Abstract
Plant-based foods rich in phenolic phytochemicals are among the promising strategies to counteract age-related changes in lipid profile. Aronia melanocarpa (AM) fruits are a rich source of phenolic compounds possessing lipid-modulating effects. The present study investigated the effect of 3-month supplementation of AM-based functional beverages on the lipid profile of healthy aging rats. Male Wistar rats (n = 40) were separated into five groups: (YC) young controls (2-month-old); (AC) adult controls (13-month-old); (A) adult animals supplemented with pure AM extract; (A + P) adult animals supplemented with pectin-enriched (1%) AM extract; (A + H) adult animals supplemented with AM extract enriched with a herbal mixture. Total cholesterol (TC), triglycerides (TG), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C) and atherogenic indices were investigated at the end of the study. Adult controls demonstrated age-related dyslipidemia resulting in decreased HDL-C, and increased TG and TC/HDL index. The supplemented groups showed a significant increase in HDL-C levels: A + P (1.49 mmol/L) and A + H (1.61 mmol/L), respectively, vs. AC (1.09 mmol/L), p < 0.05. The TC/HDL-C and LDL-C/HDL-C indices were decreased in the A + P and A + H groups in comparison to the AC group (p < 0.05). These results indicate that supplementation with polyphenol-rich AM beverages can successfully alter HDL-C levels and this effect is further potentiated by pectin and herbs.
1. Introduction
Age and sex are physiological factors that strongly affect plasma lipid levels in a number of species. Dyslipidemia is a metabolic alteration characterized by elevated fasting blood levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and decreased levels of high-density lipoprotein cholesterol (HDL-C). Dyslipidemia is known to be a risk factor for developing insulin resistance, endothelial dysfunction, hypertension and, most of all, cardiovascular disease (CVD) [1]. Furthermore, dyslipidemia is a proven independent risk factor for vascular pathology because even when other risk factors are absent, atherosclerosis progresses. The latter is considered to be the cause of more than half of the cases of ischemic heart disease in the world [2]. HDL-C is one of the key factors determining cardiovascular risk. The protective role of HDL-C was determined for the first time in an epidemiologic study in 1970, when Framingham convincingly proved that HDL-C is the strongest predictor of developing ischemic heart disease in men and women over 49 years of age. Recent evidence has proven that a very small change in HDL-C—around 0.26 mmol/L—leads to a reduction in risk by 2% in men and 3% in women [3,4].
Low HDL-C levels present an unresolved treatment problem. It has been found that lifestyle changes increase HDL-C, and many of the mechanisms underlying its occurrence have been revealed. The latter include physical activity—5–10% (increased lipoprotein lipase, pre-β-HDL, activated reverse cholesterol transport); cessation of smoking—5–10% (raised lecithin-cholesterol acyltransferase, activated reverse cholesterol transport, inhibited cholesterol ester transfer protein); weight loss—5–20% (increased lecithin-cholesterol acyltransferase and lipoprotein lipase, activated reverse cholesterol transport); moderate alcohol consumption—5–15% (increased member 1 of human transporter sub-family ABCA, apolipoprotein A-1, paraoxonase, reduced cholesterol ester transfer protein); Mediterranean diet/unsaturated fatty acids—0–5% (increased atheroprotective lipoproteins). The only group of drugs that modulate HDL-C levels with a proven clinical benefit in lowering cardiovascular incidents is HMG-coenzyme A reductase inhibitors (statins). Among all statins, only two are of practical significance—Rosuvastatin (increases HDL-C up to 10%) and Pitavastatin (increases HDL-C up to 29%). Increasing HDL-C independently with the use of four new drugs has been shown to increase cardiovascular and total mortality. Statin intolerance is a major clinical problem, including myalgia, myositis, hepatotoxicity, statin-dependent diabetes mellitus and rhabdomyolysis. Many of the mechanisms underlying its occurrence have been studied. It is the Achilles’ heel of statin therapy, frequently resulting in the discontinuation of statins. According to the latest evidence, the established statin intolerance is close to 3–5% [5].
Plant-based foods rich in phenolic phytochemicals are among the promising strategies counteracting age-related changes in lipid profile [6,7,8]. Polyphenolic compounds are found in all plants and are quantitatively a substantial type of antioxidants consumed with food. Research interest in these compounds is immense due to the numerous therapeutic effects they exert, mainly in conditions related to oxidative stress [9]. The fruits of black chokeberry, Aronia melanocarpa (AM), are among the richest sources of polyphenols in the plant kingdom. Polyphenols, especially antocyanins and proanthocyanidins, are the main group of biologically active components in AM berries [9,10]. They are the main contributors to the antioxidative properties of the plant. Apart from polyphenols, black chokeberry is a source of glucose, fructose, sucrose, sorbitol and pectin. Analyses have shown that AM fruit contains relatively large amounts of K and Zn, along with certain amounts of Na, Ca, Mg and Fe. Apart from minerals, other substances have been found, such as B1, B2, B6, and C vitamins, niacin, pantothenic acid, folic acid, alpha and beta tocopherol and carotenoids. Beta-sitosterol and campesterol have been identified among the triterpenes. The organic acids contained in the fruit include citric, malic, shikimic and ascorbic acid [9,11]. The antioxidative potential of AM has been confirmed by numerous in vitro studies and in vivo models, where it has been frequently associated with other medicinal properties. Other proven effects are the antihypertensive, lipid-lowering and anti-inflammatory effects of black chokeberry [12,13,14]. In the literature, no adverse or toxic effects of AM juice, fruit and extracts have been reported [9].
Research has found lipid-regulating properties in AM; however, most studies use different disease models, and evidence of the effect of black chokeberry juice or extracts in models of healthy aging animals is scarce. In a previous study of ours, the supplementation of aging rats with pure black chokeberry juice ad libitum, at a dose of 64 mL/kg per day, improved their lipid profile by lowering TC and LDL, as compared to the adult controls. However, high content of fructose, glucose and sorbitol in the juice resulted in a significant increase in the body weight of the animals under study. In order to avoid such an effect and look for the possibility of applying AM products in functional nutrition, we decided to conduct a new experiment with a modified fixed dose of 10 mL/kg, and variants including enrichment with pectin and herbal extracts [15].
