Functionality of MC88- and MPC85-Enriched Skim Milk: Impact of Shear Conditions in Rotor/Stator Systems and High-Pressure Homogenizers on Powder Solubility and Rennet Gelation Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. MC88- and MPC85-Enriched Skim Milk
2.2. Shear Treatments
2.3. Particle Size Measurements
2.4. Rennet Gelation Properties
2.5. Serum Loss and Casein, Whey Protein, and Casein Macropeptide Recovery in the Sweet Whey
2.6. Statistical Analyses
3. Results and Discussion
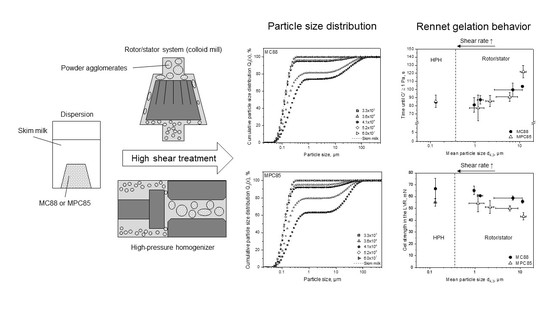
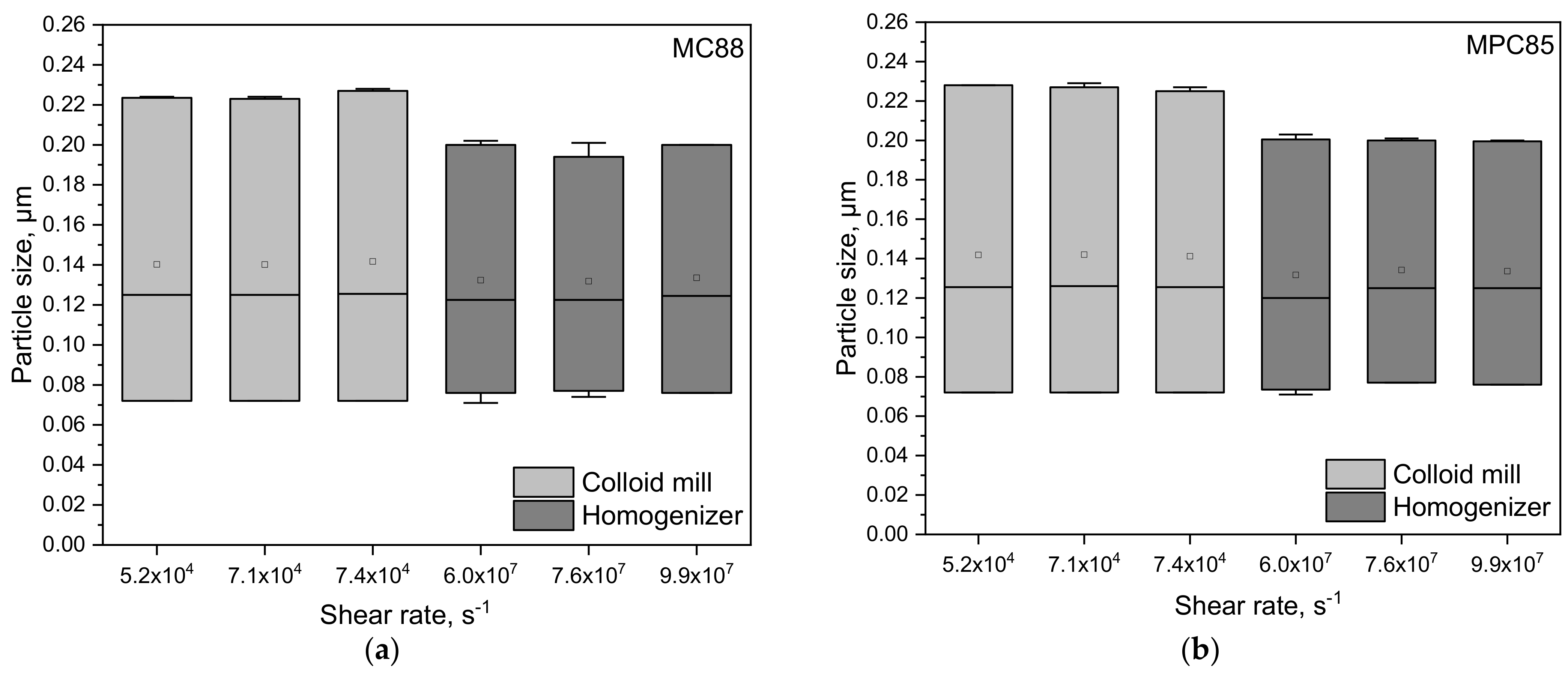
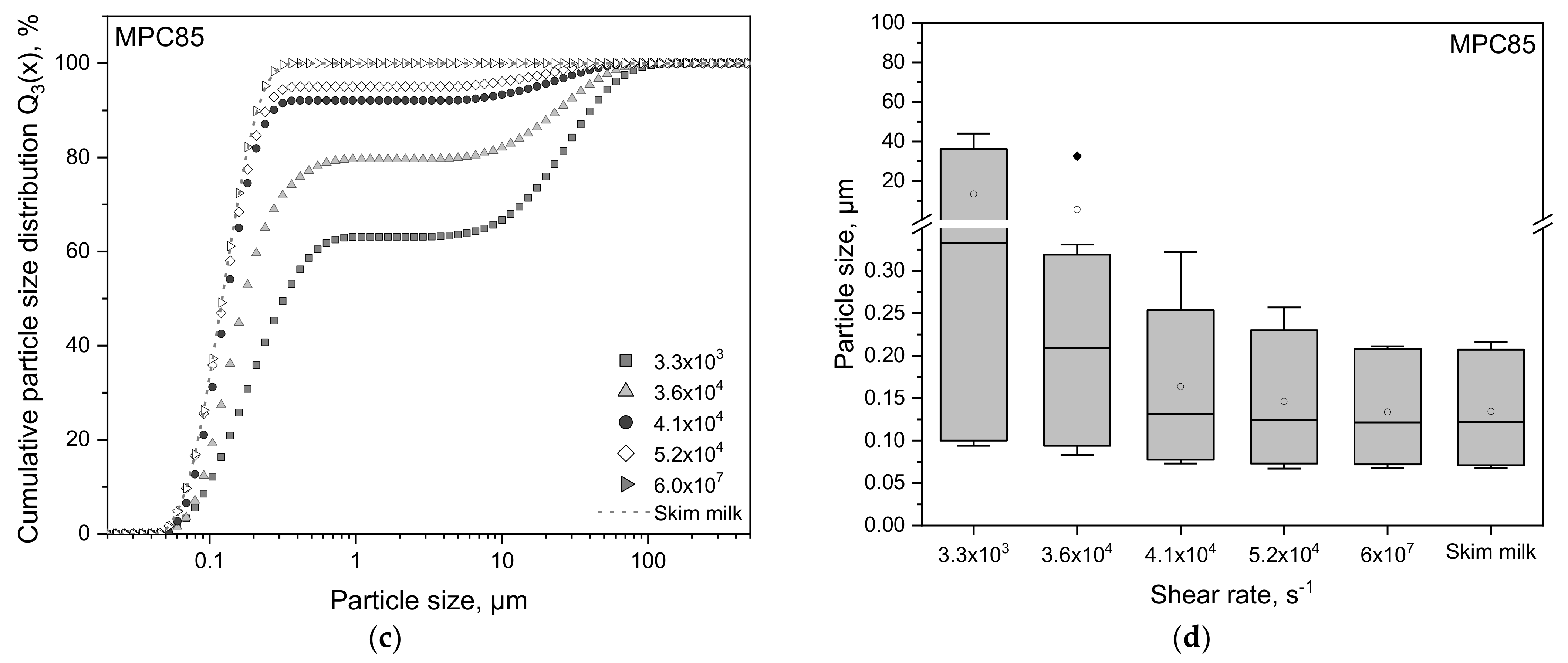
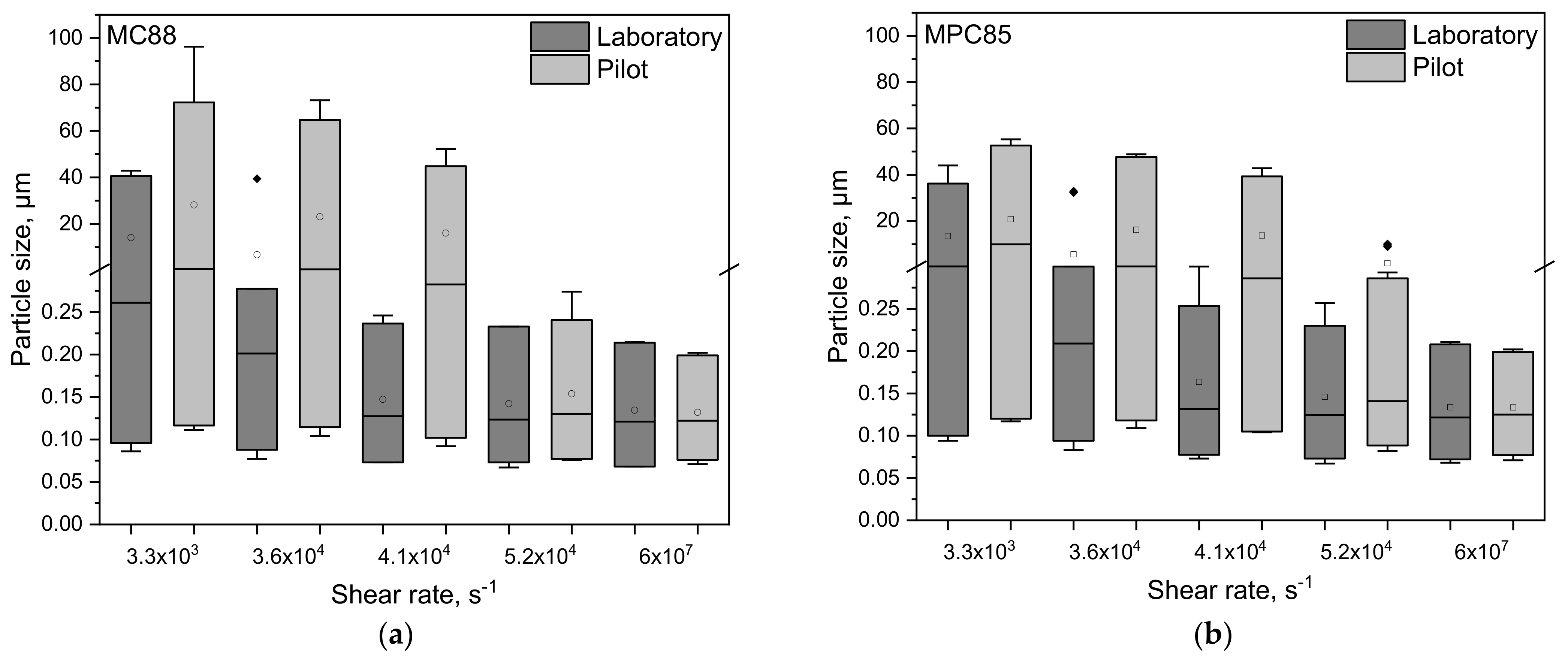
3.1. Particle Size Distributions of Protein-Enriched Skim Milk as a Function of Shear Rate
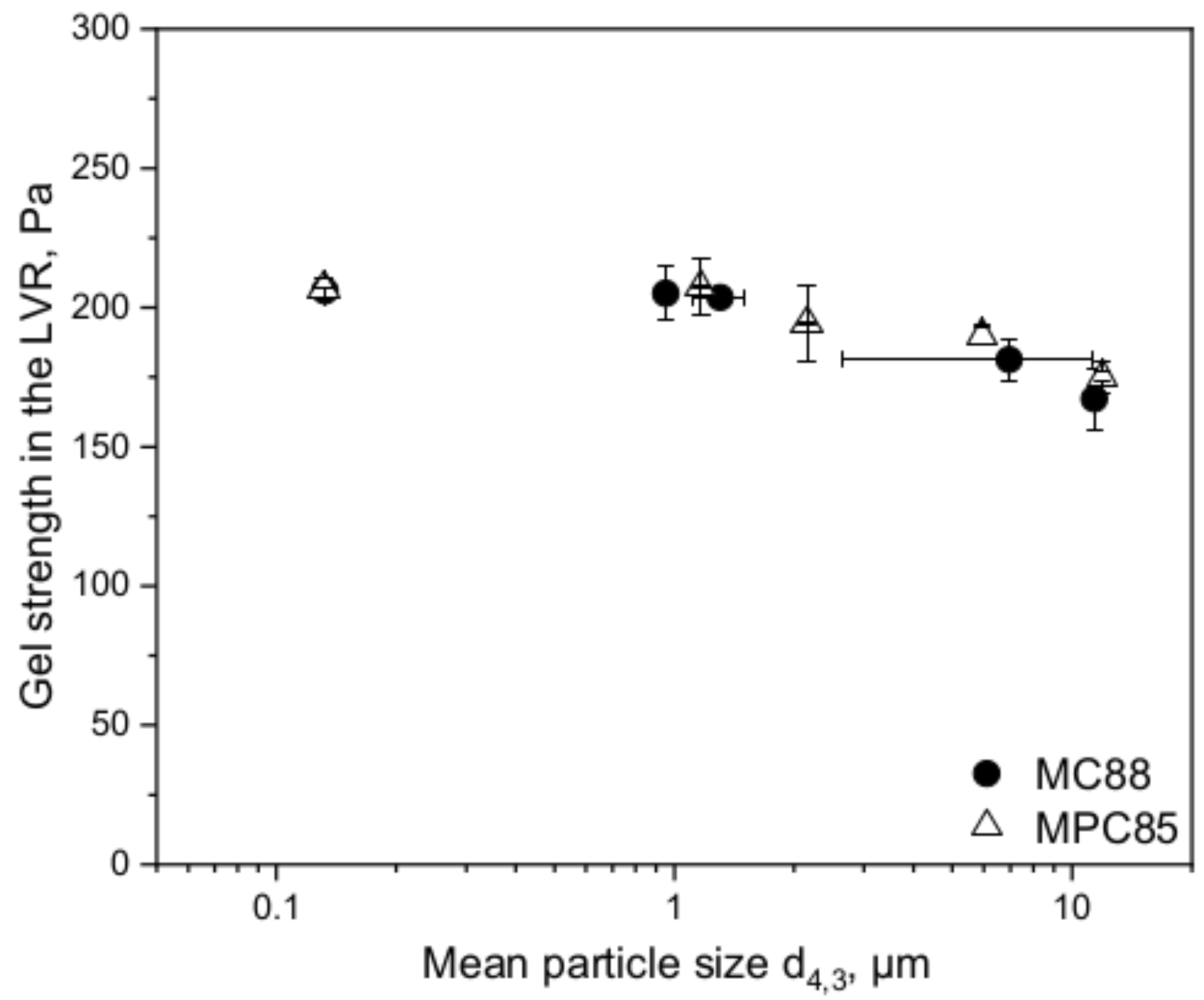
3.2. Functionality of MC88- and MPC85-Enriched Skim Milk: Rennet Gelation Behavior
3.2.1. Gelation Time
3.2.2. Gel Strength and Casein, Whey Protein, and CMP Recovery in the Sweet Whey
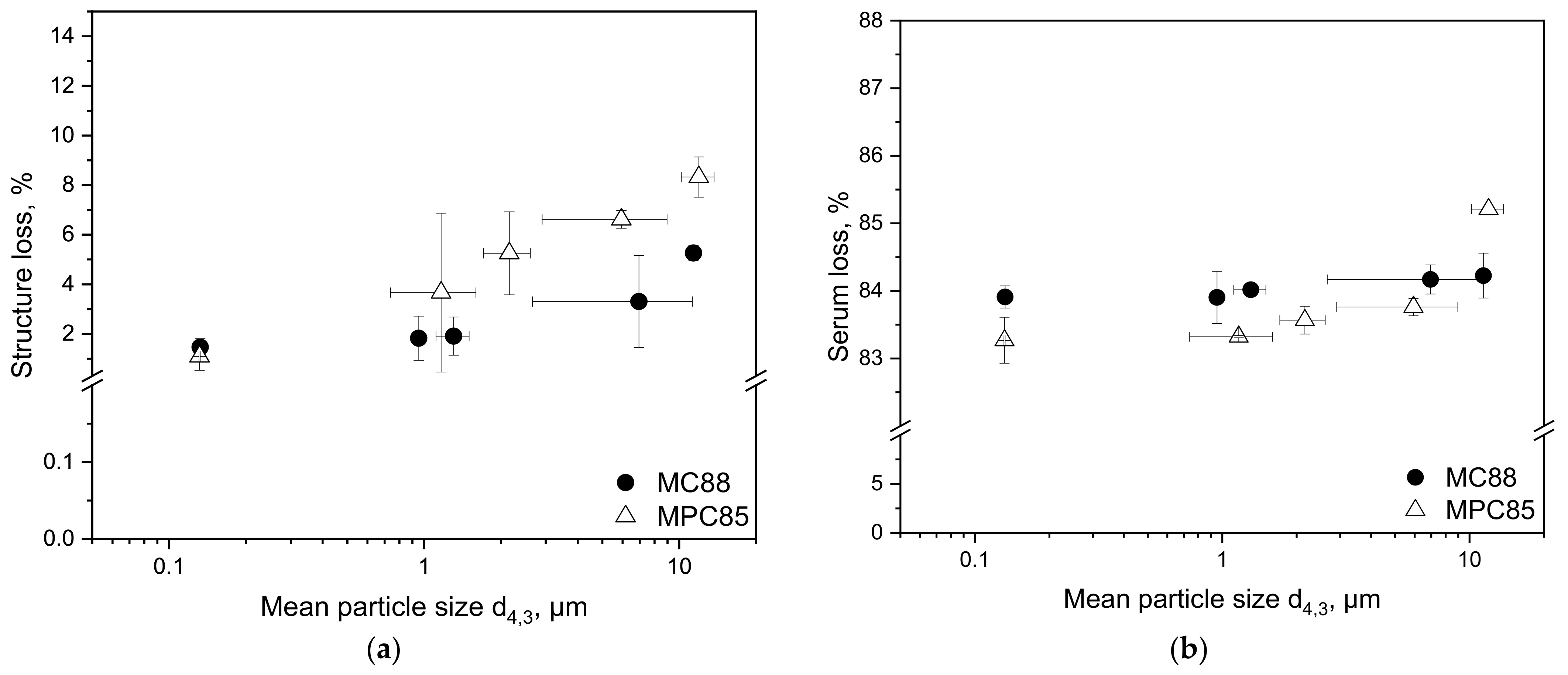
3.2.3. Structure Loss upon Deformation and Serum Loss
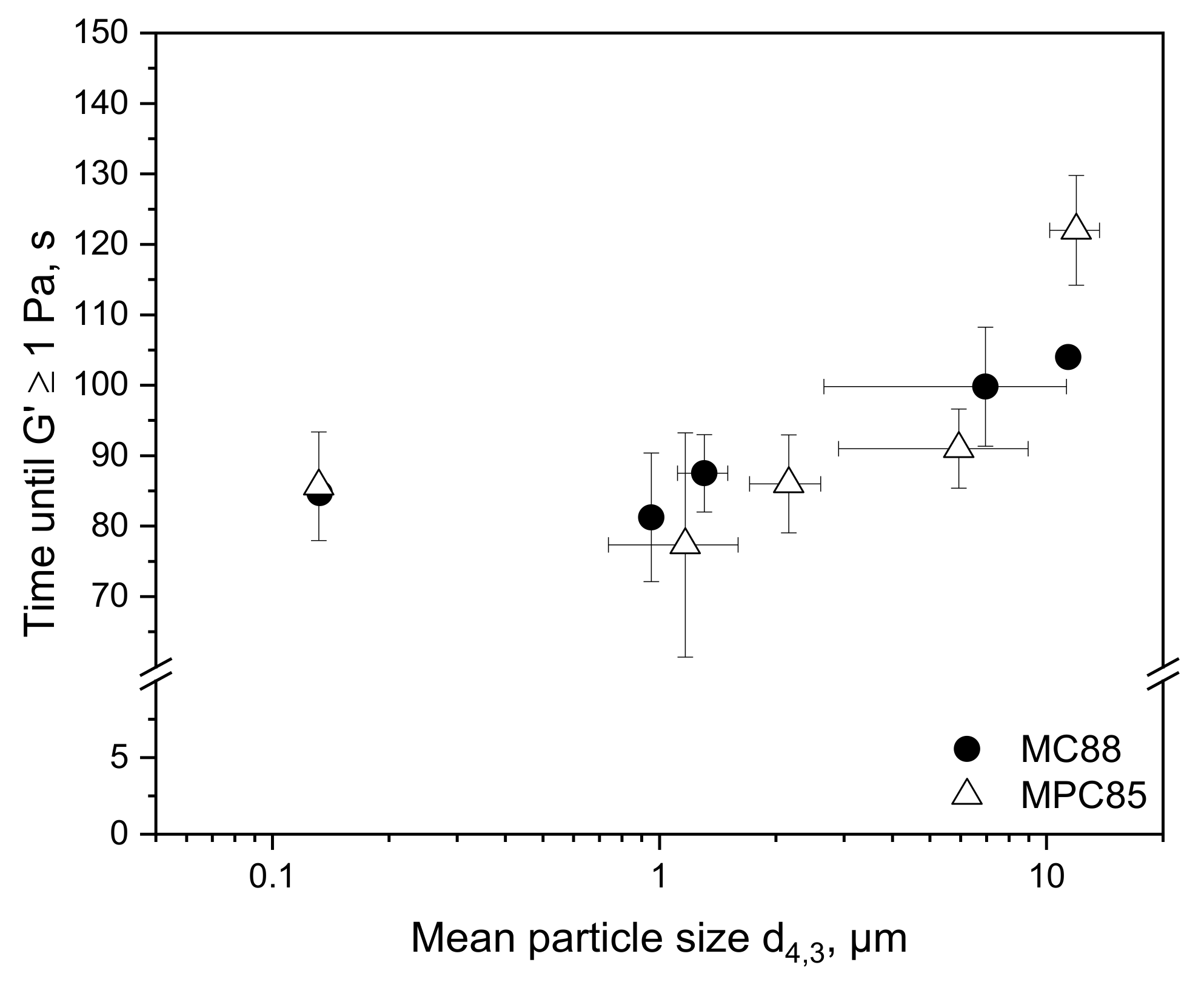
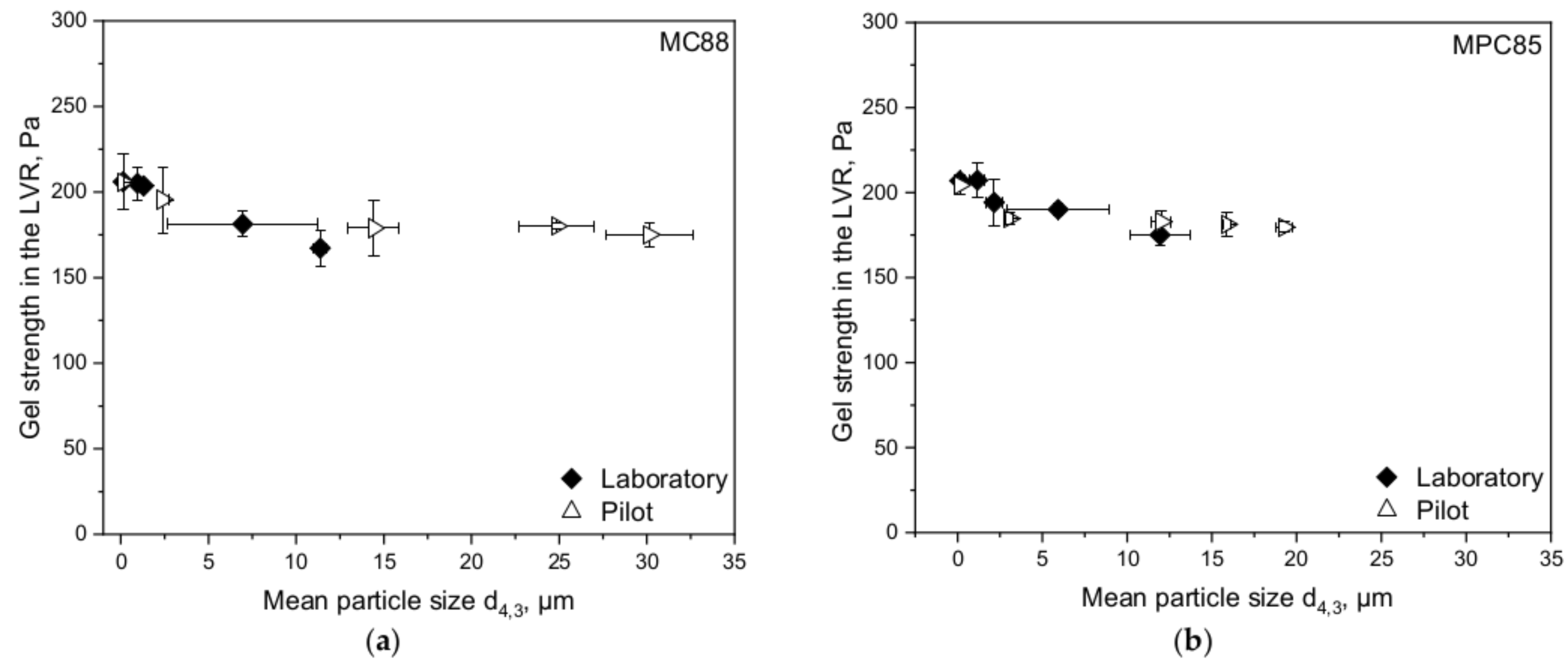
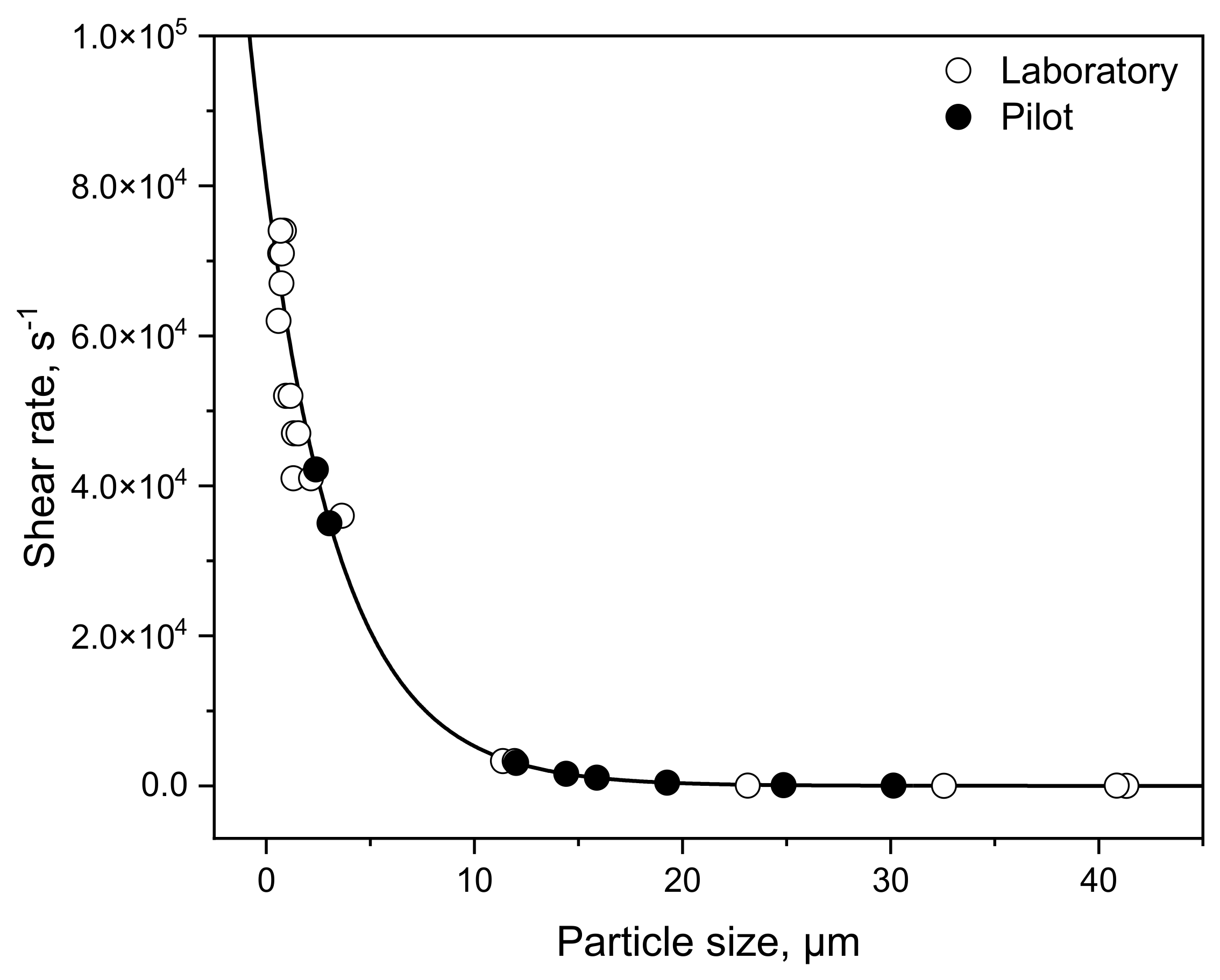
3.3. Transferability from Laboratory to Pilot Scale: Correlation between Shear Rate and Particle Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anema, S.G.; Pinder, D.N.; Hunter, R.J.; Hemar, Y. Effects of Storage Temperature on the Solubility of Milk Protein Concentrate (MPC85). Food Hydrocoll. 2006, 20, 386–393. [Google Scholar] [CrossRef]
- Singh, H. Interactions of Milk Proteins during the Manufacture of Milk Powders. Le Lait 2007, 87, 413–423. [Google Scholar] [CrossRef]
