Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Handling and Storage
2.2. Hydrothermal Processing of Legumes
2.3. Determination of the Textural Properties of Boiled and Uncooked Legumes
2.4. Simulated Oral-Gastro-Intestinal In Vitro Digestion of Uncooked and Boiled Legumes
2.5. Total Starch Measurement of Undigested Legumes Using a Glucose Oxidase/Peroxidase (GOPOD) Assay
2.6. Quantification of Free Glucose in the Legume Digesta Using a GOPOD Assay to Determine the Starch Digestibility of Boiled Legume
2.7. Quantification of Free Alpha Amino Groups in the Legume Digesta Using an O-Phthalaldehyde Assay to Determine the Protein Digestibility of Boiled Legume
2.8. Data Analysis
3. Results and Discussion
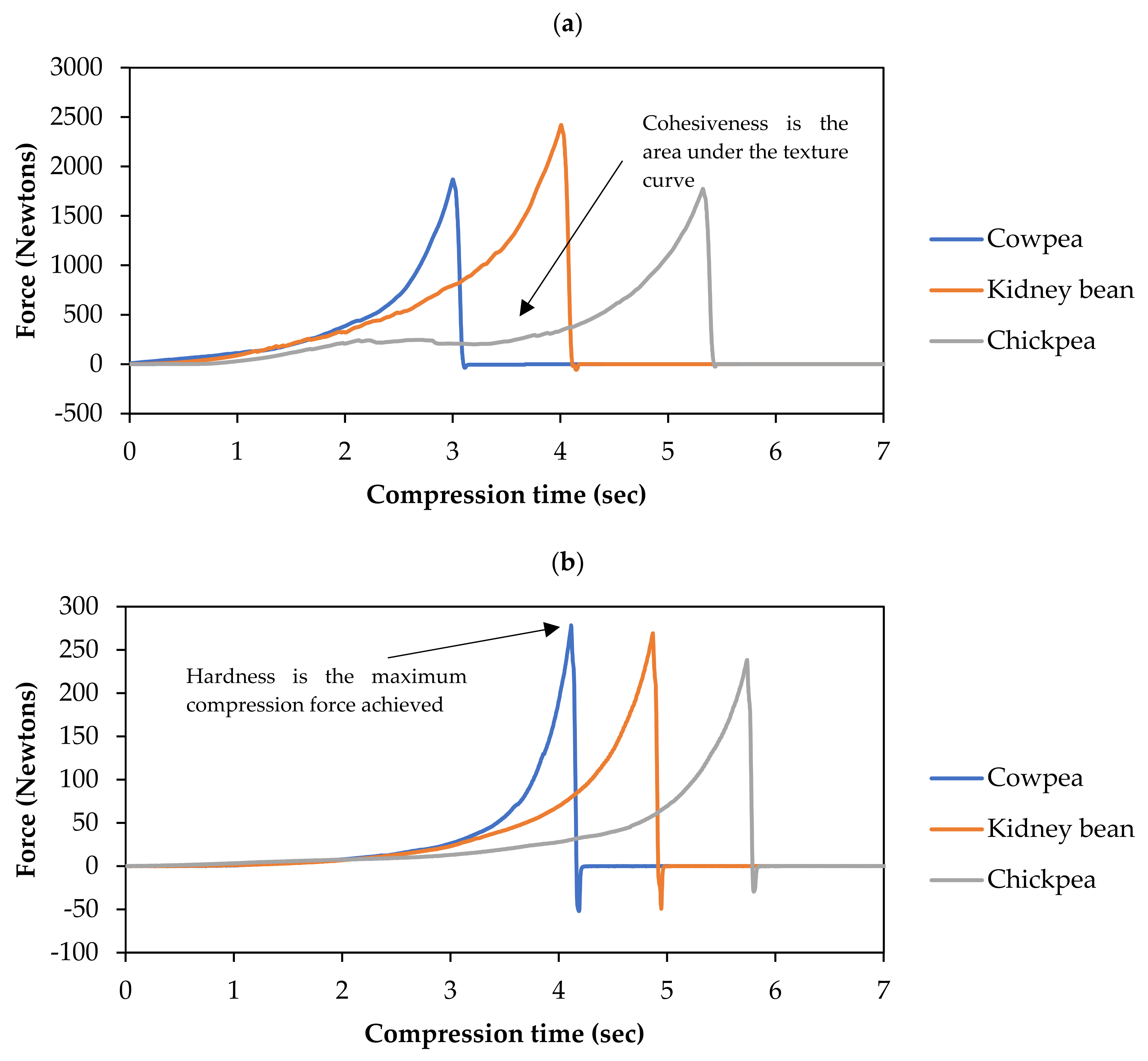
3.1. Texture Changes of Cowpea, Chickpea and Kidney Bean at Different Durations of Hydrothermal Processing
3.2. Starch Digestibility of Cowpeas, Chickpeas and Kidney Beans as Affected by the Duration of Hydrothermal Processing
3.2.1. Starch Digestibility of Legumes during In Vitro Gastric Digestion
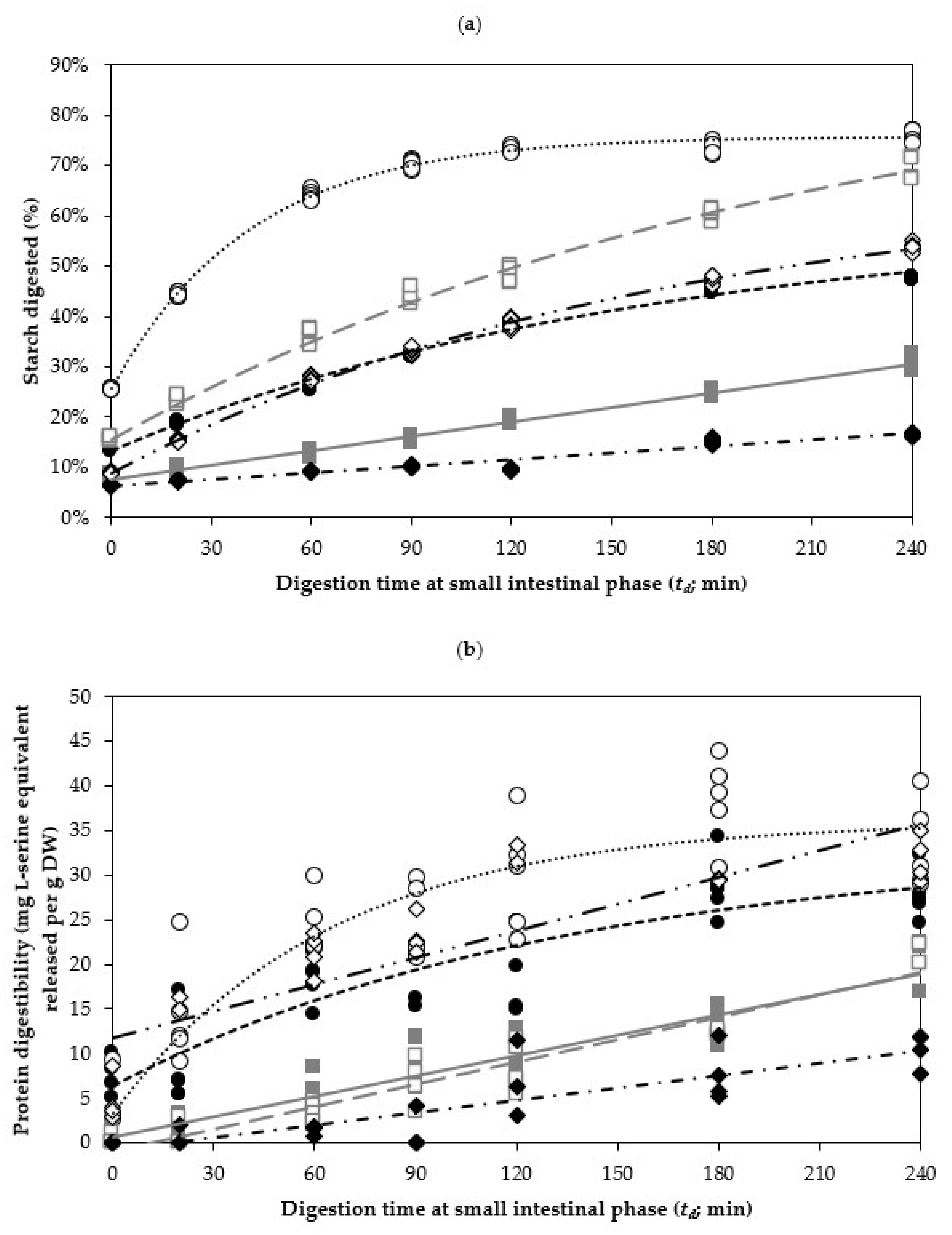
3.2.2. Kinetics of In Vitro Starch Digestibility of Legumes at the Small Intestinal Phase
3.3. Protein Digestibility of Cowpea, Chickpea and Kidney Bean as Affected by the Duration of Hydrothermal Processing
3.3.1. Protein Digestibility of Legumes during the In Vitro Gastric Digestion
3.3.2. Kinetics of the In Vitro Protein Digestibility of Legumes at the Small Intestinal Phase
3.4. The Relationship Between Texture and Starch and Protein Digestibility of Boiled Cowpeas, Chickpeas and Kidney Beans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Venn, B.; Mann, J. Cereal grains, legumes and diabetes. Eur. J. Clin. Nutr. 2004, 58, 1443–1461. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.H. Encyclopedia of Food and Culture; Charles Scribner’s Sons Imprint: New York, NY, USA, 2002. [Google Scholar]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; Van De Velde, F.; De Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G.; Cappelloni, M.; Pusztai, A. Perspectives into factors limiting in vivo digestion of legume proteins: antinutritional compounds or storage proteins? J. Agric. Food Chem. 2000, 48, 742–749. [Google Scholar] [CrossRef]
- Foos, K.M. US FDA/CFSAN bad bug book: Foodborne pathogenic microorganisms and natural toxins handbook. Choice 2006, 44, 328. [Google Scholar]
- Martínez-Villaluenga, C.; Frias, J.; Vidal-Valverde, C. Alpha-Galactosides: Antinutritional factors or functional ingredients? Crit. Rev. Food Sci. Nutr. 2008, 48, 301–316. [Google Scholar] [CrossRef]
