Improving the Functionality of Proso Millet Protein and Its Potential as a Functional Food Ingredient by Applying Nitrogen Fertiliser
Abstract
1. Introduction
2. Materials and Methods
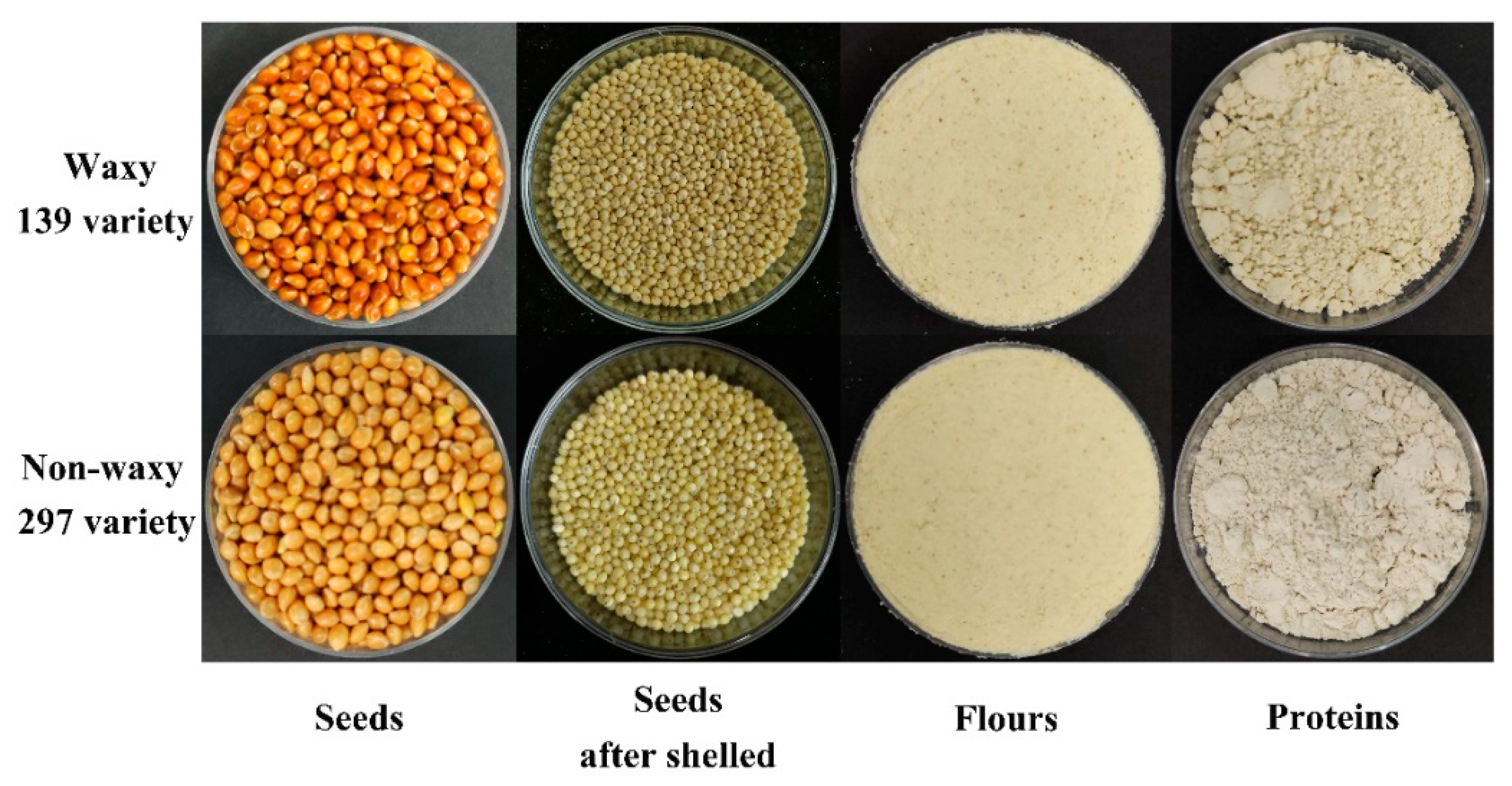