A number of fruits (apples, citruses, quinces, etc.) contain pectin, which has lipid-lowering properties. Pectin normalized body weight in rats following a lipid-rich diet, along with dyslipidemia, hyperglycemia, hyperinsulinemia, metabolic endotoxemia and systemic inflammation [16]. Aside from this, various herbs used in traditional medicine have been found to exert a beneficial effect on lipid levels [17,18,19]. Elderflowers (Sambucus nigra) and rose hips (Rosa canina) are known to be rich in polyphenolic compounds belonging to different classes and exhibit a biological activity associated with lipid metabolism [20,21]. At present, we are tempted to apply substances other than drugs in controlling HDL-C, but evidence in the literature is insufficient and controversial [22,23]. Regulating dyslipidemia with functional beverages rich in phenolic phytochemicals is among the promising management strategies of age-related dyslipidemia [24]. Therefore, the aim of the present study was to investigate the effect of three AM-based functional beverages on the anthropometric parameters and lipid profile of healthy adult rats.
2. Materials and Methods
2.1. Plant Materials
Aronia melanocarpa berries were supplied by the licensed farmer Todor Petkov (Kazanlak, Stara Zagora district, Bulgaria) in the stage of full maturity in August 2017. Fresh fruits were packed in polyethylene bags, frozen immediately and stored at −18 °C until the time of juice production. To prepare the beverage, 5 kg of frozen fruit were defrosted at room temperature and homogenized in a laboratory blender.
Rosa canina (rose hip) fruits were harvested from the Rhodope Mountains in the autumn of 2016, frozen at −18 °C and freeze-dried for 96 h in Alpha 1–4 LDplus laboratory freeze dryer. After that, dried rose hips were deseeded and stored in paper bags at room temperature until the time of production of the functional beverage.
Dried elder (Sambucus nigra) flowers were purchased from a local pharmacy in Plovdiv, Bulgaria, ground to a fine powder in a laboratory mill and stored in paper bags prior to preparation of the extract.
2.2. Preparation and Characterization of Functional Beverages
2.2.1. Preparation of Pure Aronia (A) Extract
To prepare the aronia extract, 400 g of fruit homogenate were mixed with 600 mL ultrapure water, transferred to a brown-glass bottle and incubated in a thermostatic water bath shaker (NUVE, Asagi Ovecler Ankara, Turkey) for 1 h, at 60 °C. After this, the mixture was filtered through a cheese cloth and the liquid phase was centrifuged (20 min, 6200× g) in a Megafuge 1.0R bench top centrifuge (Heraerus Instruments, Hanau, Germany).
2.2.2. Preparation of Aronia and Pectin (A + P) Beverage
To prepare the aronia beverage combined with pectin, 10 g apple pectin was dissolved in 1 L of aronia extract obtained by the procedure described in Section 2.2.1.
2.2.3. Preparation of Aronia with Herbs (A + H) Beverage
For the preparation of the aronia beverage with herbs, 360 g of aronia homogenate were mixed with 40 g rosehips husks (without seeds), 20 g elder flower powder and 600 mL ultrapure water. The mixture was transferred to a brown-glass bottle and incubated in a thermostatic water bath shaker (NUVE, Asagi Ovecler Ankara, Turkey) for 1 h, at 60 °C. After this, the mixture was filtered through a cheese cloth and the liquid phase was centrifuged (20 min, 6200× g) in a Megafuge 1.0R bench top centrifuge (Heraerus Instruments, Hanau, Germany).
2.2.4. Determination of Total Polyphenols
Total polyphenols were determined by the method of Singleton and Rossi (1965), using the Folin–Ciocalteu reagent. Gallic acid was used for the calibration curve and the results were expressed as gallic acid equivalents (GAE) per liter of beverage.
2.2.5. Antioxidant Activity by Oxygen Radical Absorbance Capacity (ORAC) Assay
ORAC was measured by the method of Ou et al. (2001). ORAC analyses were carried out on a FLUOstar OPTIMA plate reader (BMG Labtech, Ortenberg, Germany) with an excitation wavelength of 485 nm and emission wavelength of 520 nm. The results were expressed as μmol of Trolox equivalents per liter (μmol TE/L).
Polyphenol content and ORAC antioxidant activity of the AM-based functional beverages under study are shown in Table 1.

Table 1.
Polyphenol content and ORAC antioxidant activity of aronia-based functional beverages.
2.3. Experimental Animals
The present study included 40 male Wistar rats. The animals were bred in the vivarium of the Medical University, Plovdiv under standard laboratory conditions (housed in polypropylene cages in a controlled environment under the following conditions: a temperature of 22 ± 3 °C, a 12-h light/dark cycle, and relative humidity of 60 ± 5%). Taking into account the hormonal influences in females, we used only male rats. The animals were divided into 5 groups (n = 8). Eight two-month-old rats and eight 13-month-old rats were designated as young controls (YC) and adult controls (AC), respectively. Three groups were given orally polyphenol-rich drinks diluted with drinking water in the course of 3 months, the supplemented animals being the same age as the adult controls. The groups were as follows: (A) 100% AM extract in a dose of 10 mL/kg; (A + P)—AM extract enriched with 1% pectin in a dose of 10 mL/kg; (A + H)—AM extract enriched with a herbal mixture (rosehip fruit and elderflowers) in a dose of 10 mL/kg. The rats were kept in compliance with all the experimental procedures recommended by the European Commission for protection and welfare of laboratory animals. The experimental protocol was approved by the Committee on Ethical Treatment of Animals of the Bulgarian Agency for Food Safety (No. 193/2018). All animals received humane care in compliance with the “Principles of laboratory animal care” formulated by the National Society for Medical Research and the “Guide for the care and use of laboratory animals” prepared by the National Institute of Health (NIH publication No. 86-23, revised 1996).
2.4. Anthropometrical Measurements
Body measurements were taken at the end of the experiment—body weight (g), body length (cm) and heart weight (g). Body mass index (body weight (g)/length2 (cm2)) and heart weight index (heart weight (g)/body weight (g) × 100) were calculated.
2.5. Biochemical Measurements
At the end of the experiment, all animals were anesthetized with i.m. Ketamin (90 mg∙kg) + Xilazine (10 mg∙kg) and then decapitated. Immediately after decapitation, blood was collected, placed in a centrifuge tube and allowed to clot, so that serum could be obtained. The latter was removed by centrifugation at 1400× g for 10 min. Serum triglycerides (TG, mmol/L) and total cholesterol (TC, mmol/L) were determined by the enzyme-colorimetric method. Serum levels of high-density lipoprotein cholesterol (HDL-C, mmol/L) and low-density lipoprotein cholesterol (LDL-C, mmol/L) were determined by the direct enzyme-colorimetric method (Beckman Coulter AU 480 chemistry analyzer). The atherogenic indices were determined as TC-C/HDL-C ratio and LDL-C/HDL-C ratio.
