Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Preparation
2.2. Animals
2.3. Experimental Design and Drug Treatment
- Vc = Volume of paw edema in control animals
- Vt = Volume of paw edema in treated animals.
2.4. Sample Preparation
2.5. Inflammatory Biomarkers
2.6. Hematological Parameters
2.7. Oxidant Assays
2.8. Antioxidant Assays
2.9. Histological Examination
2.10. In Silico Molecular Docking Assay
2.11. Statistical Analysis
3. Results
3.1. Anti-Inflammatory Activity
3.2. Inflammatory Biomarkers
3.3. Hematological Parameters
3.4. Oxidative Stress Assessment
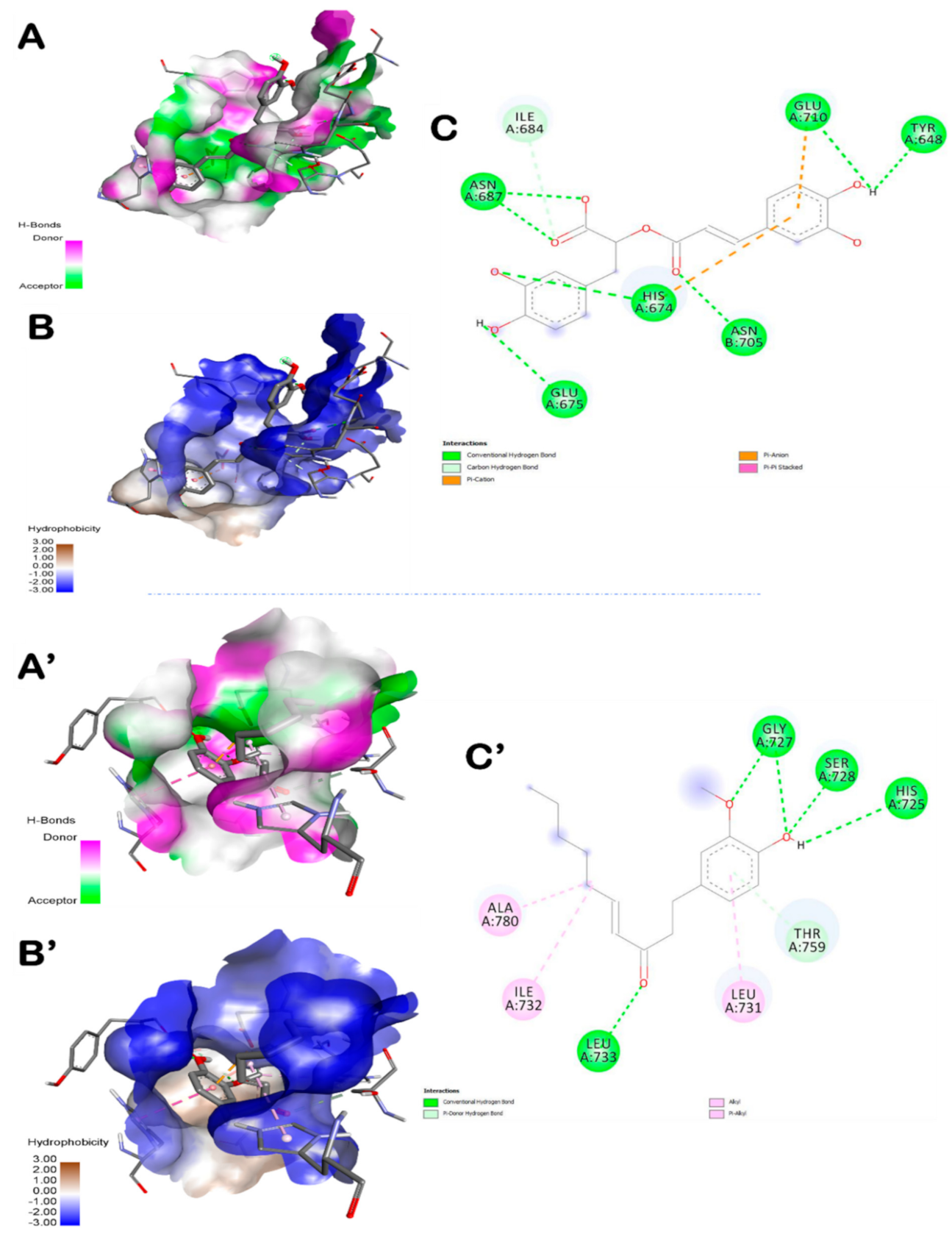
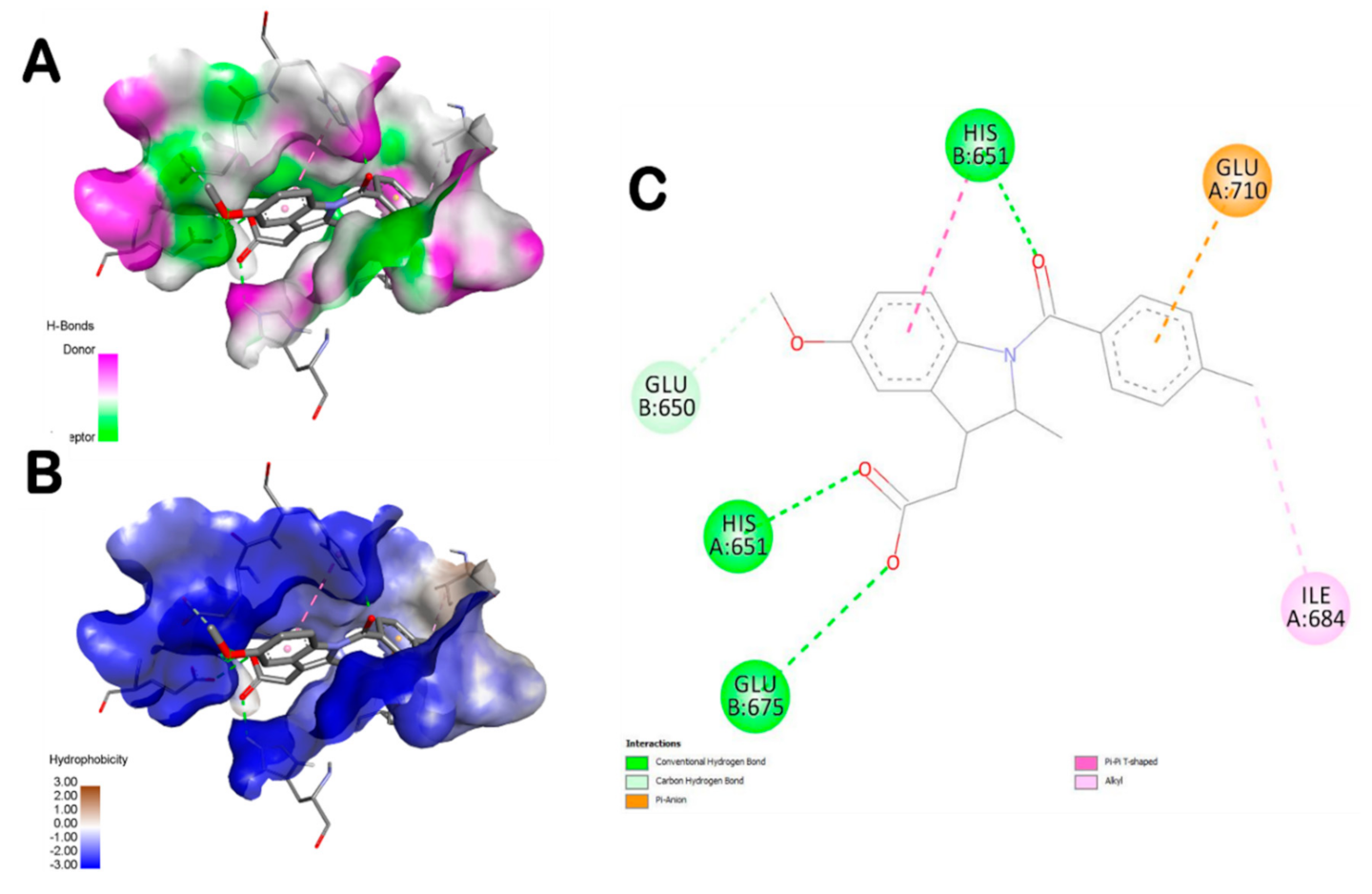
3.5. Molecular Docking Findings
3.6. Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiiflammatory Drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Borza, C.; Muntean, D.; Dehelean, C.; Săvoiu, G.; Şerban, C.; Simu, G.; Andoni, M.; Butur, M.; Drăgan, S. Oxidative Stress and Lipid Peroxidation–A Lipid Metabolism Dysfunction. Lipid Metab. 2013. [Google Scholar] [CrossRef]
- Badraoui, R.; Abdelmoula, N.B.; Sahnoun, Z.; Fakhfakh, Z.; Rebai, T. Effect of Subchronic Exposure to Tetradifon on Bone Remodelling and Metabolism in Female Rat. Comptes Rendus Biol. 2007, 330, 897–904. [Google Scholar] [CrossRef]
- Badraoui, R.; Sahnoun, Z.; Abdelmoula, N.B.; Hakim, A.; Fki, M.; Rebaï, T. May Antioxidants Status Depletion by Tetradifon Induce Secondary Genotoxicity in Female Wistar Rats via Oxidative Stress? Pestic. Biochem. Physiol. 2007, 88, 149–155. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Badraoui, R.; Blouin, S.; Moreau, M.F.; Gallois, Y.; Rebai, T.; Sahnoun, Z.; Baslé, M.; Chappard, D. Effect of Alpha Tocopherol Acetate in Walker 256/B Cells-Induced Oxidative Damage in a Rat Model of Breast Cancer Skeletal Metastases. Chem. Biol. Interact. 2009, 182, 98–105. [Google Scholar] [CrossRef]
- James, M.W.; Hawkey, C.J. Assessment of Non-Steroidal Anti-Inflammatory Drug (NSAID) Damage in the Human Gastrointestinal Tract. Br. J. Clin. Pharmacol. 2003, 56, 146–155. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The Role of Flavonoids in Autoimmune Diseases: Therapeutic Updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Duerschmied, D.; Suidan, G.L.; Demers, M.; Herr, N.; Carbo, C.; Brill, A.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet Serotonin Promotes the Recruitment of Neutrophils to Sites of Acute Inflammation in Mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef]