- Guinee, T.P.; O’Kennedy, B.T.; Kelly, P.M. Effect of Milk Protein Standardization Using Different Methods on the Composition and Yields of Cheddar Cheese. J. Dairy Sci. 2006, 89, 468–482. [Google Scholar] [CrossRef]
- Auldist, M.J.; Coats, S.; Sutherland, B.J.; Mayes, J.J.; McDowell, G.H.; Rogers, G.L. Effects of Somatic Cell Count and Stage of Lactation on Raw Milk Composition and the Yield and Quality of Cheddar Cheese. J. Dairy Res. 1996, 63, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.M.; Tamime, A.Y. Seasonal Trends in the Efficiency of Recovery of Milk Fat and Casein in Cheese Manufacture. Int. J. Dairy Technol. 1987, 40, 64–66. [Google Scholar] [CrossRef]
- O’Brien, B.; Mehra, R.; Connolly, J.F.; Harrington, D. Seasonal Variation in the Composition of Irish Manufacturing and Retail Milks: 4. Minerals and Trace Elements. Ir. J. Agric. Food Res. 1999, 38, 53–64. [Google Scholar]
- O’Keeffe, A.M. Seasonal and Lactational Influences on Moisture Content of Cheddar Cheese. Fr. J. Food Sci. Technol. 1984, 8, 27–37. [Google Scholar]
- Baldwin, A.J.; Truong, G.N.T. Development of Insolubility in Dehydration of Dairy Milk Powders. Food Bioprod. Process. 2007, 85, 202–208. [Google Scholar] [CrossRef]
- Oldfield, D.; Singh, H. Functional properties of milk powders. In Encapsulated and Powdered Foods; Onwulata, C., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 365–386. [Google Scholar]
- Crowley, S.V.; Desautel, B.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Rehydration Characteristics of Milk Protein Concentrate Powders. J. Food Eng. 2015, 149, 105–113. [Google Scholar] [CrossRef]