- Birk, Y. Protein proteinase inhibitors in legume seeds--overview. Arch. Lat. Nutr. 1996, 44, 26S–30S. [Google Scholar]
- Shimoyamada, M.; Ikedo, S.; Ootsubo, R.; Watanabe, K. Effects of soybean saponins on chymotryptic hydrolyses of soybean proteins. J. Agric. Food Chem. 1998, 46, 4793–4797. [Google Scholar] [CrossRef]
- Macrae, R.; Robinson, R.K.; Sadler, M.J. Encyclopaedia of Food Science, Food Technology, and Nutrition; Academic Press: London, UK; San Diego, CA, USA, 1993. [Google Scholar]
- Jansman, A.J.M. Tannins in feedstuffs for simple-stomached animals. Nutr. Res. Rev. 1993, 6, 209–236. [Google Scholar] [CrossRef]
- Jansman, A.J.M.; Enting, H.; Verstegen, M.W.A.; Huisman, J. Effect of condensed tannins in hulls of faba beans (Vicia faba L.) on the activities of trypsin (EC 2.4.21.4) and chymotrypsin (EC 2.4.21.1) in digesta collected from the small intestine of pigs. Br. J. Nutr. 1994, 71, 627–641. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D. Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT—Food Sci. Technol. 2009, 42, 1113–1118. [Google Scholar] [CrossRef]
- Naidoo, T.; Gerrano, A.; Mellem, J. The effect of processing on in vitro protein and starch digestibility and predictive glycaemic index of five Vigna unguiculata (cowpea) cultivars. Ann. Univ. Dunarea De Jos Galati Fascicle VI Food Technol. 2017, 41, 31–41. [Google Scholar]
- Madodé, Y.E.; Nout, M.J.R.; Bakker, E.-J.; Linnemann, A.R.; Hounhouigan, D.J.; Van Boekel, M.A.J.S. Enhancing the digestibility of cowpea (Vigna unguiculata) by traditional processing and fermentation. LWT—Food Sci. Technol. 2013, 54, 186–193. [Google Scholar] [CrossRef]
- Kiers, J.L.; Nout, R.M.J.; Rombouts, F.M. In vitro digestibility of processed and fermented soya bean, cowpea and maize. J. Sci. Food Agric. 2000, 80, 1325–1331. [Google Scholar] [CrossRef]
- Xu, Y.; Cartier, A.; Obielodan, M.; Jordan, K.; Hairston, T.; Shannon, A.; Sismour, E. Nutritional and anti-nutritional composition, and in vitro protein digestibility of Kabuli chickpea (Cicer arietinum L.) as affected by differential processing methods. J. Food Meas. Charact. 2016, 10, 625–633. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Loosveldt, B.; Karimi, S.N.; Hendrickx, M.; Grauwet, T. Effect of process-induced common bean hardness on structural properties of in vivo generated boluses and consequences for in vitro starch digestion kinetics. Br. J. Nutr. 2019, 122, 388–399. [Google Scholar] [CrossRef]