- Akacha, A.; Badraoui, R.; Rebai, T.; Zourgui, L. Effect of Opuntia Ficus Indica Extract on Methotrexate-Induced Testicular Injury: A Biochemical, Docking and Histological Study. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.C.; Hung, C.T.; Cheng, K.C.; Wu, C.Y.; Chen, Y.C.; Wu, Y.J.; Liu, W.; Chiu, C.C. Protective Effects of Lycium barbarum Extracts on UVB-Induced Damage in Human Retinal Pigment Epithelial Cells Accompanied by Attenuating ROS and DNA Damage. Oxid. Med. Cell Longev. 2018, 2018, 4814928. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.; Ribeiro, A.P.D.; Offenbacher, S.; Loewy, Z.G. Anti-Inflammatory Effects of Vitamin E in Response to Candida albicans. Microorganisms 2020, 8, 804. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Kim, K.Y. Inhibition of Proinflammatory Cytokines in Cutibacterium acnes-Induced Inflammation in HaCaT Cells by Using Buddleja davidii Aqueous Extract. Int. J. Inflam. 2020, 2020, 8063289. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Rebai, T.; Elkahoui, S.; Alreshidi, M.; Veettil, V.N.; Noumi, E.; Al-Motair, K.A.; Aouadi, K.; Kadri, A.; De Feo, V.; et al. Allium subhirsutum L. as a Potential Source of Antioxidant and Anticancer Bioactive Molecules: HR-LCMS Phytochemical Profiling, In Vitro and In Vivo Pharmacological Study. Antioxidants 2020, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zammel, N.; Amri, N.; Chaabane, R.; Rebai, T.; Badraoui, R. Proficiencies of Zingiber officinale against Spine Curve and Vertebral Damage Induced by Corticosteroid Therapy Associated with Gonadal Hormone Deficiency in a Rat Model of Osteoporosis. Biomed. Pharmacother. 2018, 103, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Semerci, A.; İnceçayır, D.; Mammadova, V.; Hoş, A.; Tunç, K. Antimicrobial Activities of Allium staticiforme and Allium subhirsutum. Bangladesh J. Pharmacol. 2020, 15, 19–23. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially Processed Dry Ginger (Zingiber officinale): Composition and Effects on LPS-Stimulated PGE2 Production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Kikuzaki, H.; Hisamoto, M.; Nakatani, N. Antioxidant Properties of Gingerol Related Compounds from Ginger. Biofactors 2004, 21, 293–296. [Google Scholar] [CrossRef]
