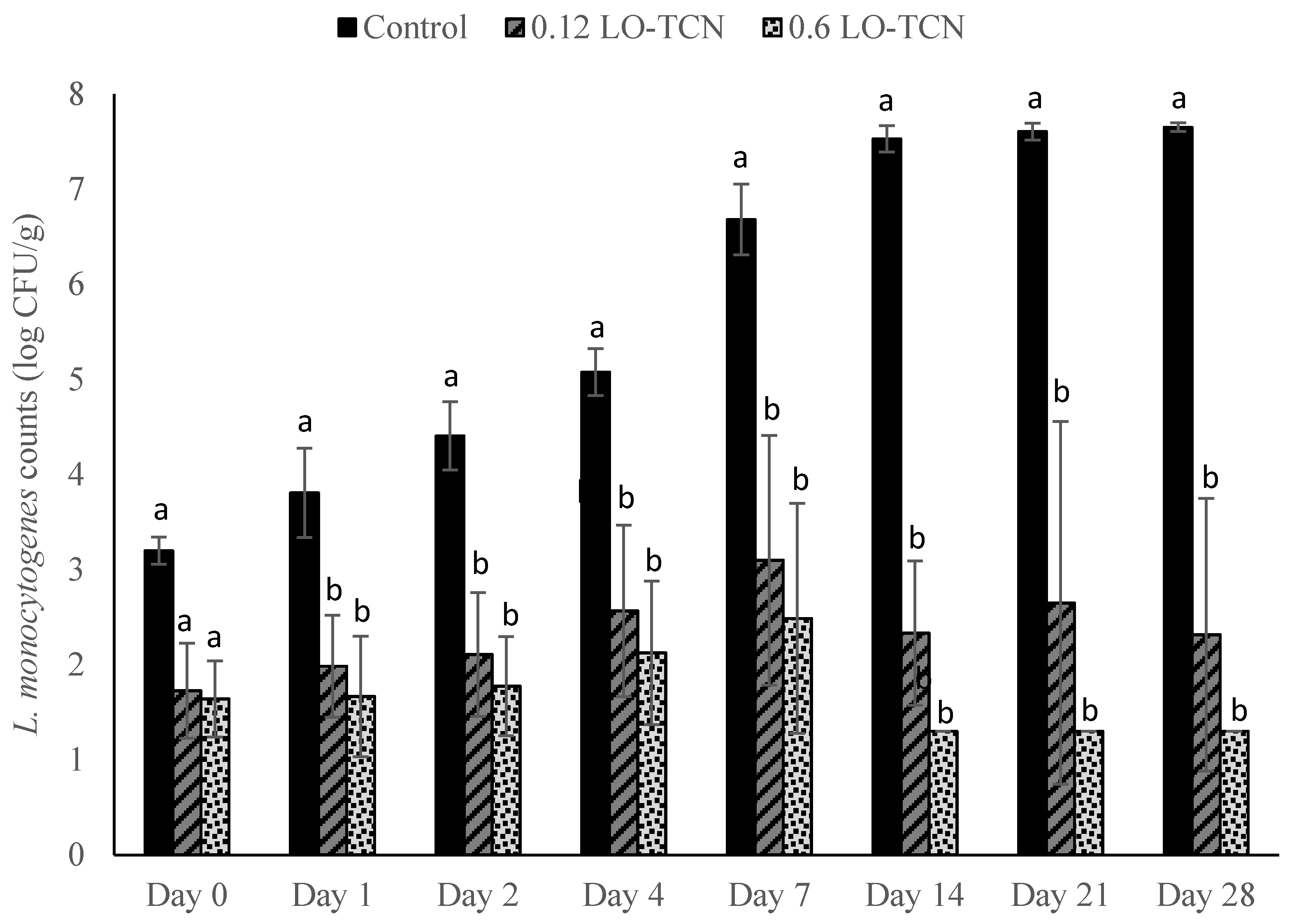
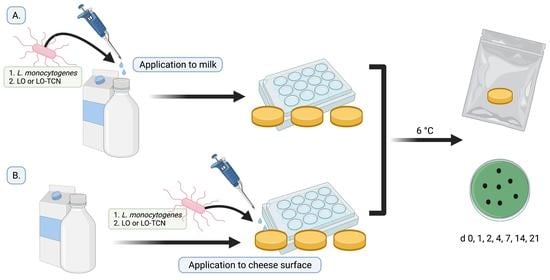
2.3. Application of LO and TCN for Inhibition of L. monocytogenes in the Milk
Cheese was made as described in the earlier section with the following modifications. During the first step, the prepared L. monocytogenes cocktail was inoculated into the milk at final concentrations of approximately either 1 log CFU/mL or 1 CFU/100 mL. The bottles were inverted to ensure distribution of the inoculum throughout the milk. Following the one-hour incubation step to heat the milk, either LO (LactoYield, Chr. Hansen, Milwaukee, WI, USA) on its own or a combination of LO-TCN (VWR International, Solon, OH, USA) were added to each inoculated treatment. Separate experiments were performed using the LO treatment on its own or the LO-TCN combined treatment. Lactose oxidase was added into the milk to reach a final concentration of either 0.12 or 0.6 g/L in the milk. Sodium thiocyanate was added into the milk to reach a final concentration of 14 mg/L. A positive control with no added LO was used for each inoculum, and a negative control with no added LO or inoculum was used. The samples were stored at 6 °C for the entirety of the trial. For each of these LO and LO-TCN experiments, batches of cheese were made in triplicate to measure the pH using an edge meter (Hanna Instruments, Woonsocket, RI, USA) and water activity (aW) with an Aqualab meter (METER Group, Pullman, WA, USA) in duplicate throughout the storage conditions of the treatment following the same sampling period as microbiological analysis.