- Baldwin, A.J. Insolubility of Milk Powder Products—A Minireview. Dairy Sci. Technol. 2010, 90, 169–179. [Google Scholar] [CrossRef]
- Crowley, S.V.; Megemont, M.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Heat Stability of Reconstituted Milk Protein Concentrate Powders. Int. Dairy J. 2014, 37, 104–110. [Google Scholar] [CrossRef]
- Warncke, M.; Kulozik, U. Impact of Temperature and High Pressure Homogenization on the Solubility and Rheological Behavior of Reconstituted Dairy Powders of Different Composition. Powder Technol. 2020, 376, 285–295. [Google Scholar] [CrossRef]
- Ferrer, M.A.; Hill, A.R.; Corredig, M. Rheological Properties of Rennet Gels Containing Milk Protein Concentrates. J. Dairy Sci. 2008, 91, 959–969. [Google Scholar] [CrossRef]
- Martin, G.J.O.; Williams, R.P.W.; Dunstan, D.E. Comparison of Casein Micelles in Raw and Reconstituted Skim Milk. J. Dairy Sci. 2007, 90, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.J.O.; Williams, R.P.W.; Dunstan, D.E. Effect of Manufacture and Reconstitution of Milk Protein Concentrate Powder on the Size and Rennet Gelation Behaviour of Casein Micelles. Int. Dairy J. 2010, 20, 128–131. [Google Scholar] [CrossRef]
- Kieferle, I.; Hiller, K.; Kulozik, U.; Germann, N. Rheological Properties of Fresh and Reconstituted Milk Protein Concentrates under Standard and Processing Conditions. J. Colloid Interface Sci. 2019, 537, 458–464. [Google Scholar] [CrossRef]
- Lin, Y.; Kelly, A.L.; O’Mahony, J.A.; Guinee, T.P. Effects of Milk Heat Treatment and Solvent Composition on Physicochemical and Selected Functional Characteristics of Milk Protein Concentrate. J. Dairy Sci. 2018, 101, 6799–6813. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration of High-Protein-Containing Dairy Powder: Slow- and Fast-Dissolving Components and Storage Effects. Dairy Sci. Technol. 2010, 90, 335–344. [Google Scholar] [CrossRef]