- Gwala, S.; Wainana, I.; Pallares Pallares, A.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Texture and interlinked post-process microstructures determine the in vitro starch digestibility of Bambara groundnuts with distinct hard-to-cook levels. Food Res. Int. 2019, 120, 1–11. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Rousseau, S.; Chigwedere, C.M.; Kyomugasho, C.; Hendrickx, M.; Grauwet, T. Temperature-pressure-time combinations for the generation of common bean microstructures with different starch susceptibilities to hydrolysis. Food Res. Int. 2018, 106, 105–115. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Alvarez Miranda, B.; Truong, N.Q.A.; Kyomugasho, C.; Chigwedere, C.M.; Hendrickx, M.; Grauwet, T. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food Funct. 2018, 9, 6544–6554. [Google Scholar] [CrossRef]
- Benelam, B. Satiation, satiety and their effects on eating behaviour. Nutr. Bull. 2009, 34, 126–173. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Dhital, S.; Bhattarai, R.R.; Gorham, J.; Gidley, M.J. Intactness of cell wall structure controls the in vitro digestion of starch in legumes. Food Funct. 2016, 7, 1367–1379. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballèvre, O.; Beaufrère, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, 340–348. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part, I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Caprioli, G.; Giusti, F.; Ballini, R.; Sagratini, G.; Vila-Donat, P.; Vittori, S.; Fiorini, D. Lipid nutritional value of legumes: Evaluation of different extraction methods and determination of fatty acid composition. Food Chem. 2016, 192, 965–971. [Google Scholar] [CrossRef]
- Zhang, B.; Tamura, M.; Berger-Doyle, J.; Chen, P. Comparison of instrumental methods for measuring seed hardness of food-grade soybean. J. Texture Stud. 2008, 39, 28–39. [Google Scholar] [CrossRef]
- Song, J.-Y.; An, G.-H.; Kim, C.-J. Color, texture, nutrient contents, and sensory values of vegetable soybeans [Glycine max (L.) Merrill] as affected by blanching. Food Chem. 2003, 83, 69–74. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrire, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Oey, I.; Bremer, P.; Silcock, P.; Carne, A. Proteolytic pattern, protein breakdown and peptide production of ovomucin-depleted egg white processed with heat or pulsed electric fields at different pH. Food Res. Int. 2018, 108, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Megazyme. Total Starch Assay Procedure; Megazyme: Bray, Ireland, 2019. [Google Scholar]