2.6. Statistical Analysis
The data obtained were processed statistically using SPSS 21. The results are presented as mean ± standard error of mean (SEM). Paired sample t-test, independent sample t-test and one-way ANOVA followed by Tukey’s test were used for the parametric analysis at a normal distribution level. Wilcoxon signed rank test and Mann–Whitney U tests were used for the non-parametric analysis. p < 0.05 was considered as statistically significant.
3. Results
3.1. Anthropometric Parameters
3.1.1. Somatometric Parameters
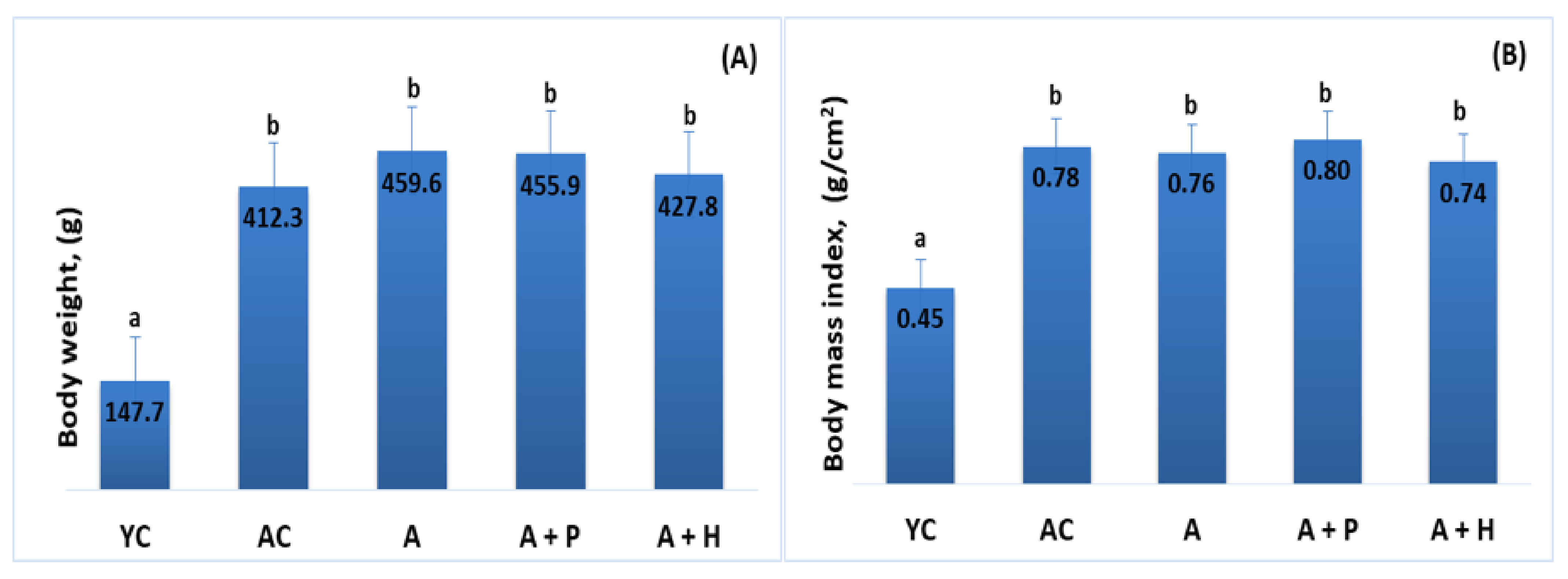
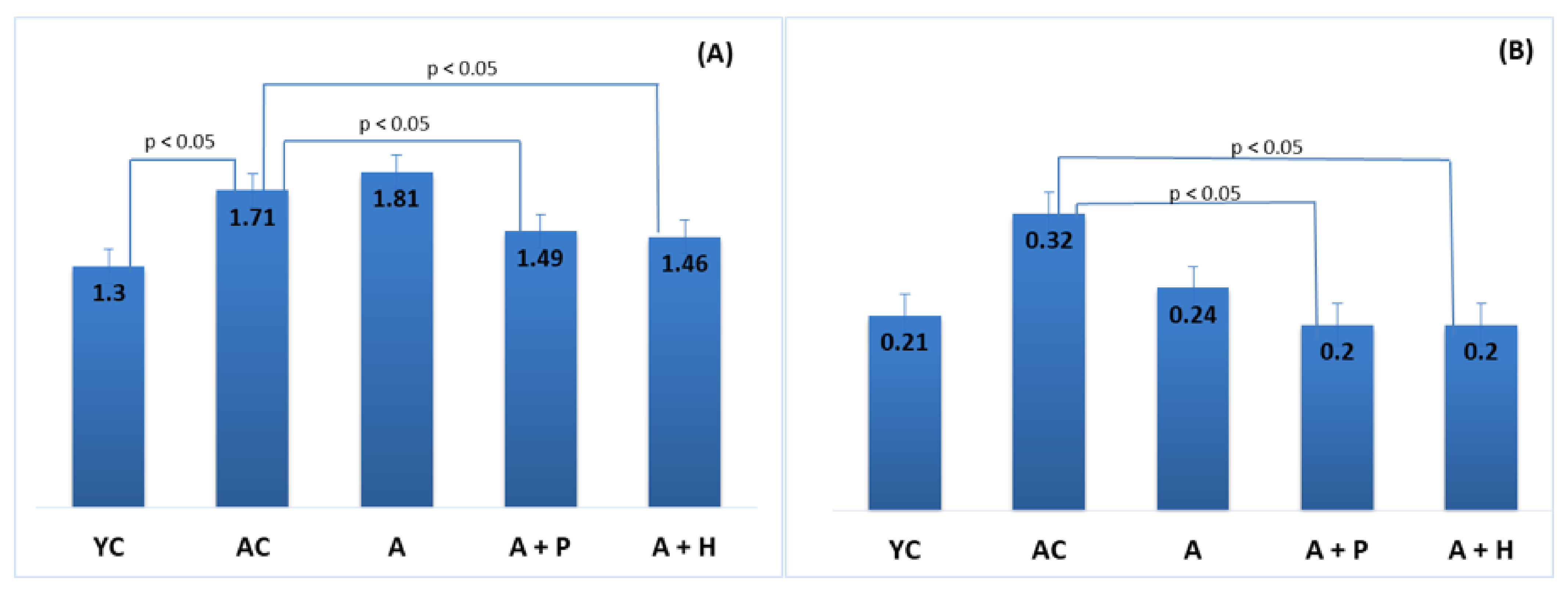
At the end of the experiment, body weight and body mass index were increased in the adult animals (both control and supplemented groups), as compared to the young controls (p < 0.05), which is a natural occurrence in the aging process. Body weight and body mass index in the adult-supplemented animals did not change significantly, as compared to the adult controls (Figure 1A,B).