- Haksar, A.; Sharma, A.; Chawla, R.; Kumar, R.; Arora, R.; Singh, S.; Prasad, J.; Gupta, M.; Tripathi, R.P.; Arora, M.P.; et al. Zingiber officinale Exhibits Behavioral Radioprotection against Radiation-Induced CTA in a Gender-Specific Manner. Pharmacol. Biochem. Behav. 2006, 84, 179–188. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.K.; Al-Sawan, S.M.; Alnaqeeb, M.A.; Khan, I.; Ali, M. The Use of Ginger (Zingiber officinale Rosc.) as a Potential Anti-Inflammatory and Antithrombotic Agent. Prostaglandins Leukot Essent Fatty Acids 2002, 67, 475–478. [Google Scholar] [CrossRef]
- Young, H.-Y.; Luo, Y.-L.; Cheng, H.-Y.; Hsieh, W.-C.; Liao, J.-C.; Peng, W.-H. Analgesic and Anti-Inflammatory Activities of [6]-Gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef]
- Goyal, C.; Ahuja, M.; Sharma, S.K. Preparation and Evaluation of Anti-Inflammatory Activity of Gugulipid-Loaded Proniosomal Gel. Acta Pol. Pharm. 2011, 68, 147–150. [Google Scholar]
- Sinha, P.; Clements, V.K.; Fulton, A.M.; Ostrand-Rosenberg, S. Prostaglandin E2 Promotes Tumor Progression by Inducing Myeloid-Derived Suppressor Cells. Cancer Res. 2007, 67, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Morse, L.R.; Stolzmann, K.; Nguyen, H.P.; Jain, N.B.; Zayac, C.; Gagnon, D.R.; Tun, C.G.; Garshick, E. Association between Mobility Mode and C-Reactive Protein Levels in Men with Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2008, 89, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Clauss, A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens; Karger: Basel, Switzerland, 1957. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. In Methods in Enzymology; Oxygen Radicals in Biological Systems Part B: Oxygen Radicals and Antioxidants; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 421–431. [Google Scholar]
- Kayali, R.; Cakatay, U.; Akçay, T.; Altuğ, T. Effect of Alpha-Lipoic Acid Supplementation on Markers of Protein Oxidation in Post-Mitotic Tissues of Ageing Rat. Cell Biochem. Funct. 2006, 24, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. [13]Catalase in vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-Induced Liver Necrosis. Protective Role of Glutathione and Evidence for 3,4-Bromobenzene Oxide as the Hepatotoxic Metabolite. PHA 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Badraoui, R.; Adnan, M.; Bardakci, F.; Alreshidi, M.M. Chloroquine and Hydroxychloroquine Interact Differently with ACE2 Domains Reported to Bind with the Coronavirus Spike Protein: Mediation by ACE2 Polymorphism. Molecules 2021, 26, 673. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.