- Lassé, M.; Deb-Choudhury, S.; Haines, S.; Larsen, N.; Gerrard, J.A.; Dyer, J.M. The impact of pH, salt concentration and heat on digestibility and amino acid modification in egg white protein. J. Food Compos. Anal. 2015, 38, 42–48. [Google Scholar] [CrossRef]
- Luyten, H.; Plijter, J.; Van Vliet, T. Crispy/crunchy crusts of cellular solid foods: A literature review with discussion. J. Texture Stud. 2004, 35, 445–492. [Google Scholar] [CrossRef]
- Lillford, P. The impact of food structure on taste and digestibility. Food Funct. 2016, 7, 4131–4136. [Google Scholar] [CrossRef]
- Chigwedere, C.M.; Nkonkola, C.M.; Rai, S.; Kyomugasho, C.; Kermani, Z.J.; Pallares, A.P.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Cotyledon pectin molecular interconversions explain pectin solubilization during cooking of common beans (Phaseolus vulgaris). Food Res. Int. 2019, 116, 462–470. [Google Scholar] [CrossRef]
- Rizvi, A.; Tong, C. Fractional conversion for determining texture degradation kinetics of vegetables. J. Food Sci. 1997, 62, 1–7. [Google Scholar] [CrossRef]
- Chigwedere, C.M.; Olaoye, T.F.; Kyomugasho, C.; Kermani, Z.J.; Pallares, A.P.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Mechanistic insight into softening of Canadian wonder common beans (Phaseolus vulgaris) during cooking. Food Res. Int. 2018, 106, 522–531. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D.; Nyachoti, C.M. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT—Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Hoover, R.; Manuel, H. Effect of heat—moisture treatment on the structure and physicochemical properties of legume starches. Food Res. Int. 1996, 29, 731–750. [Google Scholar] [CrossRef]
- Edwards, C.H.; Warren, F.J.; Campbell, G.M.; Gaisford, S.; Royall, P.G.; Butterworth, P.J.; Ellis, P.R. A study of starch gelatinisation behaviour in hydrothermally-processed plant food tissues and implications for in vitro digestibility. Food Funct. 2015, 6, 3634–3641. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Liu, L.; Wang, S.; Copeland, L. Structural orders of wheat starch do not determine the in vitro enzymatic digestibility. J. Agric. Food Chem. 2017, 65, 1697–1706. [Google Scholar] [CrossRef]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans. Food Chem. 2019, 286, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.R.; Tresina, P.S.; Daffodil, E.D. Antinutritional factors in legume seeds: Characteristics and determination. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 211–220. [Google Scholar]