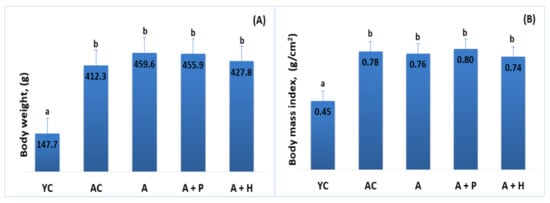
Figure 1.
(A) Body weight and (B) body mass index of the animals from the experimental groups. The results are presented as mean values ± SEM. Different small letters indicate statistically significant differences (p < 0.05): a and b = YC vs. AC; YC vs. A; YC vs. A + P; YC vs. A + H.
3.1.2. Organometric Parameters
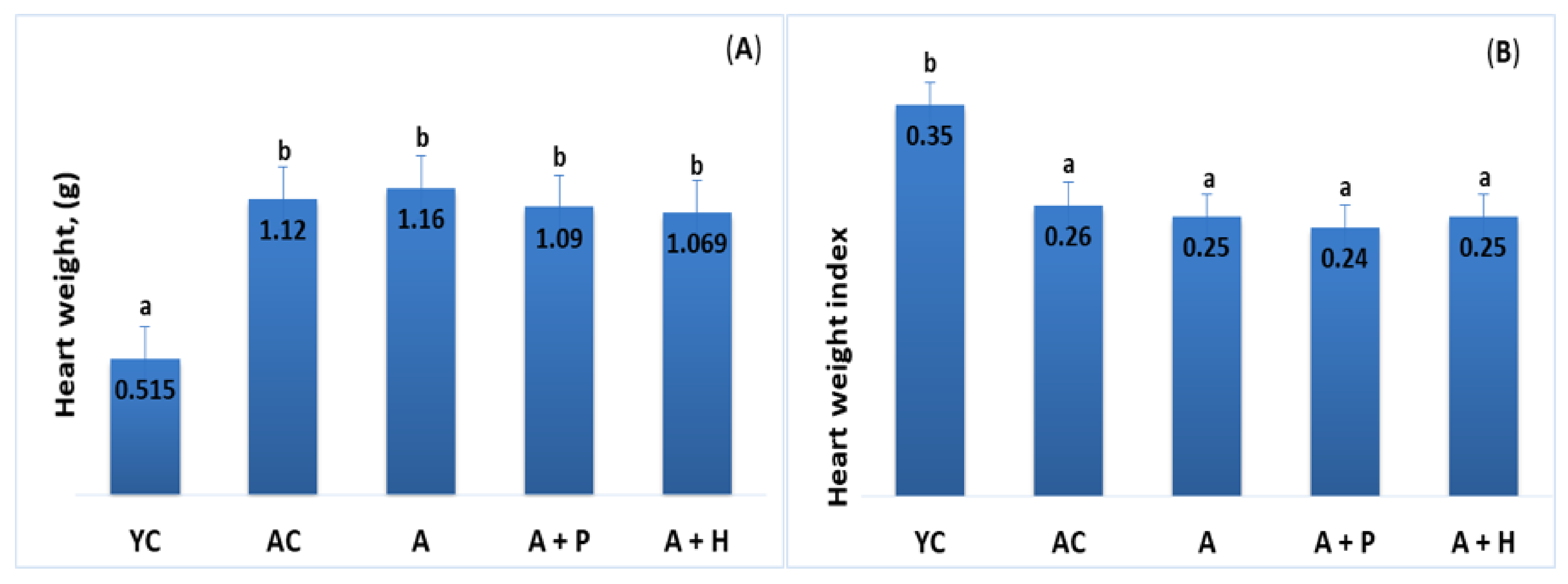
The analysis of heart weight showed an increase in the adult animals (both control and supplemented groups), as compared to the young group (p < 0.05). For example, YC (0.515 g) vs. AC (1.120 g), (p < 0.05), YC (0.515 g) vs. A (1.160 g), (p < 0.05). No significant difference was found in these parameters when comparing individuals from the supplemented adult groups (Figure 2A,B).
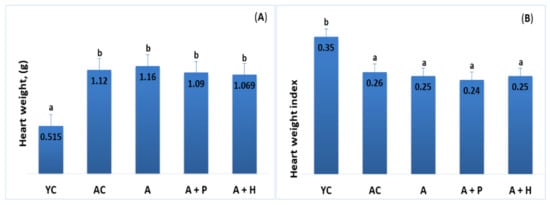
Figure 2.
(A) Heart weight and (B) heart weight index of the animals from the experimental groups. The results are presented as mean values ± SEM. Different small letters indicate statistically significant differences (p < 0.05): a and b = YC vs. AC; YC vs. A; YC vs. A + P; YC vs. A + H.