- Havea, P. Protein Interactions in Milk Protein Concentrate Powders. Int. Dairy J. 2006, 16, 415–422. [Google Scholar] [CrossRef]
- Schokker, E.P.; Church, J.S.; Mata, J.P.; Gilbert, E.P.; Puvanenthiran, A.; Udabage, P. Reconstitution Properties of Micellar Casein Powder: Effects of Composition and Storage. Int. Dairy J. 2011, 21, 877–886. [Google Scholar] [CrossRef]
- Chandrapala, J.; Martin, G.J.O.; Kentish, S.E.; Ashokkumar, M. Dissolution and Reconstitution of Casein Micelle Containing Dairy Powders by High Shear Using Ultrasonic and Physical Methods. Ultrason. Sonochem. 2014, 21, 1658–1665. [Google Scholar] [CrossRef]
- Harvey, J. Protein Fortification of Cheese Milk Using MPC-Yield Improvement and Product Quality. Aust. J. Dairy Technol. 2006, 61, 183–185. [Google Scholar]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and Coagulation of Caseins and Whey Proteins in Concentrated Skim Milk Heated by Direct Steam Injection. Dairy Sci. Technol. 2017, 96, 807–826. [Google Scholar] [CrossRef]
- Schmitz-Schug, I.; Gianfrancesco, A.; Kulozik, U.; Foerst, P. Physical State, Molecular Mobility and Chemical Stability of Powdered Dairy Formulations. Food Res. Int. 2013, 53, 268–277. [Google Scholar] [CrossRef]
- Bowen, R. Unraveling the Mysteries of Shear-Sensitive Mixing Systems. Chem. Eng. 1986, 93, 55–63. [Google Scholar]
- Rawle, A. Basic Principles of Particle. Surf. Coat. Int. Part A Coat. J. 2003, 86, 58–65. [Google Scholar]