-H.; Park, H.H. Crystal Structure of TIR Domain of TLR6 Reveals Novel Dimeric Interface of TIR-TIR Interaction for Toll-like Receptor Signaling Pathway. J. Mol. Biol. 2014, 426, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-Induced Mouse Paw Oedema Is Biphasic, Age-Weight Dependent and Displays Differential Nitric Oxide Cyclooxygenase-2 Expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Capasso, F.; Dunn, C.J.; Yamamoto, S.; Willoughby, D.A.; Giroud, J.P. Further Studies on Carrageenan-Induced Pleurisy in Rats. J. Pathol. 1975, 116, 117–124. [Google Scholar] [CrossRef]
- Heller, A.; Koch, T.; Schmeck, J.; van Ackern, K. Lipid Mediators in Inflammatory Disorders. Drugs 1998, 55, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Drug Screening Methods: Preclinical Evaluation of New Drugs; Jaypee: New Delhi, India, 2009; ISBN 978-81-8448-618-6. [Google Scholar]
- Brooks, P.M.; Day, R.O. Nonsteroidal Antiinflammatory Drugs--Differences and Similarities. N. Engl. J. Med. 1991, 324, 1716–1725. [Google Scholar] [CrossRef]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of Ginger Constituents and Synthetic Analogues on Cyclooxygenase-2 Enzyme in Intact Cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- Srinivasan, K. Ginger Rhizomes (Zingiber officinale): A Spice with Multiple Health Beneficial Potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Hsiang, C.-Y.; Lo, H.-Y.; Huang, H.-C.; Li, C.-C.; Wu, S.-L.; Ho, T.-Y. Ginger Extract and Zingerone Ameliorated Trinitrobenzene Sulphonic Acid-Induced Colitis in Mice via Modulation of Nuclear Factor-ΚB Activity and Interleukin-1β Signalling Pathway. Food Chem. 2013, 136, 170–177. [Google Scholar] [CrossRef]
- Frondoza, C.G.; Sohrabi, A.; Polotsky, A.; Phan, P.V.; Hungerford, D.S.; Lindmark, L. An in Vitro Screening Assay for Inhibitors of Proinflammatory Mediators in Herbal Extracts Using Human Synoviocyte Cultures. In Vitro Cell Dev. Biol. Anim. 2004, 40, 95–101. [Google Scholar] [CrossRef]
- Tripathi, S.; Bruch, D.; Kittur, D.S. Ginger Extract Inhibits LPS Induced Macrophage Activation and Function. BMC Complementary Altern. Med. 2008, 8, 1. [Google Scholar] [CrossRef]
- Murata, H.; Shimada, N.; Yoshioka, M. Current Research on Acute Phase Proteins in Veterinary Diagnosis: An Overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Vazquez, E.; Navarro, M.; Salazar, Y.; Crespo, G.; Bruges, G.; Osorio, C.; Tortorici, V.; Vanegas, H.; López, M. Systemic Changes Following Carrageenan-Induced Paw Inflammation in Rats. Inflamm. Res. 2015, 64, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute Phase Reaction and Acute Phase Proteins. J. Zhejiang Univ. Sci. B 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The Interactions between Inflammation and Coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; de Jonge, E.; Levi, M. Regulatory Role of Cytokines in Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2001, 27, 639–651. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Moliterno, D.J. The Role of the Platelet in the Pathogenesis of Atherothrombosis. Am. J. Cardiovasc Drugs 2005, 5, 399–408. [Google Scholar] [CrossRef]
- Busso, N.; Chobaz-Péclat, V.; Hamilton, J.; Spee, P.; Wagtmann, N.; So, A. Essential Role of Platelet Activation via Protease Activated Receptor 4 in Tissue Factor-Initiated Inflammation. Arthritis Res. Ther. 2008, 10, R42. [Google Scholar] [CrossRef]
- Davies, S.A.; Goldberg, A.L. Proteins Damaged by OxygenRadicals Are Rapidly Degraded in Extracts of Red Blood Cells. J. Biol. Chem. 1987, 262, 8227–8234. [Google Scholar] [CrossRef]
- Mzid, M.; Badraoui, R.; Khedir, S.B.; Sahnoun, Z.; Rebai, T. Protective Effect of Ethanolic Extract of Urtica Urens, L. against the Toxicity of Imidacloprid on Bone Remodeling in Rats and Antioxidant Activities. Biomed. Pharmacother. 2017, 91, 1022–1041. [Google Scholar] [CrossRef]
- Rahmouni, F.; Saoudi, M.; Amri, N.; El-Feki, A.; Rebai, T.; Badraoui, R. Protective Effect of Teucrium Polium on Carbon Tetrachloride Induced Genotoxicity and Oxidative Stress in Rats. Arch. Physiol. Biochem. 2018, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Rebai, T. Effect of malignant ascites on antioxidative potency of two tumoral cells-induced bone metastases: Walker 256/B and MatLyLu. Exp. Toxicol. Pathol. 2012, 1–2, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Badraoui, R.; Alrashedi, M.M.; El-May, M.V.; Bardakci, F. Acute respiratory distress syndrome: A life threatening associated complication of SARS-CoV-2 infection inducing COVID-19. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alderman, C.J.J.; Shah, S.; Foreman, J.C.; Chain, B.M.; Katz, D.R. The Role of Advanced Oxidation Protein Products in Regulation of Dendritic Cell Function. Free Radic. Biol. Med. 2002, 32, 377–385. [Google Scholar] [CrossRef]
- Del Mastro, R.; Thaw, H.H.; Björk, J.; Planker, M.; Arfors, K.E. Free Radicals as Mediators of Tissue Injury. Acta Physiol. Scand. Suppl. 1980, 492, 43–57. [Google Scholar]