3.2. Lipidogram
3.2.1. Serum Lipids and Atherogenic Indexes—Comparison between Control Groups
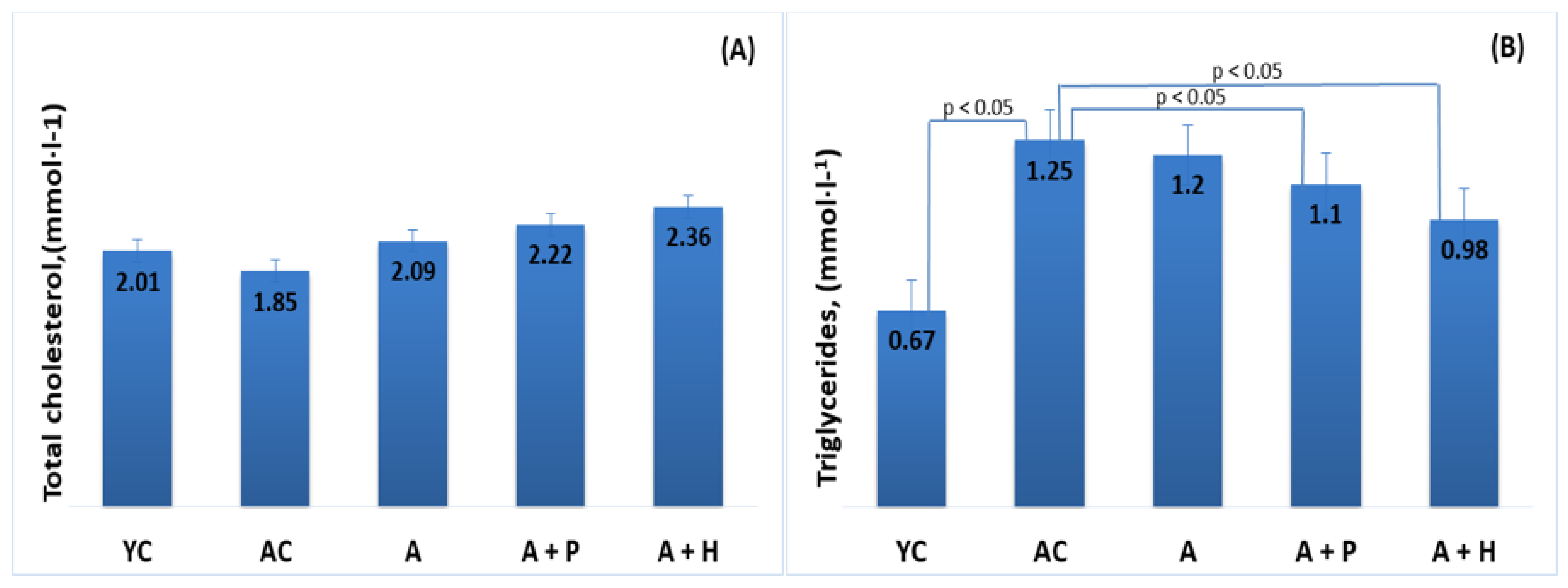
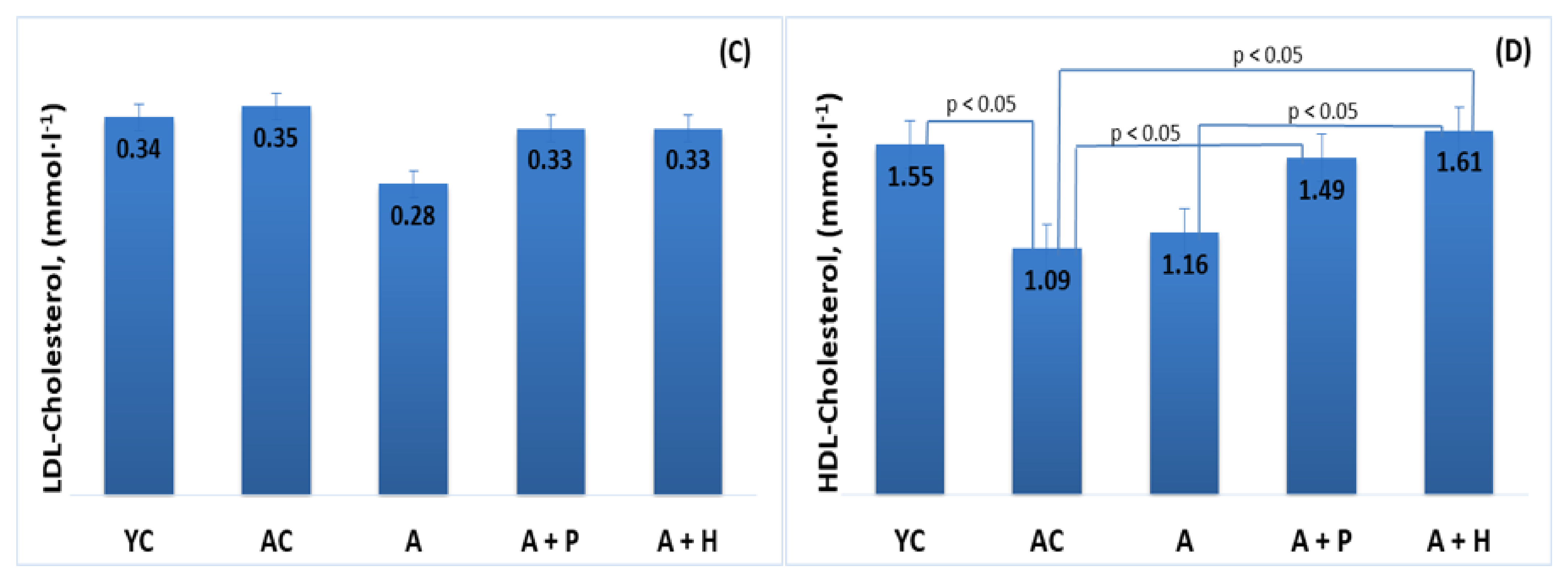
The results showed significant differences in the lipid parameters of the young and adult controls, confirming the spontaneous age-related dyslipidemia. Triglycerides (0.67 mmol·L−1) and HDL-C (1.55 mmol·L−1) of YC were significantly different (p < 0.05) in comparison to the values found in AC—1.25 mmol·L−1 and 1.09 mmol·L−1, respectively—resulting in a significantly lower (p < 0.05) TC/HDL index in YC (1.29), as compared to AC (1.71) (Figure 3B,D and Figure 4A). However, no significant differences were observed in TC (2.01 mmol·L−1 vs. 1.85 mmol·L−1) and LDL-cholesterol (0.34 mmol/L vs. 0.35 mmol/L) levels in the young and adult controls (Figure 3A–D).
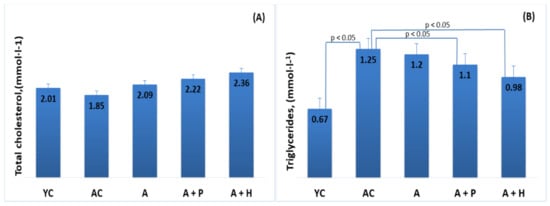
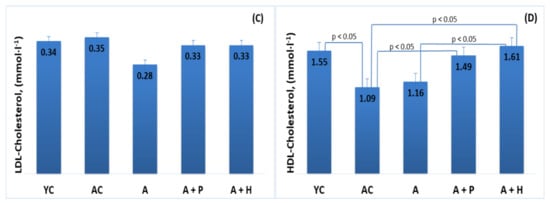
Figure 3.