2.4. Enumeration of Samples for Microbiological Analysis
For each trial, cheese samples were enumerated for microbiological analysis using the same method. Samples were enumerated on days 0, 1, 2, 4, 7, 14, 21, and 28 for L. monocytogenes counts. Each sample was aseptically added to a Whirl-Pak filter bag (Nasco, Fort Atkinson, WI, USA), and a 1:10 (w/v) dilution using PBS was performed. The samples were digested at normal speed for 60 s using a Seward Stomacher 400 Lab Blender Series (VWR International, Solon, OH, USA). The digested samples were diluted in 9 mL PBS blanks to the appropriate dilution and vortexed. Then, dilutions were spread-plated on Modified Oxford Agar (MOX) plates in duplicate and incubated for 48 h at 37 °C. L. monocytogenes counts were taken after 48 h of incubation. Each experiment was performed at least in triplicate.
2.5. Preparation of LO and TCN Solutions for Surface Application
The LO and TCN solutions for surface application were prepared as follows. A 16% lactose solution was combined with 5 mL of a 0.24 g/L LO solution and filter sterilized through a 0.20 μm surfactant-free cellulose acetate filter (Corning Inc., Corning, NY, USA). A 1.2 g/L LO solution was combined with a 16% lactose solution and filter sterilized through a 0.20 μm surfactant-free cellulose acetate filter (Corning Inc.). Final concentrations of 0.12 g/L LO 8% lactose and 0.6 g/L LO 8% lactose in solution were produced when combined. A 14 mg/L solution of TCN was made and filter sterilized through a 0.20 μm surfactant-free cellulose acetate filter (Corning Inc., Corning, NY, USA). Each treatment was stored at 6 °C for the entirety of the trial. For each of these LO and LO-TCN surface experiments, batches of cheese were made to measure the pH and aW in duplicate throughout the storage conditions of the treatment following the same sample period as microbiological analysis.