- Sut, S.; Maggi, F.; Bruno, S.; Badalamenti, N.; Quassinti, L.; Bramucci, M.; Beghelli, D.; Lupidi, G.; Dall’Acqua, S. Hairy Garlic (Allium subhirsutum) from Sicily (Italy): LC-DAD-MSn Analysis of Secondary Metabolites and In Vitro Biological Properties. Molecules 2020, 25, 2837. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Sharma, M.; Pillai, K.K.; Aeri, V.; Sheliya, M.A. Inhibitory Effect of Careya Arborea on Inflammatory Biomarkers in Carrageenan-Induced Inflammation. Pharm. Biol. 2015, 53, 437–445. [Google Scholar] [CrossRef]
- 6Fatehi-Hassanbad, Z.; Gholamnezhad, Z.; Jafarzadeh, M.; Fatehi, M. The Anti-Inflammatory Effects of Aqueous Extract of Ginger Root in Diabetic Mice. DARU J. Pharm. Sci. 2005, 13, 70–73. [Google Scholar]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and Its Active Compounds in Cancer Therapy: From Folk Uses to Nano-Therapeutic Applications. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Tao, Y.; Li, W. Cyclooxygenase-2 Inhibitors in Ginger (Zingiber officinale). Fitoterapia 2011, 82, 38–43. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, W.; Qin, Y.; Gu, W.; Xue, Y.; Tang, Y.; Xu, H.; Wang, H.; Zhang, C.; Wang, C.; et al. 6-Gingerol Stabilized the p-VEGFR2/VE-Cadherin/β-Catenin/Actin Complex Promotes Microvessel Normalization and Suppresses Tumor Progression. J. Exp. Clin. Cancer Res. 2019, 38, 285. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, D. Assessment of Malathion Toxicity on Cytophysiological Activity, DNA Damage and Antioxidant Enzymes in Root of Allium Cepa Model. Sci. Rep. 2020, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, R.; Chen, J.; Fu, C.; Lin, J.; Wu, G. Anti-Inflammatory Activity of Radix Angelicae Biseratae in the Treatment of Osteoarthritis Determined by Systematic Pharmacology and in Vitro Experiments. Exp. Ther. Med. 2021, 21. [Google Scholar] [CrossRef]
- Zhou, Y.; Banday, A.H.; Hruby, V.J.; Cai, M. Development of N-Acetylated Dipalmitoyl-S-Glyceryl Cysteine Analogs as Efficient TLR2/TLR6 Agonists. Molecules 2019, 24, 3512. [Google Scholar] [CrossRef]
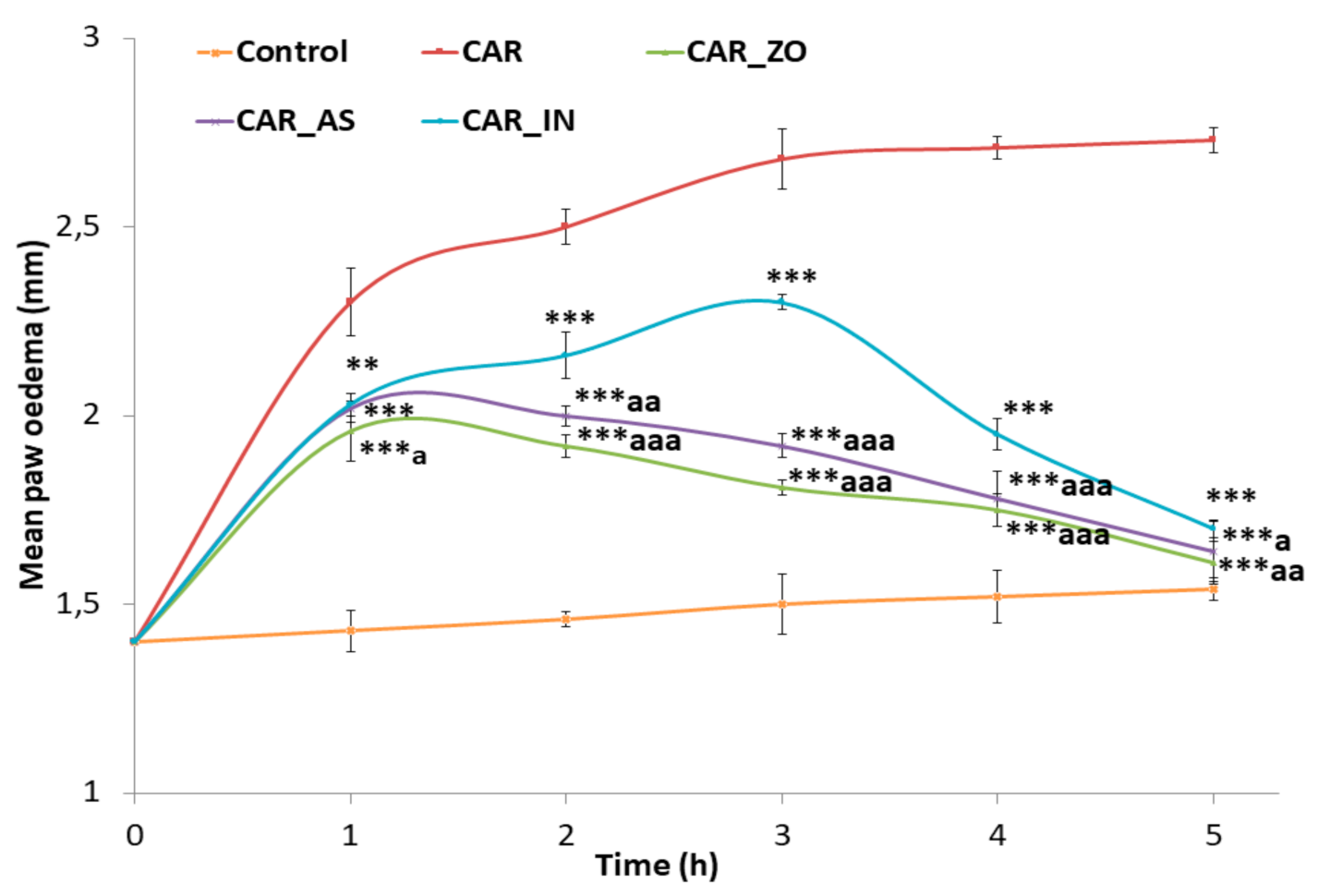
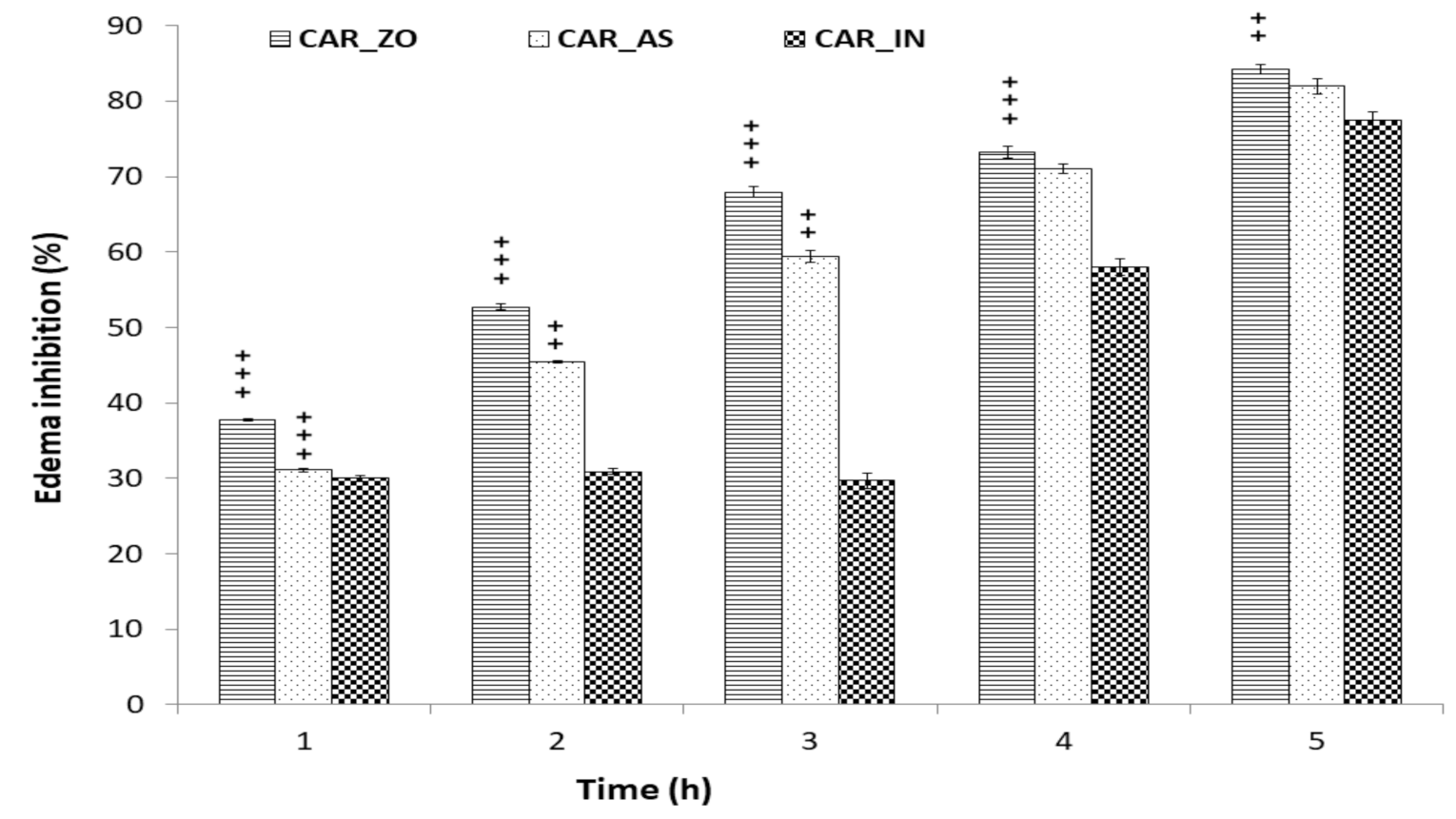


| Parameters | Control | CAR | CAR_ZO | CAR_AS | CAR_IN |
|---|---|---|---|---|---|
| CRP (mg/L) | 0.79 ± 0.012 | 1.043 ± 0.015 *** | 0.813 ± 0.015 ### | 0.84 ± 0.02 ### | 0.857 ± 0.009 *### |
| Fibrinogen (g/L) | 1.743 ± 0.024 | 2.047 ± 0.009 *** | 1.807 ± 0.003 ### | 1.86 ± 0.2 ### | 1.863 ± 0.029 *## |
| Parameters | Control | CAR | CAR_ZO | CAR_AS | CAR_IN |
|---|---|---|---|---|---|
| WBC (103/µL) | 12.95 ± 0.386 | 19.1 ± 0.785 *** | 13.27 ± 0.549 ### | 14.33 ± 0.94 *### | 14.175 ± 0.437 ## |