Serum (A) total cholesterol, (B) triglycerides, (C) LDL-cholesterol and (D) HDL-cholesterol of the animals from the experimental groups. The results are presented as mean values ± SEM.
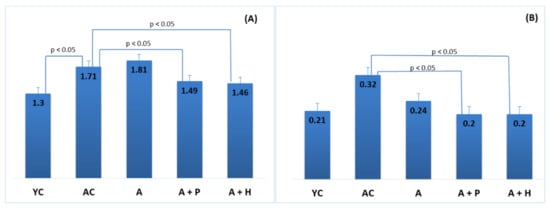
Figure 4.
Atherogenic indices: (A) TC/HDL ratio and (B) LDL/HDL ratio.
3.2.2. Serum Lipids and Atherogenic Indexes—Comparison between Supplemented and Control Groups
Supplementation with functional drinks led to certain statistically significant changes in the lipid profiles of the supplemented animals, as compared to the adult controls. For instance, the HDL-C values observed in the A + P and A + H groups (1.49 mmol/L and 1.61 mmol/L, respectively) were higher than that of the AC group (1.09 mmol/L), (p < 0.05), whereas the atherogenic TC/HDL index in the A + P and A + H groups (1.49 and 1.46, respectively) was significantly lower than the one found in AC (1.71), (p < 0.05) (Figure 3A–D). In addition, the atherogenic index LDL/HDL was improved significantly in the A + P and A + H groups, as compared to the adult controls (Figure 4A and 4B).
4. Discussion
4.1. Somatometry
From the results, it is evident that body weight alterations in the animals increase with age—a fact that has been found by other authors as well. Body weight and body mass index change significantly with the aging process, which is due to an increase in the body adipose tissue, on the one hand, and to changes in the lean mass, on the other [25,26,27]. It is also clear that AM supplementation in a dose of 10 mL·kg−1 does not lead to significant body weight alterations. These results showed that supplementation with functional beverages did not have an effect on the body mass index—the criterion for normal body structure. The significant differences between the weight index of the internal organs of the animals from the young and adult groups (supplemented and not supplemented) are an indication of the age-related atrophy of these organs. The absence of significant differences between the weight indices of the supplemented animals and the adult controls suggests that the weight of an organ changes in correlation to the overall body weight as a result of supplementation, which has been described by other authors [28]. Furthermore, the supplementation did not significantly affect the somatometric parameters; thus, they cannot be associated with changes in the lipid profile.
4.2. Age-Related Dyslipidemia
The occurrence of age-related dyslipidemia in healthy middle-aged rats was confirmed by the significant differences in the lipid profile, as compared to the young group (Figure 3 A–D). The results obtained in the current study correspond to the evidence presented by other authors [2]. It is a proven fact that total cholesterol and LDL plasma levels increase as part of the normal process of aging, while HDL-C level decreases with age [29,30]. The potential mechanisms underlying the age-related disturbance in the lipoprotein metabolism of humans and animals, which have not been fully clarified yet, are associated with changes in the liver sinusoidal endothelium, postprandial lipidemia, insulin resistance induced by free fatty acids, as well as changes in growth hormone, androgens in men and the expression and activity of peroxisome proliferator-activated receptor (PPAR) [2].
4.3. Lipid-Lowering Properties of Aronia
The current study involved healthy normally aging animals and followed the natural development of age-related dyslipidemia. Studies in animals and humans have shown that AM berries effectively modulate lipid metabolism [31,32,33]. In other experiments involving spontaneous or induced hyperlipidemias, the lipid-lowering effects of black chokeberry are more marked, as compared to our experiment. Valcheva-Kuzmanova et al. have reported that AM juice lowers diet-induced increase in serum TC, LDL-C and TG, an effect resulting from the large amount of polyphenols contained in the juice [29,30,31]. The possible underlying mechanisms of the lipid-lowering properties of flavonoids are as follows: cholesterol uptake suppression (by silymarin and tea catechins); improved lipoprotein catabolism (by cyanides); increased bile efflux, elimination of bile cholesterol and bile acids (proven for narginin) [30]. Quercetin, a flavonoid contained in black chokeberry, has an inhibiting effect on the enzymes that take part in the process of cholesterol synthesis and esterification [31].
Research on the mechanisms of AM lipid-lowering effect has found that black chokeberry extracts exert an effect on the expression of genes regulating cholesterol synthesis, binding and elimination in a dose-dependent manner in humans. The data obtained by Kim et al. suggest that the hypolipidemic effects of AM extract are likely to be due to an increased apical efflux of LDL-derived cholesterol and to decreased chylomicron formation in the intestines [34].
A particularly interesting finding of the study is the increase in the HDL-C levels in the groups supplemented with combined beverages—pectin-enriched AM and AM enriched with a herbal mixture. Such an effect on HDL-C is in the focus of attention of present-day research. In epidemiological studies, HDL-C is considered to be a risk marker for cardiovascular disease, so inducing elevation of HDL-C levels is a controversial issue. Since many of the drugs influencing HDL-C levels either exhibit serious side effects, or do not lower the risk of developing cardiovascular diseases, there is an active search for alternative means other than medications [35]. An experiment involving administration of a cyanidin-3-O-β-glucoside to mice by an oral gavage (50 mg/kg body weight) has found that the substance markedly increased serum HDL-C and apo A-1 level, as well as reduced atherosclerosis [36]. On the other hand, a diet-induced obesity model in rats has proven that supplementation with two polyphenols—resveratrol and quercetin—leads to a significant lowering of TC, TG and LDL-C levels, without affecting HDL-C [37]. In an experiment involving a hyperlipidemic model of rats, the supplementation of Fragaria nilgerrensis Schlecht. and Centella asiatica (L.) Urban in combination significantly decreased the levels of TG, TC, LDL-C, apolipoprotein B and hepatic malondialdehyde, but increased serum HDL-C, apolipoprotein A1 and hepatic superoxide dismutase [18]. A compound (polydatin) isolated from Polygonum cuspidatum Sieb., a widely used herb in traditional Chinese medicine, has been found to influence the lipid and glucose metabolism in mice, significantly lowering serum TG and LDL-C levels, simultaneously increasing HDL-C levels, as compared to the control mice [17]. In our study, pectin-enriched AM juice exerted a potentiated effect on the lipid profile. This is the first time when such a combination was used in the natural process of aging of rats. Various studies have proven the lipid-lowering properties of pectin. An experiment involving a 4-week administration of pectin from various sources to people with moderate hypercholesterolemia revealed its lipid-lowering effect. The levels of homocysteine and C-reactive protein were not affected [38]. Studying the effects of pectin on apoE −/− mice kept on a high lipid diet has shown a decrease in the total and LDL cholesterol levels, along with a reduction in atherosclerotic lesions [39]. A 90-day experiment with pectin supplementation in mice has proven lowering of TC, LDL-C and TG serum levels, liver malondialdehyde levels and inflammation, as well as a reduction in body weight in hypercholesterolemic animals. The lipid-lowering effects of pectin are associated with its role in the excretion of bile acids and the reduction in their intestinal reabsorption, increase in the viscosity of the intestinal content, decrease in fatty acid absorption and a possible alteration in the activity of digestive enzymes [40].