- Rawle, A.; Kippax, P. Partikelanalyse Mittels Laserbeugung Neu Geregelt. Schüttgut 2010, 16, 82. [Google Scholar]
- Anema, S.G.; Lowe, E.K.; Kim, S.; Klostermeyer, H. Effect of the PH of Skim Milk at Heating on Milk Concentrate Viscosity. Int. Dairy J. 2014, 39, 336–343. [Google Scholar] [CrossRef]
- Bouvier, J.M.; Collado, M.; Gardiner, D.; Scott, M.; Schuck, P. Physical and Rehydration Properties of Milk Protein Concentrates: Comparison of Spray-Dried and Extrusion-Porosified Powders. Dairy Sci. Technol. 2013, 93, 387–399. [Google Scholar] [CrossRef]
- Sandra, S.; Corredig, M. Rennet Induced Gelation of Reconstituted Milk Protein Concentrates: The Role of Calcium and Soluble Proteins during Reconstitution. Int. Dairy J. 2013, 29, 68–74. [Google Scholar] [CrossRef]
- Ji, J.; Fitzpatrick, J.; Cronin, K.; Maguire, P.; Zhang, H.; Miao, S. Rehydration Behaviours of High Protein Dairy Powders: The Influence of Agglomeration on Wettability, Dispersibility and Solubility. Food Hydrocoll. 2016, 58, 194–203. [Google Scholar] [CrossRef]
- Dumpler, J.; Kulozik, U. Heat-Induced Coagulation of Concentrated Skim Milk Heated by Direct Steam Injection. Int. Dairy J. 2016, 59, 62–71. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Taylor, M.W.; Singh, H. Effect of Preheating and Other Process Parameters on Whey Protein Reactions during Skim Milk Powder Manufacture. Int. Dairy J. 2005, 15, 501–511. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Stabilization of Protein Structure by Sugars. Biochemistry 1982, 21, 6536–6544. [Google Scholar] [CrossRef] [PubMed]