| RBC (106/µL) | 8.1 ± 0.071 | 7.43 ± 0.025 *** | 7.958 ± 0.040 ### | 8.22 ± 0.41 ### | 7.873 ± 0.071 *## |
| PLT (103/mm3) | 482.5 ± 7.773 | 924 ± 5.292 *** | 505.5 ± 7.171 ###++ | 569 ± 46.72 *### | 581.5 ± 16.102 ***### |
| Control | CAR | CAR_ZO | CAR_AS | CAR_IN | |
|---|---|---|---|---|---|
| Skin Tissue | |||||
| TBARS (nmoles /mg protein) | 0.404 ± 0.02 | 1.076 ± 0.047 *** | 0.455 ± 0.019 ###+ | 0.467 ± 0.051 ###+ | 0.604 ± 0.03 **### |
| AOPP (nmoles/mg protein) | 0.613 ± 0.025 | 1.05 ± 0.037 *** | 0.624 ± 0.016 ### | 0.633 ± 0.074 ### | 0.754 ± 0.019 **### |
| SOD (Units/mg protein) | 4.917 ± 0.373 | 1.934 ± 0.179 *** | 4.321 ± 0.148 ###++ | 3.692 ± 0.566 ### | 3.724 ± 0.062 *## |
| CAT (µmoles/min/mg protein) | 7.675 ± 0.407 | 2.697 ± 0.163 *** | 7.237 ± 0.196 ###++ | 6.551 ± 0.892 *###+ | 6.232 ± 0.132 *### |
| GPx (µmoles/min/mg protein) | 1.292 ± 0.045 | 0.591 ± 0.036 *** | 1.185 ± 0.059 ###+ | 0.966 ± 0.093 *### | 0.944 ± 0.014 ***### |
| GSH (µg GSH/mg protein) | 1.181 ± 0.021 | 0.673 ± 0.021 *** | 1.251 ± 0.039 ###++ | 0.846 ± 0.063 *###+ | 0.993 ± 0.028 **### |
| Erythrocytes | |||||
| TBARS (nmoles/mg protein) | 1.881 ± 0.066 | 3.044 ± 0.12 *** | 1.9 ±0.036 ###++ | 2.07 ± 0.102###++ | 2.254 ± 0.105 *## |
| AOPP (nmoles/mg protein) | 0.938 ± 0.056 | 1.738 ± 0.07 *** | 0.949 ± 0.036 ###++ | 1.017 ± 0.053###++ | 1.176 ± 0.069 *## |
| SOD (Units/mg protein) | 21.111 ± 0.832 | 14.717 ± 0.384 *** | 20.158 ± 0.62 ### | 19.461 ± 0.663### | 18.506 ± 0.285 *### |
| CAT (µmoles/min/mg protein) | 16.404 ± 0.551 | 9.402 ± 0.522 *** | 17.154 ± 0.256 ###+++ | 15.086 ± 0.774 *### | 14.081 ± 0.495 *## |
| GPx (µmoles/min/mg protein) | 3.373 ± 0.07 | 1.065 ± 0.61 *** | 3.727 ± 0.138 ###+++ | 3.022 ± 0.427 ###++ | 2.34 ± 0.181 **### |
| GSH (µg/mg protein) | 2.296 ± 0.04 | 1.446 ± 0.038 *** | 2.443 ± 0.12 ###++ | 1.983 ± 0.056 *### | 1.943 ± 0.057 **### |
| Plant/Compound | Binding Affinity (kcal/mol) | Intermolecular INTERACTIONS | |
|---|---|---|---|
| Conventional Hydrogen Bonds | Closest Interacting Residue (Distance, Å) | ||
| Zingiber officinale rosea L. | |||
| 6-Gingerol | −7.1 | 4 | His651 (2.321) |
| 8-Gingerol | −6.6 | 3 | Glu710 (2.208) |
| 10-Gingerol | −6.0 | 3 | Tyr648 (2.128) |
| 6-Shogaol | −7.2 | 5 | Ser728 (2.105) |
| Caffeic Acid | −6.1 | 3 | Lys769 (1.980) |
| Rosmarinic Acid | −8.6 | 7 | His674 (2.061) |
| Syringic Acid | −5.4 | 5 | Ser728 (2.071) |
| Amentoflavone | −10.8 | 5 | His674 (2.044) |
| Ferulic Acid | −6.3 | 3 | Gln757 (2.363) |
| Allium subhirsutum L. | |||