The results from the present research showed that the administration of AM juice in combination with a herbal mixture have a potentiating effect on HDL-C. This is likely to be due to the rich polyphenolic content and the synergism with the other biologically active substances. The elderflowers have a high polyphenolic content, of which phenolic acids, quercetin, kaempferol, catechin, epicatechin and narginin constitute a major part. They can affect disease processes by counteracting oxidative stress, leading to a positive effect on blood pressure and blood sugar levels, stimulating the immune system, increasing plasma enzymatic antioxidant activity and lowering uric acid [20]. Rose hip has rich polyphenolic content and an abundance of vitamins C, E, and B and carotenoids, which exhibit a synergistic antioxidative effect. In recent years, research has revealed the potential of its medicinal properties in a number of conditions, such as hyperlipidemia, hepatotoxicity, inflammatory processes, renal impairment, arthritis, obesity and cancer [21]. A potential mechanism underlying the positive effect on carbohydrate and lipid metabolism is the activation of adenosine monophosphate-activated protein kinase (AMPK) in hepatic cells [41]. Millar et al. have revealed the mechanisms underlying the effect of flavonoids on HDL-C levels, emphasizing their role in reverse cholesterol transport, high-density lipoprotein metabolism and function. Having in mind that inflammation participates in inducing HDL-C particle dysfunction, flavonoids can improve HDL-C function through a reduction in oxidative stress and inflammation [42].
4.4. Clinical Aspects
The optimal balance between “bad” (LDL-C) and “good” (HDL-C) in the atherogenic process is part of the history of medicine. Numerous epidemiologic findings have confirmed the fact that low HDL-C levels are associated with high cardiovascular mortality [3,4,43]. On the other hand, the antiatherogenic effects of HDL-C have been proven—it participates in reverse cholesterol transport, exhibits anti-inflammatory, anti-apoptotic, anti-thrombogenic effects, and increases NO levels [44,45,46]. Modulation of HDL-C has proved to be quite a challenge and, at present, it remains an unresolved therapeutic problem [22,23,47]. Research in the field of HDL-C physiology and pathophysiology is of great interest and focuses on establishing new treatment strategies. An individual increase in HDL-C by medications is not accepted at present, except for statins, which elevate it up to 10–15% (Rosuvastatin, Pitavastatin). Increasing HDL-C levels by means other than drugs is of significant clinical importance for medical practice. Body weight reduction, diet, continuous aerobic training and cessation of smoking are important but insufficient to optimize HDL-C levels. The results of the present study convincingly demonstrated that Aronia melanocarpa in combination with pectin or a herbal mixture has the potential to be used not only for prophylaxis, but also as an adjunct functional food in the diet for the purposes of managing dyslipidemia.
The results obtained in the present experimental investigation regarding the significant elevation of HDL cholesterol following supplementation with black chokeberry extract require further clinical studies involving patients. Furthermore, such an elevation in HDL-C could have an extensive clinical application under the conditions of the COVID-19 pandemic. There is evidence that HDL-C plays an important part in the normal functioning of the human immune system [48]. Recent data reported in the literature have shown a substantial correlation between the low levels of HDL-C and the risk of developing COVID-19 infection, as well as the severity of its clinical course [49,50]. All the specialized publications regarding this issue showed that people infected with coronavirus had lowered HDL serum levels. Experts advise paying special attention to patients with compromised HDL levels, since this is likely to result in other pathologic complications in the course of a COVID-19 infection. The experimental model of HDL-C modulation by black chokeberry extract became the basis of another study of ours involving patients with COVID-19. This study is being carried out at present with the aim of investigating the effect of AM extract in patients with low baseline levels of HDL cholesterol on the risk of developing COVID-19 infection and the severity of its course.
5. Conclusions
The results of the present study indicate that nutrition-relevant doses of functional beverages based on Aronia melanocarpa could counteract spontaneous age-related dyslipidemia in healthy adult rats by modulating serum HDL-cholesterol levels. The addition of pectin or herbs further increases Aronia melanocarpa lipid-lowering effect. More specifically, the combination of aronia with rosehip and elderflower extracts had the most pronounced HDL-cholesterol increasing effect. Therefore, the functional beverages studied can be used as a potential means for prophylaxis and as an adjunct functional food in the diet for the purposes of managing dyslipidemia.