- Nguyen, G.T.; Sopade, P.A. Modeling starch digestograms: Computational characteristics of kinetic models for in vitro starch digestion in food research. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1422–1445. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; Björck, I.M.; Asp, N.G. Incomplete digestion of legume starches in rats: A study of precooked flours containing retrograded and physically inaccessible starch fractions. J. Nutr. 1992, 122, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Noah, L.; Guillon, F.; Bouchet, B.; Buléon, A.; Molis, C.; Gratas, M.; Champ, M. Digestion of carbohydrate from white beans (Phaseolus vulgaris L.) in healthy humans. J. Nutr. 1998, 128, 977–985. [Google Scholar] [CrossRef]
- Ajeigbe, H.A.; Ihedioha, D.; Chikoye, D. Variation in physico-chemical properties of seed of selected improved varieties of Cowpea as it relates to industrial utilization of the crop. Afr. J. Biotechnol. 2008, 7, 3639–3644. [Google Scholar]
- Van Boekel, M.A.J.S. Kinetic modeling of food quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Degen, L.; Phillips, S. Variability of gastrointestinal transit in healthy women and men. Gut 1996, 39, 299–305. [Google Scholar] [CrossRef]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. A closer look to cell structural barriers affecting starch digestibility in beans. Carbohydr. Polym. 2018, 181, 994–1002. [Google Scholar] [CrossRef]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2015, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.M.; Oliveira, J.T.A. Antinutritional properties of plant lectins. Toxicon 2004, 44, 385–403. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation methods of soybean trypsin inhibitor—A review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Laurena, A.C.; Van Den, T.; Mendoza, E.M.T. Effects of condensed tannins on the in vitro protein digestibility of cowpea [Vigna unguiculata (L.) Walp.]. J. Agric. Food Chem. 1984, 32, 1045–1048. [Google Scholar] [CrossRef]
- Chitra, U.; Singh, U.; Venkateswara Rao, P. Phytic acid, in vitro protein digestibility, dietary fiber, and minerals of pulses as influenced by processing methods. Plant Foods Hum. Nutr. 1996, 49, 307–316. [Google Scholar] [CrossRef]
- Van Der Poel, A.F.B. Effect of processing on antinutritional factors and protein nutritional value of dry beans (Phaseolus vulgaris L.). A review. Anim. Feed. Sci. Technol. 1990, 29, 179–208. [Google Scholar] [CrossRef]