| Methyl N-(amethylbutyryl) glycine | −5.3 | 3 | His674 (2.187) |
| Bis (2-hydroxypropyl) amine | −4.4 | 3 | Tyr648 (2.279) |
| Cepharanthine | −6.7 | 1 | Asn705 (1.829) |
| (22S)-1alpha,22,25-trihydroxy-26,27-dimethyl-23,23,24,24-tetradehydro-24ahomovitamin D3/(22S)-1al | −7.7 | 2 | Glu650 (2.312) |
| L-4-Hydroxy-3-methoxy-amethylphenylalanine | −6.1 | 5 | Glu675 (2.204) |
| N-(2-fluro-ethyl) arachidonoyl amine | −7.1 | 1 | His674 (2.731) |
| Dihydrodeoxystreptomycin | −6.2 | 7 | Glu650 (2.069) |
| 6 alpha-hydroxy castasterone | −6.7 | 4 | Glu710 (2.045) |
| C16 Sphinganine | −5.2 | 3 | Glu650 (2.172) |
| 3beta,7alpha,12alpha-Trihydroxy-5alpha-cholestan-26-oic acid | −6.6 | 4 | Glu710 (1.930) |
| 4-Oxomytiloxanthin | −7.4 | 3 | Asn705 (2.298) |
| Sebacic acid | −5.3 | 5 | Tyr714 (1.866) |
| Tuberonic acid | −6.0 | 5 | His713 (2.200) |
| 6-Deoxo castasterone | −6.6 | 4 | Gly681 (2.156) |
| 2,2-difluoro-hexadecanoic acid | −5.3 | 5 | Tyr714 (2.009) |
| Indomethacin | −6.2 | 3 | His651 (1.969) |
| Compound Name | Interacting Residues in the Pocket Region of TLR6 |
|---|---|
| 6-Shogaol | Ile684, Asn687, Glu675, His674, Asn705, Glu710, Tyr648 |
| Rosmarinic acid | Ala780, Ile732, Leu733, Leu731, Thr759, His725, Ser728, Gly727 |
| L-4-Hydroxy-3-methoxy-amethylphenylalanine | His651, Glu675, Lys682, Asn687 |
| Dihydrodeoxystreptomycin | Glu650, His651, Glu710, Ser709, Glu675, Gln708 |
| Indomethacin (Reference) | Glu675, His651, Glu650, Glu710, Ile684 |
| Bold amino acids: interacting with the correspondent compound via conventional H-bonds. Italics’ amino acids: same interacting residues as for the reference compound. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. https://doi.org/10.3390/foods10061383
Zammel N, Saeed M, Bouali N, Elkahoui S, Alam JM, Rebai T, Kausar MA, Adnan M, Siddiqui AJ, Badraoui R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods. 2021; 10(6):1383. https://doi.org/10.3390/foods10061383
Chicago/Turabian StyleZammel, Nourhene, Mohd Saeed, Nouha Bouali, Salem Elkahoui, Jahoor M. Alam, Tarek Rebai, Mohd A. Kausar, Mohd Adnan, Arif J. Siddiqui, and Riadh Badraoui. 2021. "Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study" Foods 10, no. 6: 1383. https://doi.org/10.3390/foods10061383
APA StyleZammel, N., Saeed, M., Bouali, N., Elkahoui, S., Alam, J. M., Rebai, T., Kausar, M. A., Adnan, M., Siddiqui, A. J., & Badraoui, R. (2021). Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods, 10(6), 1383. https://doi.org/10.3390/foods10061383