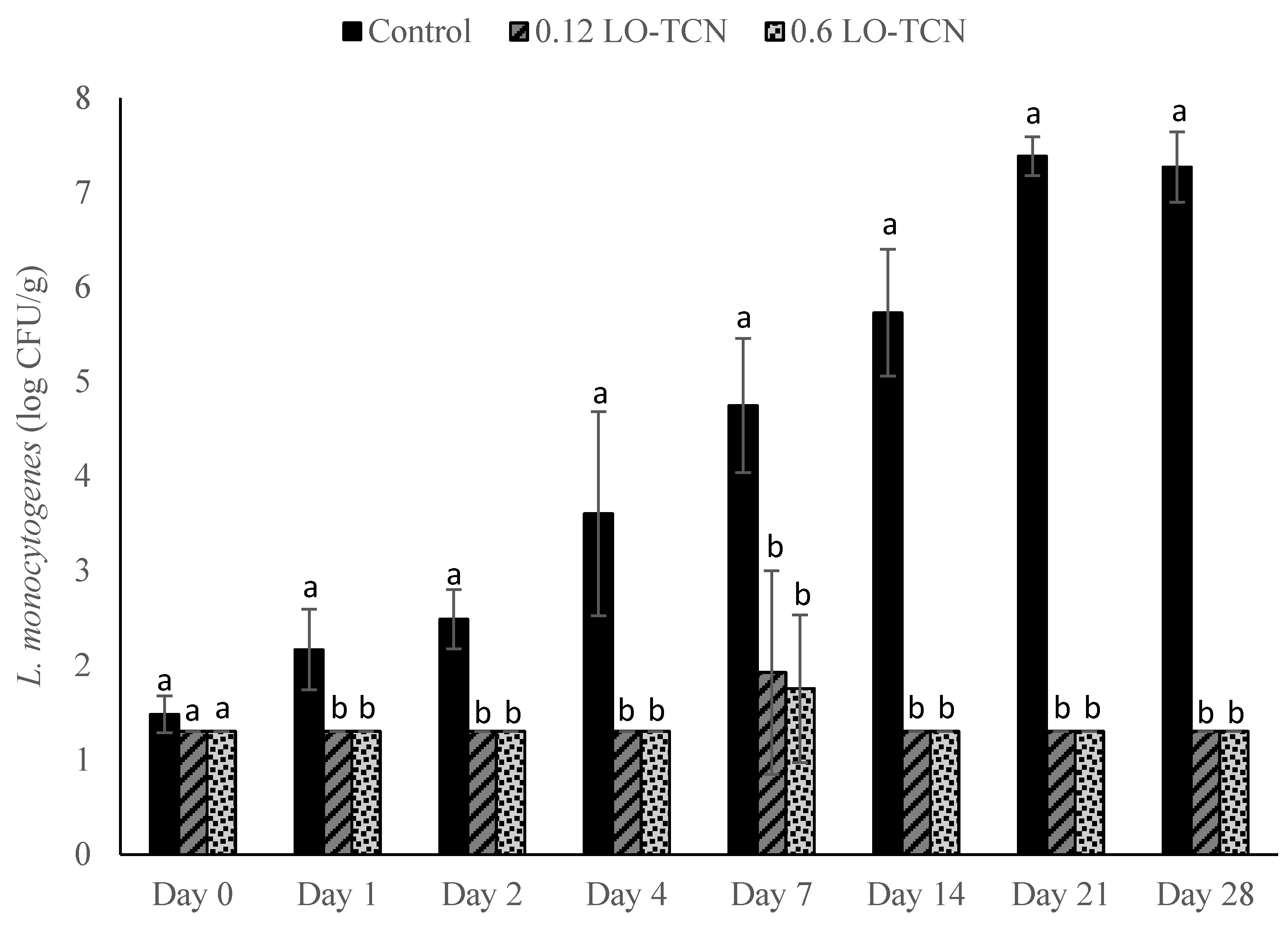
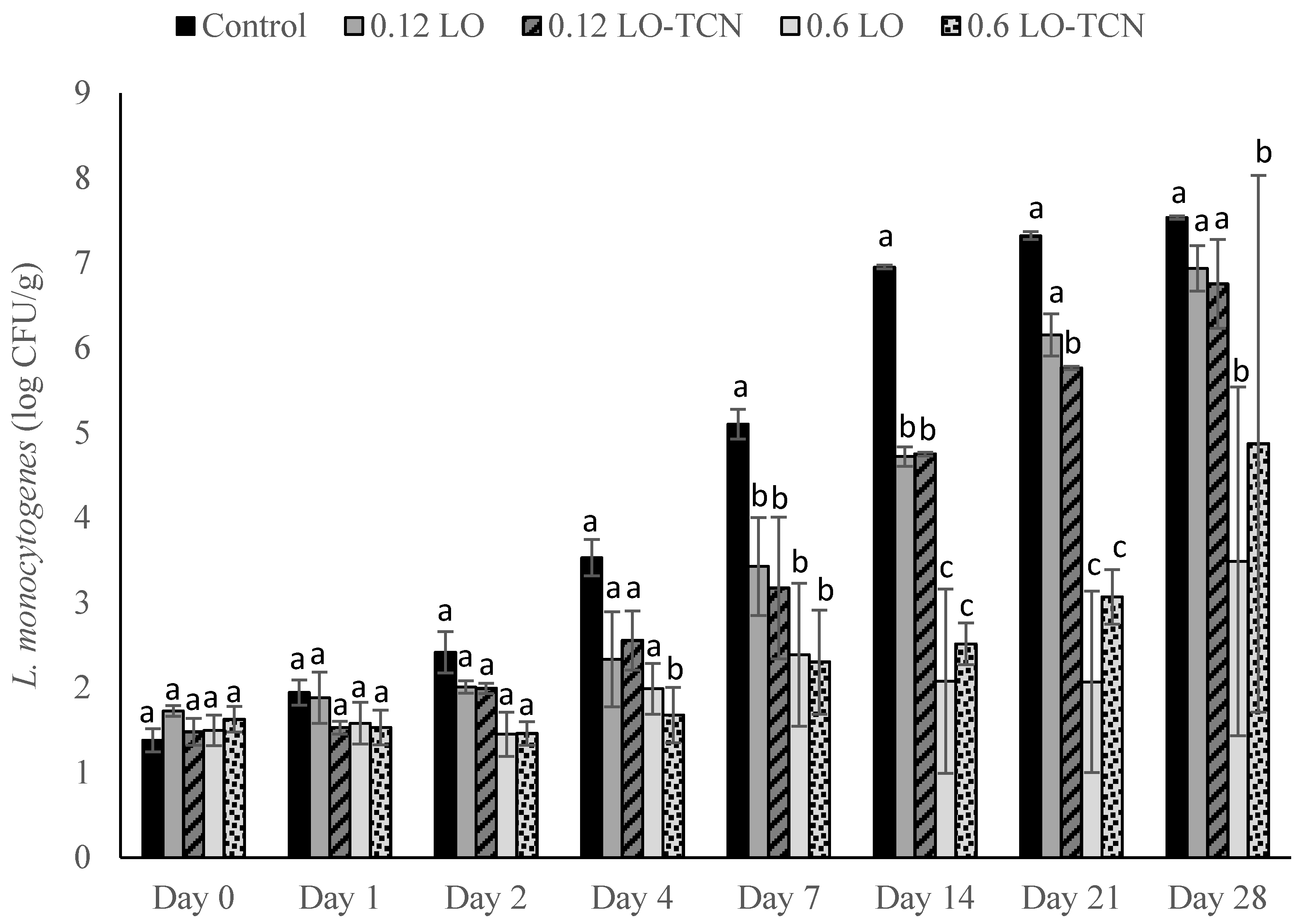
2.6. Surface Application of LO and TCN for Inhibition of L. monocytogenes on Cheese Surface
Laboratory-scale cheese was produced using the method described in the cheese-making section. Following the overnight press, 100 µL of L. monocytogenes cocktail was inoculated on the surface of the cheese to obtain final concentrations of approximately 200 and 2 CFU/g. Cheese with no inoculum was included as a negative control. The inoculum was allowed to attach for approximately 45 min.
For each experiment, the following treatments were added to the cheese. For the LO treatments, 100 μL of either the 0.12 g/L LO or 0.6 g/L LO and 8% lactose solution was dispensed onto the surface of the cheese. For the TCN treatment, the respective LO solution was dispensed onto the surface of the cheese with 100 μL of TCN solution. For the negative and positive control, 100 µL of sterile MilliQ water was dispensed onto the cheese. Cheese samples were enumerated as described in the previous section.
2.7. Statistical Analysis
Each experiment was repeated in triplicate. All statistical analyses were performed using R software (Version 3.5.2, R Development Core Team, Vienna, Austria). Analysis of Variance and Tukey’s Honest Significant Difference tests were performed at each time point to determine log differences in L. monocytogenes counts between all treatments and the positive control. The same tests were performed at each time point to determine differences in pH and aW values between all treatments and the negative control.