- Wu, W.; Williams, W.P.; Kunkel, M.E.; Acton, J.C.; Wardlaw, F.B.; Huang, Y.; Grimes, L.W. Thermal effects on in vitro protein quality of red kidney bean (Phaseolus vulgaris L.). J. Food Sci. 1994, 59, 1187–1191. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Damodaran, S.; German, B. Physicochemical and functional properties of oilseed proteins with emphasis on soy proteins. New Protein Foods (USA) 1985, 107–179. [Google Scholar]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility−digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Koriyama, T.; Sato, Y.; Iijima, K.; Kasai, M. Influences of soaking temperature and storage conditions on hardening of soybeans (Glycine max) and red kidney beans (Phaseolus vulgaris). J. Food Sci. 2017, 82, 1546–1556. [Google Scholar] [CrossRef]
 represents cowpeas;
represents cowpeas; 
 represents chickpeas;
represents chickpeas; 
 represents kidney beans. The lines represent the fitted values by a first-order fractional conversion model, see Equation (2), to the hydrothermal processing time (N = 5 measurements).
represents kidney beans. The lines represent the fitted values by a first-order fractional conversion model, see Equation (2), to the hydrothermal processing time (N = 5 measurements).
 represents cowpeas;
represents cowpeas; 
 represents chickpeas;
represents chickpeas; 
 represents kidney beans. The lines represent the fitted values by a first-order fractional conversion model, see Equation (2), to the hydrothermal processing time (N = 5 measurements).
represents kidney beans. The lines represent the fitted values by a first-order fractional conversion model, see Equation (2), to the hydrothermal processing time (N = 5 measurements).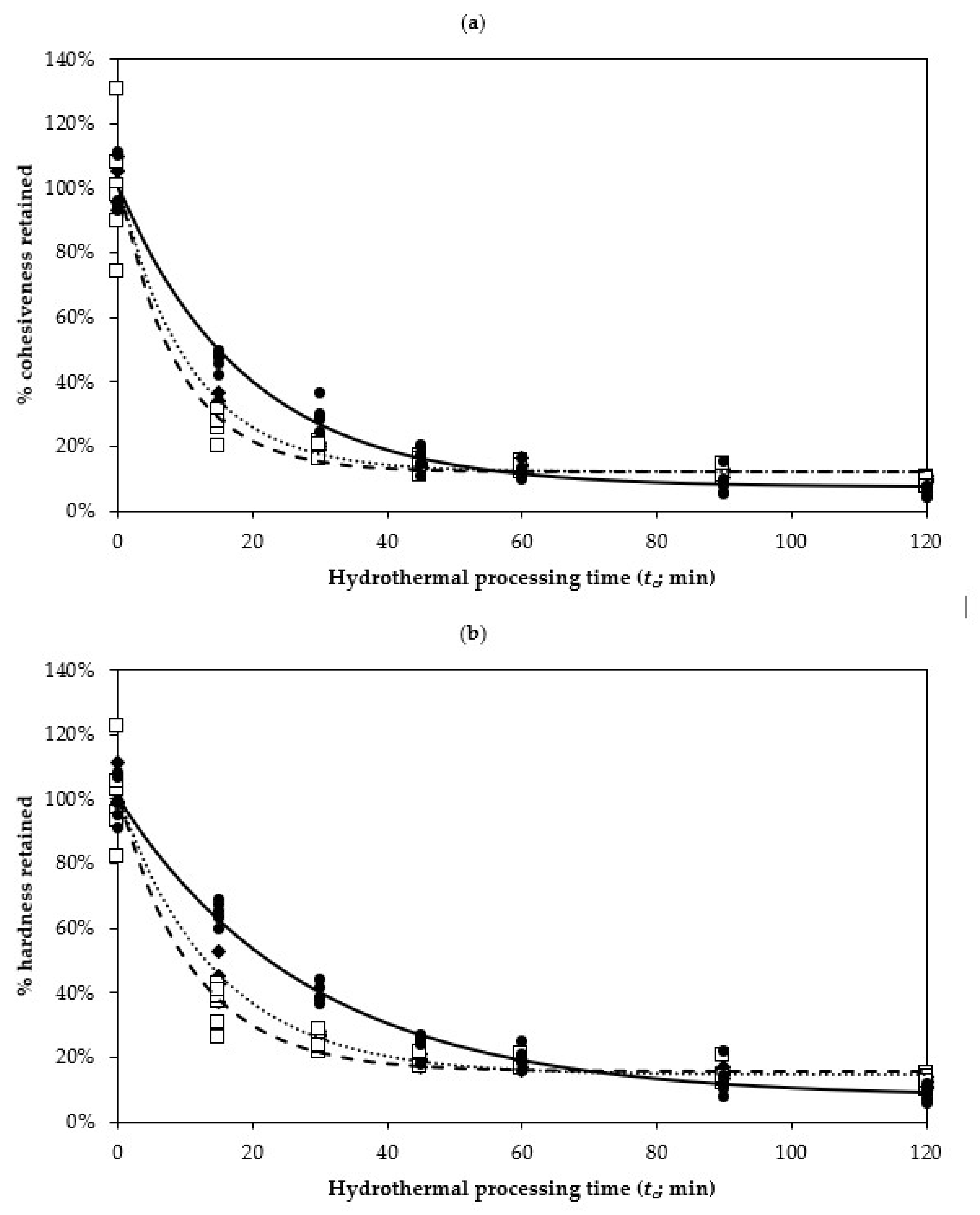

 ; Chickpeas boiled for 15 min (CHI15)
; Chickpeas boiled for 15 min (CHI15) 
 ; Kidney beans boiled for 15 min (KID15)
; Kidney beans boiled for 15 min (KID15) 
 ; Cowpeas boiled for 120 min (COW120)
; Cowpeas boiled for 120 min (COW120) 
 ; Chickpeas boiled for 120 min (CHI120)
; Chickpeas boiled for 120 min (CHI120) 
 ; Kidney beans boiled for 120 min (KID120)
; Kidney beans boiled for 120 min (KID120) 
 .
.

 ; Chickpeas boiled for 15 min (CHI15)
; Chickpeas boiled for 15 min (CHI15) 
 ; Kidney beans boiled for 15 min (KID15)
; Kidney beans boiled for 15 min (KID15) 
 ; Cowpeas boiled for 120 min (COW120)
; Cowpeas boiled for 120 min (COW120) 
 ; Chickpeas boiled for 120 min (CHI120)
; Chickpeas boiled for 120 min (CHI120) 
 ; Kidney beans boiled for 120 min (KID120)