- Timasheff, S.N. Protein Hydration, Thermodynamic Binding, and Preferential Hydration. Biochemistry 2002, 41, 13473–13482. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Jelen, P. Thermal Stability of Whey Proteins—A Calorimetric Study. J. Dairy Sci. 1985, 68, 2847–2852. [Google Scholar] [CrossRef]
- Garrett, J.M.; Stairs, R.A.; Annett, R.G. Thermal Denaturation and Coagulation of Whey Proteins: Effect of Sugars. J. Dairy Sci. 1988, 71, 10–16. [Google Scholar] [CrossRef]
- Jou, K.D.; Harper, W.J. Effect of Di-Saccharides on the Thermal Properties of Whey Proteins Determined by Differential Scanning Calorimetry (DSC). Milchwissenschaft 1996, 51, 509–512. [Google Scholar]
- Plock, J.; Spiegel, T.; Kessler, H.G. Influence of the Lactose Concentration on the Denaturation Kinetics of Whey Proteins in Concentrated Sweet Whey. Milchwissenschaft 1998, 53, 389–393. [Google Scholar]
- Spiegel, T. Whey Protein Aggregation under Shear Conditions–Effects of Lactose and Heating Temperature on Aggregate Size and Structure. Int. J. Food Sci. Technol. 1999, 34, 523–531. [Google Scholar] [CrossRef]
- Warncke, M.; Kieferle, I.; Nguyen, T.M.; Kulozik, U. Impact of Heat Treatment, Casein/Whey Protein Ratio and Protein Concentration on Rheological Properties of Milk Protein Concentrates Used for Cheese Production. J. Food Eng. 2021. under review. [Google Scholar]
- McKenna, A.B. Effect of Processing and Storage on the Reconstitution Properties of Whole Milk and Ultrafiltered Skim Milk Powders. Ph.D. Thesis, Massey University, Institute of Food Nutrition and Human Health, Palmerston North, New Zealand, 2000. [Google Scholar]
- Martin, G.J.O.; Williams, R.P.W.; Choong, C.; Lee, B.; Dunstan, D.E. Comparison of Rennet Gelation Using Raw and Reconstituted Skim Milk. Int. Dairy J. 2008, 18, 1077–1080. [Google Scholar] [CrossRef]