4. Conclusions
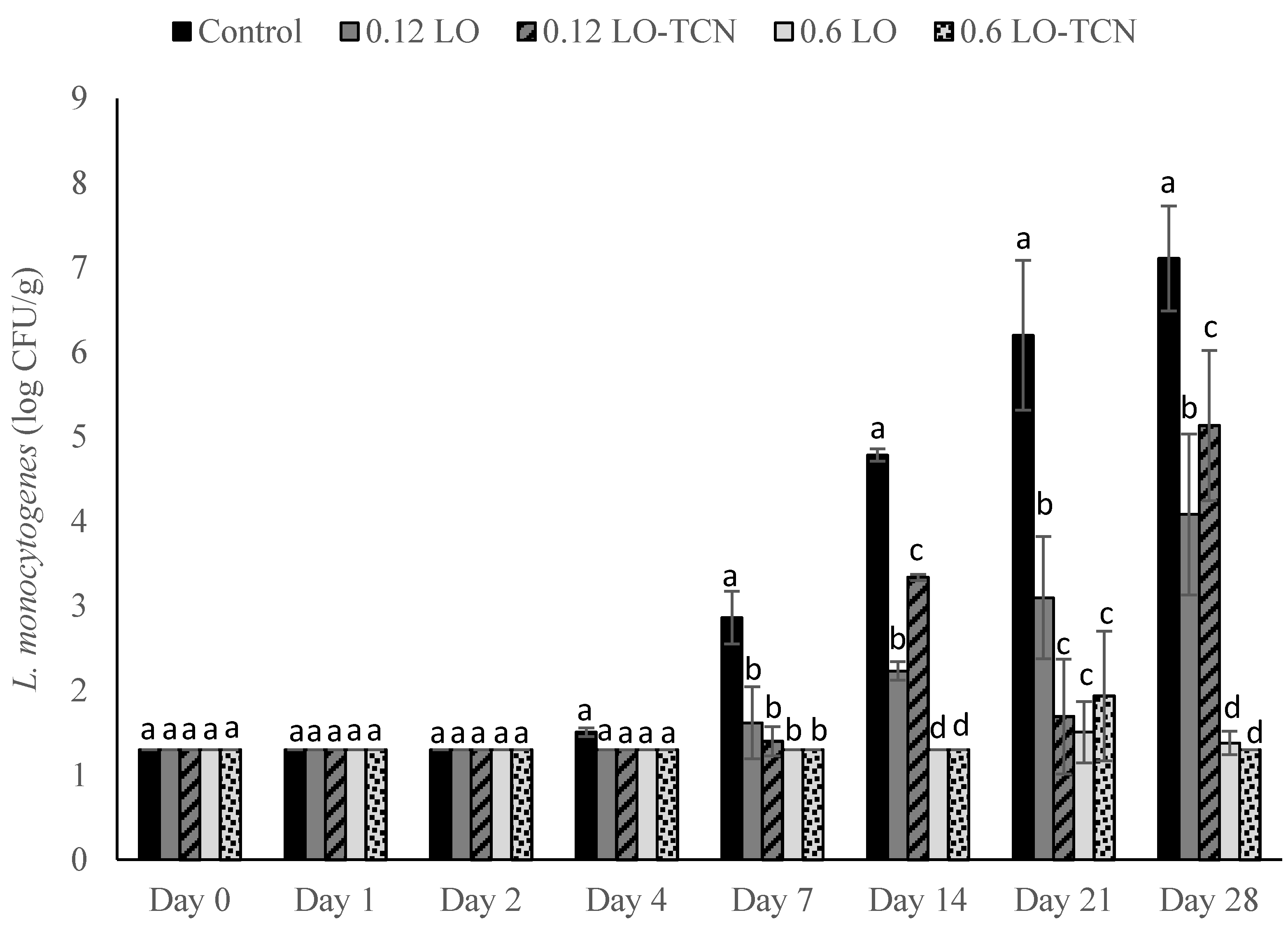
In this study, we explored the effect of lactose oxidase on its own and in combination with TCN as a method to control L. monocytogenes outgrowth in a laboratory-scale fresh cheese model. We first explored the inoculation of L. monocytogenes and the addition of LO and LO-TCN combinations into the incoming raw milk. We determined that LO and LO-TCN combinations inhibit the growth of L. monocytogenes in a concentration-dependent fashion. We determined that LO is effective as a listericidal control method with both a high (1 log CFU/mL) and a low (1 CFU/100 mL) inoculum in the milk used during the cheese-making process. These treatments did cause a significant change in the pH of the cheese, which may affect sensory analysis and should be explored further in future studies.
Then, we explored the efficacy of LO and LO-TCN combinations as a topical application for the surface contamination of queso fresco and determined that LO showed efficacy in low-level contaminant scenarios. The level of initial surface contamination by L. monocytogenes in the real world is difficult to know definitely, and it obviously varies by the conditions of the event. Our results suggests that the surface application of LO, with or without TCN supplementation, could be useful for controlling incidental low-level L. monocytogenes from the environment onto the surface of the cheese.
Overall, the aim of this study was to explore a novel method to control L. monocytogenes outgrowth in a laboratory-scale queso fresco to improve the safety of high-risk cheeses. In conjunction with good hygienic practices, LO represent a novel tool that cheesemakers could use to improve the safety of their cheeses. Further research is needed to optimize the use of LO and understand the potential synergies with other antimicrobials that can be used to control L. monocytogenes.