Author Contributions
Conceptualization, E.D., S.D., L.V.-K. and P.D.; methodology, E.D., S.D. and P.D.; resources, E.D., S.D., L.V.-K. and P.D.; writing—original draft preparation, E.D., S.K.; writing—review and editing, P.D., L.V.-K. and S.D.; supervision, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bulgarian National Science Fund, project DN 09/20 “Integrated approach for improving the quality, organoleptic properties and biological activity of new functional foods from chokeberry (Aronia melanocarpa) by copigmentation and synergy in antioxidant activity.”, managed by Petko Denev.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Jana Mladenova for the revision of the English translation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Liu, H.; Li, J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probsfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R., Jr.; Bangdiwala, S.; Tyroler, H.A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A Mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Laufs, U.; Isermann, B. Statin intolerance: Myths and facts. Eur. Heart J. 2020, 41, 3343–3345. [Google Scholar] [CrossRef]
- Ochani, P.; D’Mello, P. Antyioxidant and antihyperlipemic activity of Hibiscus sabdariffa Linn. Leaves and calyces extracts in rats. Indian J. Exp. Biol. 2009, 47, 276–282. [Google Scholar] [PubMed]
- Ajiboye, T.O.; Raji, H.O.; Adeleye, A.O.; Adigun, N.S.; Giwa, O.B.; Ojewuyi, O.B.; Oladiji, A.T. Hibiscus sabdariffa calyx palliates insulin resistance, hyperglycemia, dyslipidemiaand oxidative rout in fructose-induced metabolic syndrome rats. J. Sci. Food Agric. 2015, 1522–1531. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Kratchanov, C.; Ciz, M.; Lojek, A.; Kratchanova, M. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Comp. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkievicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Skoczyñska, A.; Jêdrychowska, I.; Porêba, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharm. Rep. 2007, 59, 177–182. [Google Scholar]
- Zapolska-Downar, D.; Hajdukiewicz, K.; Bryk, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Cebova, M.; Klimentova, J.; Janega, P.; Pechanova, O. Effect of bioactive compound of Aronia melanocarpa on cardiovascular system in experimental hypertension. Oxid. Med. Cell. Longev. 2017, 2017, 8156594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valcheva-Kuzmanova, S.; Kuzmanov, A.; Kuzmanova, V.; Tzaneva, M. Aronia melanocarpa fruit juice ameliorates the symptoms of inflammatory bowel disease in TNBS-induced colitis in rats. Food Chem. Toxicol. 2018, 113, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, E.; Delchev, S.; Peeva, Y.; Vladimirova-Kitova, L.; Kratchanova, M.; Kratchanov, C.; Denev, P. Antiatherogenic and Cardioprotective Effects of Black Chokeberry (Aronia melanocarpa) Juice in Aging Rats. Evid. Based Complement. Alternat. Med. 2015, 2015, 717439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Gao, X.; Wu, C. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 2016, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Wu, J.; Mo, J.; Guo, L.; Wu, X.; Bao, Y. Polydatin Inhibits Adipose Tissue Inflammation and Ameliorates Lipid Metabolism in High-Fat-Fed Mice. Biomed Res. Int. 2019, 2019, 7196535. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lin, Z.; Liu, Y.; Wang, X.; Wan, L.; Zhang, L.; Liu, X. Hypolipidemic effect of Fragarianilgerrensis Schlecht. medicine compound on hyperlipidemic rats. Lipids Health Dis. 2018, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 25, 1137. [Google Scholar] [CrossRef]
- Much, A.A.; Fardman, A.; Segev, S.; Klempfner, R.; Grossman, E.; Segev, A.; Maor, E. Non-pharmacological improvement in HDL-cholesterol is not associated with reduced cardiovascular risk in apparently healthy adults. J. Am. Coll. Cardiol. 2020, 75 (Suppl. 1), 1982. [Google Scholar] [CrossRef]
- Dragan, S.; Serban, C.; Banach, M. Can we change the functionality of HDL cholesterol with nonpharmacological and pharmacological agents? Curr. Med. Chem. 2014, 21, 2927–2946. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Doménech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary Polyphenol Intake is Associated with HDL-Cholesterol and A Better Profile of Other Components of the Metabolic Syndrome: A PREDIMED-Plus Sub-Study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, E.M.; Singer, A.A.; Kirakosyan, A.; Urcuyo-Llanes, D.E.; Kaufman, P.B.; Bolling, S.F. Altered hyperlipidemia, hepatic steatosis and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food 2008, 11, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolden-Hanson, T. Changes in body composition in response to challenges during aging in rats. Interdiscipl. Top Gerontol. 2010, 37, 64–83. [Google Scholar]
- Aissaoui, A.; Zizi, S.; Israili, Z.; Lyoussi, B. Hypoglycemic and hypolipidemic effects of Coriandrum sativum L. in Meriones shawi rats. J. Ethnopharmacol. 2011, 137, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Blalock, E.; Phelps, J.; Pancani, T.; Searcy, J.; Anderson, K.; Gant, J.; Popovic, J.; Avdiushko, M.; Cohen, D.; Chen, K.; et al. Effects of long-term pioglitazone treatment on peripheral and central markers of aging. PLoS ONE 2010, 5, e1040. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant. Foods Hum. Nutr. 2007, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 101–105. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tsanova-Savova, S.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Lipid-lowering effects of Aronia melanocarpa fruit juice in rats fed cholesterol-containing diets. J. Food Biochem. 2007, 31, 589–602. [Google Scholar] [CrossRef]
- Kardum, N.; Konić-Ristić, A.; Šavikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of polyphenol-rich chokeberry juice on antioxidant/pro-oxidant status in healthy subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- AIM-HIGH Investigators; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Cen, F.; Tian, F.; Li, M.J.; Zhang, Q.; Shen, H.Y.; Shen, X.C.; Zhou, M.M.; Du, J. Combination treatment with quercetin and resveratrol attenuates high fat diet induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway. Exp. Ther. Med. 2017, 14, 5942–5948. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Paim, R.T.T.; Benjamin, S.R.; Rondina, D.; Marques, M.M.M.; Viana, D.D.A.; Gonzaga, M.L.D.C.; Vieira, Í.G.P.; Mendes, F.N.P.; Rodrigues, P.A.S.; Guedes, M.I.F. Corrigendum to “Antihypercholesterolemic effects of fruit aqueous extract of Copernicia prunifera (Miller) H.E. Moore in Mice Diet-Induced Hypercholesterolemia”. Evid. Based Complement. Alternat. Med. 2017, 2017, 2486328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and other bioactive compounds of Sideritis plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.Q.; Duclos, Q.; Blesso, C. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv. Nutr. 2017, 8, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Martinez, I.; Sourlas, A. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Barter, P.J.; Nicholls, S.; Rye, K.A.; Anantharamaiah, G.M.; Navab, M.; Fogelma, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Tomás, M.; Latorre, G.; Sentí, M.; Marrugat, J. The antioxidant function of high-density lipoproteins: A new paradigm in atherosclerosis. Rev. Esp. Cardiol. 2004, 57, 557–569. [Google Scholar] [CrossRef]
- Chiesa, S.; Charakida, M. High-density lipoprotein function and dysfunction in health and disease. Cardiovasc. Drugs Ther. 2019, 33, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. HDL in infectious diseases and sepsis. In High Density Lipoproteins. Handbook of Experimental Pharmacology; Eckardstein, A., Dimitris Kardassis, D., Eds.; Springer: Cham, Switzerland; Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2015; Volume 224, pp. 154–196. [Google Scholar]
- Garg, I.; Srivastava, S.; Kumar, B. Understanding the Role of HDL during COVID-19 Infection. Coronaviruses 2021, 2, 289–290. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Zhao, X.; Dong, H.; Wu, C.; Wu, F.; Yu, B.; Lv, J.; Zhang, S.; Wu, G.; et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: An observational study. Lipids Health Dis. 2020, 19, 204. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).