; Kidney beans boiled for 120 min (KID120) 
 .
.
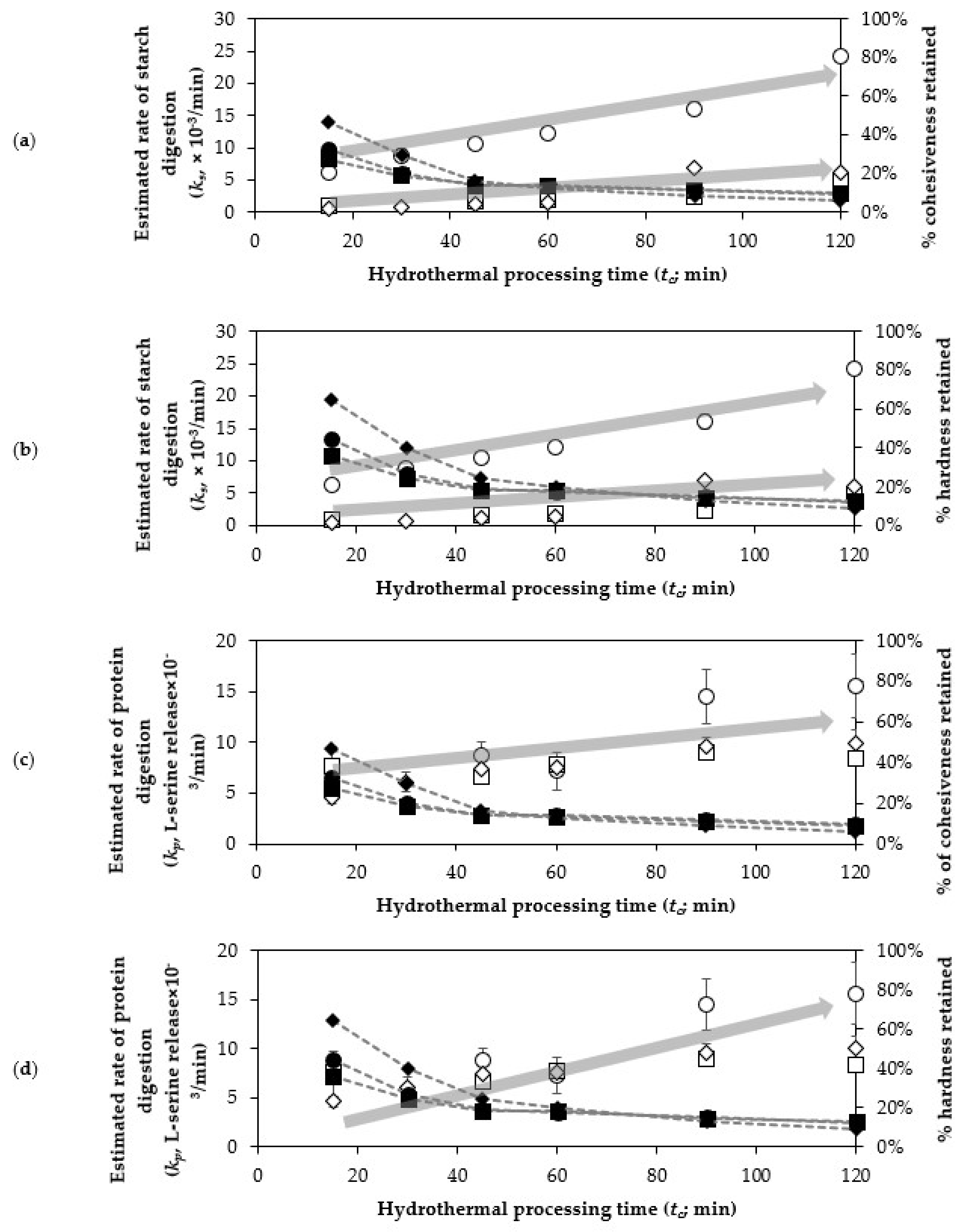
| Texture Parameter | Legume | k (×10−2/min) | Tf (%) | Corrected R2 |
|---|---|---|---|---|
| Cohesiveness | Cowpeas | 9.29 ± 0.47 | 12.29 ± 0.75 | 0.9941 |
| Chickpeas | 11.16 ± 1.28 | 12.29 ± 1.41 | 0.9704 | |
| Kidney beans | 5.23 ± 0.25 | 7.61 ± 1.07 | 0.9906 | |
| Hardness | Cowpeas | 6.78 ± 0.33 | 14.47 ± 0.90 | 0.9941 |
| Chickpeas | 8.94 ± 0.77 | 15.68 ± 1.24 | 0.9812 | |
| Kidney beans | 3.50 ± 0.16 | 7.94 ± 1.32 | 0.9934 |
| Digestion Time (td, min) | Hydrothermal Processing Time (tc, min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Uncooked | Soaked | 15 | 30 | 45 | 60 | 90 | 120 | ||
| Cowpea | 0 | 4.61 ± 0.26 A a | 5.95 ± 0.15 A b | 5.98 ± 0.96 A b | 6.69 ± 0.12 A c | 9.33 ± 0.18 A d | 10.92 ± 0.11 A e | 11.42 ± 0.27 A f | 12.55 ± 0.10 A g |
| 60 | 5.62 ± 0.18 A a | 5.90 ± 0.10 A a | 9.57 ± 0.91 B b | 9.48 ± 0.18 B b | 14.67 ± 0.22 B c | 20.49 ± 0.18 B d | 18.80 ± 0.22 B d | 19.03 ± 0.24 B e | |
| 120 | 5.72 ± 0.22 B a | 5.79 ± 0.06 A a | 10.94 ± 0.30 C b | 12.22 ± 0.20 C c | 16.63 ± 0.22 C d | 21.53 ± 0.29 C e | 20.43 ± 0.33 C f | 21.05 ± 0.37 B g | |
| Chickpea | 0 | 1.57 ± 0.10 A a | 6.73 ± 0.24 B b | 6.56 ± 0.21 A b | nd. | 6.98 ± 0.26 A b | 9.01 ± 0.28 A c | 8.39 ± 0.24 A d | 10.59 ± 0.27 A e |
| 60 | 1.95 ± 0.14 B a | 6.58 ± 0.32 AB b | 6.43 ± 0.23 A b | nd. | 6.72 ± 0.32 A b | 9.96 ± 0.19 B d | 8.37 ± 0.22 A c | 10.54 ± 0.38 A e | |
| 120 | 1.54 ± 0.14 A a | 6.24 ± 0.32 A b | 7.67 ± 0.43 B c | nd. | 7.82 ± 0.34 B c | 10.35 ± 0.49 B d | 8.90 ± 0.17 B c | 11.82 ± 0.26 B e | |
| Kidney Bean | 0 | 3.61 ± 0.17 A b | 3.61 ± 0.14 A b | 2.89 ± 0.11 A a | 4.19 ± 0.06 A c | 5.13 ± 0.04 B d | 5.91 ± 0.12 B e | 6.45 ± 0.20 B f | 5.77 ± 0.22 A e |
| 60 | 4.09 ± 0.18 B a | 3.90 ± 0.1 B a | 3.87 ± 0.13 B a | 4.73 ± 0.07 B b | 5.08 ± 0.05 B c | 6.55 ± 0.12 C f | 6.23 ± 0.20 B e | 5.79 ± 0.09 A d | |
| 120 | 3.80 ± 0.18 A a | 4.83 ± 0.15 C b | 5.96 ± 0.06 C c | 5.04 ± 0.28 C b | 3.50 ± 0.06 A a | 5.09 ± 0.10 A b | 5.70 ± 0.24 A c | 4.95 ± 0.86 A c | |
| Legume | Sample | tc (min) | ks (×10−3/min) | Cs (% Starch digested) | Corrected R2 |
|---|---|---|---|---|---|
| Cowpea | COW15 | 15 | 6.20 ± 0.40 a | 59.19 ± 1.85 a | 0.9986 † |
| COW30 | 30 | 8.80 ± 1.00 ab | 61.49 ± 2.41 a | 0.9949 † | |
| COW45 | 45 | 10.50 ± 0.30 b | 69.95 ± 0.61 b | 0.9996 † | |
| COW60 | 60 | 12.20 ± 0.40 c | 67.94 ± 0.58 b | 0.9996 † | |
| COW90 | 90 | 16.10 ± 1.10 d | 68.55 ± 0.97 b | 0.9980 † | |
| COW120 | 120 | 24.10 ± 0.50 e | 75.61 ± 0.29 c | 0.9997 † | |