- Fox, P.F.; Guineee, T.P.; Cogann, T.M.; Mcsweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Lucey, J.A.; Fox, P.F. Importance of Calcium and Phosphate in Cheese Manufacture: A Review. J. Dairy Sci. 1993, 76, 1714–1724. [Google Scholar] [CrossRef]
- Udabage, P.; McKinnon, I.R.; Augustin, M.A. Effects of Mineral Salts and Calcium Chelating Agents on the Gelation of Renneted Skim Milk. J. Dairy Sci. 2001, 84, 1569–1575. [Google Scholar] [CrossRef]


|
Casein (%, w/w) |
Whey Protein (%, w/w) |
Total Protein (%, w/w) | Casein/Whey Protein Ratio | Degree of Whey Protein Denaturation (%) |
Lactose (%, w/w) |
Minerals (%, w/w) | Total solids (%) | |
|---|---|---|---|---|---|---|---|---|
| MC88 | 78.4 ± 0.5 | 5.6 ± 0.6 | 84 | 93:7 | 65.4 ± 0.8 | 1.4 ± 0.2 | 2.7 ± 0.0 | 94.4 ± 0.0 |
| MPC85 | 70.5 ± 0.2 | 11.5 ± 0.1 | 82 | 86:14 | 42.7 ± 0.0 | 2.7 ± 0.0 | 2.4 ± 0.0 | 94.6 ± 0.0 |
| Stirred Tank | Colloid Mill/Shear Pump | Colloid Mill/Shear Pump | Colloid Mill/Shear Pump | Colloid Mill/Shear Pump | High-Pressure Homogenizer | |
|---|---|---|---|---|---|---|
| Configuration laboratory scale | 53 rpm | 3170 min−1 | 3487 min−1 | 4026 min−1 | 5088 min−1 | 100 bar |
| Configuration pilot scale | 33 rpm | 1255 min−1 | 1381 min−1 | 1594 min−1 | 2015 min−1 | 100 bar |
| Calculated shear rate (s−1) | 27 | 3.3 × 103 | 3.6 × 104 | 4.1 × 104 | 5.2 × 104 | 6.0 × 107 |
| y0 | a | b |
|---|---|---|
| 7.32755 | 8.034 × 104 | −0.27206 |
| Shear Rate Calculated by Equation (7) (s−1) | Targeted Shear Rate Calculated by Equations (2) and (3) (s−1) | Shear Rate Difference (s−1) | Shear Rate Deviation (%) | Adjusted Shear Rate Settings (s−1) |