| Chickpea | CHI15 | 15 | 0.94 ± 0.02 a | np. | 0.9840 ‡ |
| CHI45 | 45 | 1.62 ± 0.03 b | np. | 0.9841 ‡ | |
| CHI60 | 60 | 1.96 ± 0.03 c | np. | 0.9910 ‡ | |
| CHI90 | 90 | 2.34 ± 0.05 d | np. | 0.9787 ‡ | |
| CHI120 | 120 | 4.73 ± 0.34 e | 94.09 ± 3.68 | 0.9988 † | |
| Kidney bean | KID15 | 15 | 0.43 ± 0.02 a | np. | 0.9212 ‡ |
| KID30 | 30 | 0.74 ± 0.01 b | np. | 0.9952 ‡ | |
| KID45 | 45 | 1.15 ± 0.02 c | np. | 0.9918 ‡ | |
| KID60 | 60 | 1.44 ± 0.03 d | np. | 0.9797 ‡ | |
| KID90 | 90 | 6.93 ± 0.45 e | 56.37 ± 1.70 a | 0.9980 † | |
| KID120 | 120 | 6.07 ± 0.22 e | 66.92 ± 1.21 b | 0.9995 † |
| Legume | Sample | tc (min) | kp (L-Serine Release × 10−3/min) | Cp (mg L-Serine Equivalent/g DW) | Corrected R2 |
|---|---|---|---|---|---|
| Cowpea | COW15 | 15 | 7.40 ± 2.28 a | 33.25 a ± 4.44 a | 0.9653 † |
| COW45 | 45 | 8.72 ± 1.34 a | 42.96 a ± 2.47 a | 0.9908 † | |
| COW60 | 60 | 7.20 ± 1.84 a | 46.04 a ± 4.76 a | 0.9838 † | |
| COW90 | 90 | 14.50 ± 2.64 a | 39.99 a ± 1.93 a | 0.9848 † | |
| COW120 | 120 | 15.60 ± 3.16 a | 35.99 a ± 2.27 a | 0.9642 † | |
| Chickpea | CHI15 | 15 | 7.62 ± 0.55 a | np. | 0.8668 ‡ |
| CHI45 | 45 | 6.60 ± 0.67 a | np. | 0.7640 ‡ | |
| CHI60 | 60 | 7.78 ± 0.71 a | np. | 0.8013 ‡ | |
| CHI90 | 90 | 8.97 ± 0.67 a | np. | 0.8459 ‡ | |
| CHI120 | 120 | 8.40 ± 0.45 a | np. | 0.9239 ‡ | |
| Kidney bean | KID15 | 15 | 4.60 ± 0.58 a | np. | 0.7290 ‡ |
| KID30 | 30 | 6.09 ± 0.94 ab | np. | 0.6378 ‡ | |
| KID45 | 45 | 7.45 ± 1.14 ab | np. | 0.6308 ‡ | |
| KID60 | 60 | 7.53 ± 0.79 b | np. | 0.7922 ‡ | |
| KID90 | 90 | 9.58 ± 0.89 b | np. | 0.8302 ‡ | |
| KID120 | 120 | 9.96 ± 1.36 b | np. | 0.7290 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrisanapant, P.; Leong, S.Y.; Kebede, B.; Oey, I. Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans. Foods 2021, 10, 1415. https://doi.org/10.3390/foods10061415
Khrisanapant P, Leong SY, Kebede B, Oey I. Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans. Foods. 2021; 10(6):1415. https://doi.org/10.3390/foods10061415
Chicago/Turabian StyleKhrisanapant, Prit, Sze Ying Leong, Biniam Kebede, and Indrawati Oey. 2021. "Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans" Foods 10, no. 6: 1415. https://doi.org/10.3390/foods10061415
APA StyleKhrisanapant, P., Leong, S. Y., Kebede, B., & Oey, I. (2021). Effects of Hydrothermal Processing Duration on the Texture, Starch and Protein In Vitro Digestibility of Cowpeas, Chickpeas and Kidney Beans. Foods, 10(6), 1415. https://doi.org/10.3390/foods10061415