|---|---|---|---|---|
| 2.3 × 102 ± 2.0 × 102 | 3.3 × 103 | 3.1 × 103 ± 2.0 × 102 | 7.0 ± 6.1 | 2.3 × 104 ± 2.9 × 104 |
| 5.9 × 102 ± 4.9 × 102 | 3.6 × 104 | 3.5 × 104 ± 4.9 × 102 | 1.6 ± 1.4 | 5.9 × 104 ± 6.9 × 104 |
| 2.3 × 103 ± 7.3 × 102 | 4.1 × 104 | 3.9 × 104 ± 7.3 × 102 | 5.7 ± 1.8 | 2.3 × 105 ± 1.0 × 105 |
| 3.9 × 104 ± 3.6 × 103 | 5.2 × 104 | 1.3 × 104 ± 3.6 × 103 | 74.2 ± 6.9 | 3.9 × 106 ± 5.1 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warncke, M.; Kulozik, U. Functionality of MC88- and MPC85-Enriched Skim Milk: Impact of Shear Conditions in Rotor/Stator Systems and High-Pressure Homogenizers on Powder Solubility and Rennet Gelation Behavior. Foods 2021, 10, 1361. https://doi.org/10.3390/foods10061361
Warncke M, Kulozik U. Functionality of MC88- and MPC85-Enriched Skim Milk: Impact of Shear Conditions in Rotor/Stator Systems and High-Pressure Homogenizers on Powder Solubility and Rennet Gelation Behavior. Foods. 2021; 10(6):1361. https://doi.org/10.3390/foods10061361
Chicago/Turabian StyleWarncke, Malou, and Ulrich Kulozik. 2021. "Functionality of MC88- and MPC85-Enriched Skim Milk: Impact of Shear Conditions in Rotor/Stator Systems and High-Pressure Homogenizers on Powder Solubility and Rennet Gelation Behavior" Foods 10, no. 6: 1361. https://doi.org/10.3390/foods10061361
APA StyleWarncke, M., & Kulozik, U. (2021). Functionality of MC88- and MPC85-Enriched Skim Milk: Impact of Shear Conditions in Rotor/Stator Systems and High-Pressure Homogenizers on Powder Solubility and Rennet Gelation Behavior. Foods, 10(6), 1361. https://doi.org/10.3390/foods10061361