2.1. Plant Materials and Experimental Design
2.2. Protein Fractions Analysis
2.3. Extraction and Isolation of Proso Millet Protein
2.4. Amino Acid Profile
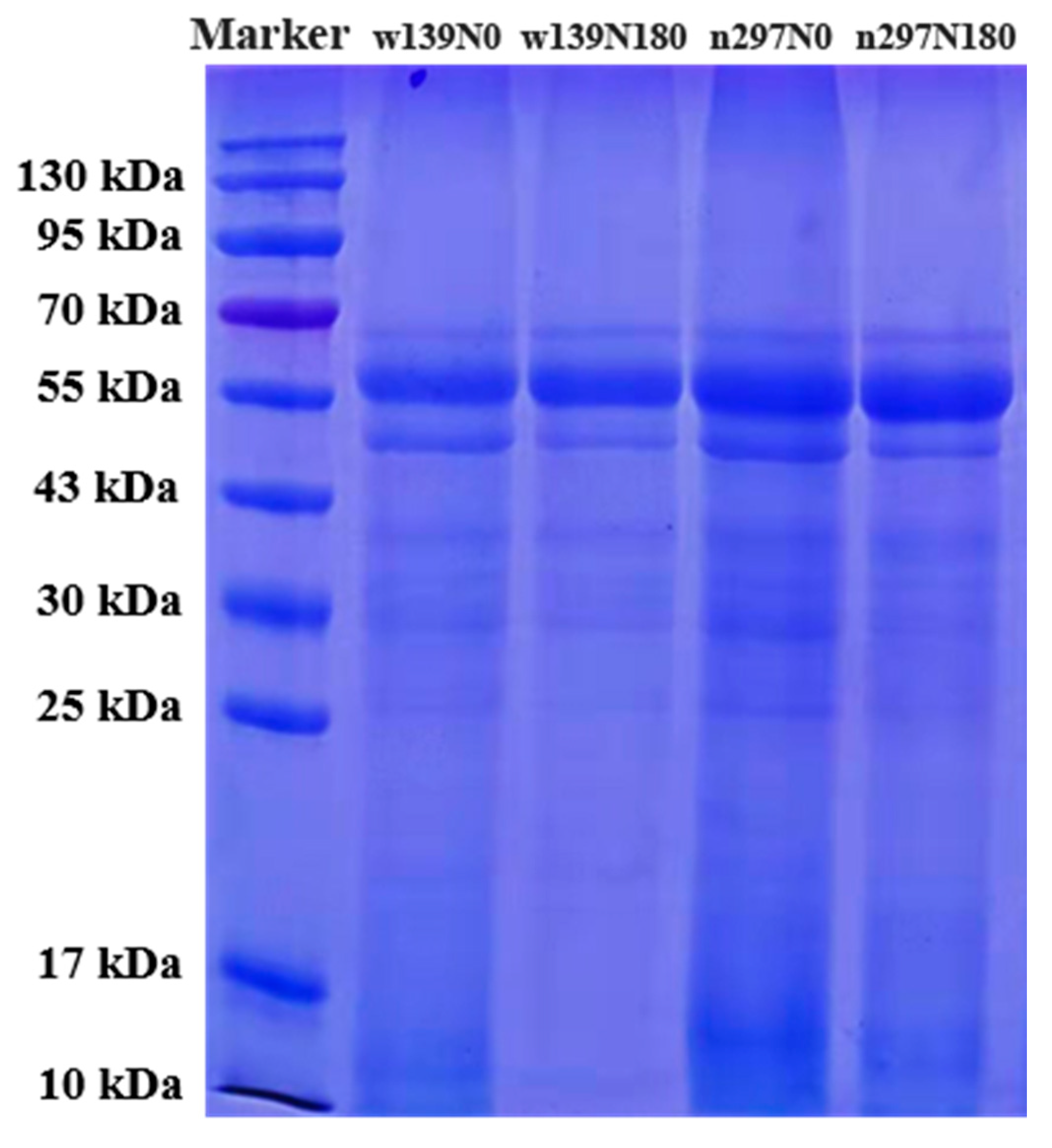
2.5. SDS-PAGE
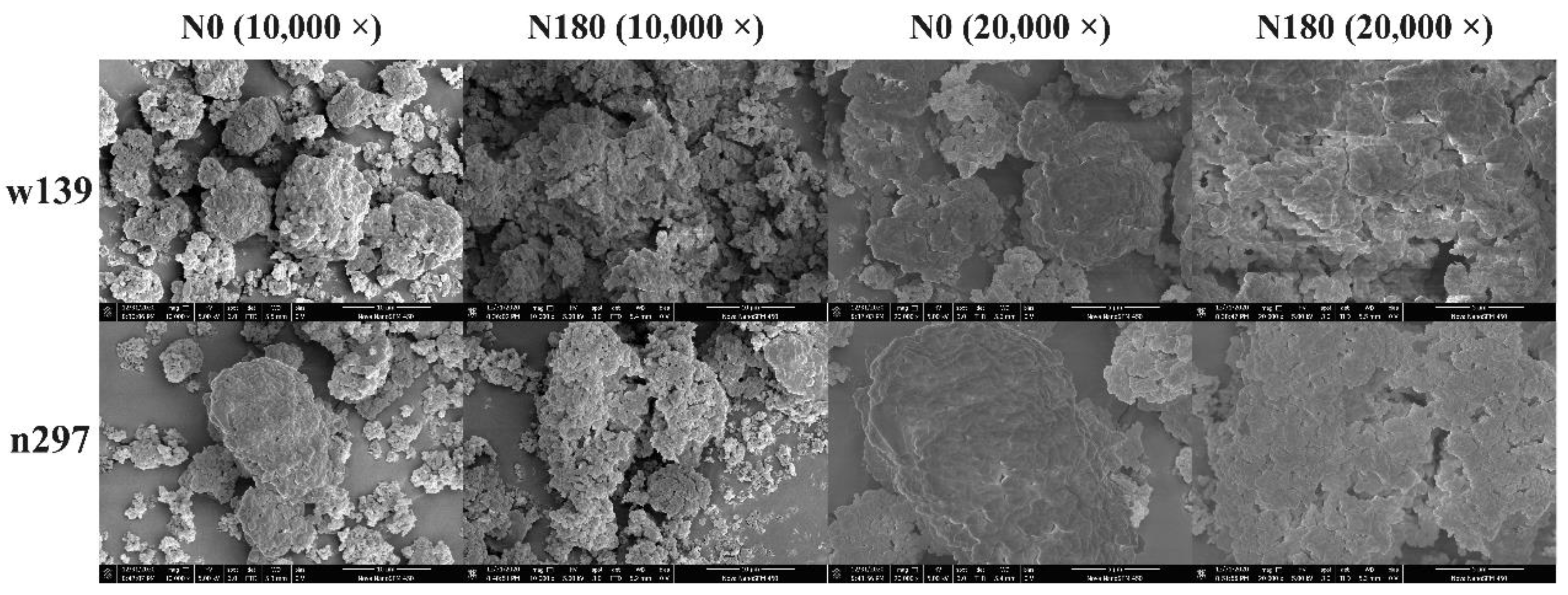
2.6. Morphological Observation and Granule Size Analysis
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Functional Properties of Proteins
2.8.1. Protein Solubility (PS), Water Holding Capacity (WHC) and Oil Absorption Capacity (OAC)
2.8.2. Emulsification Capacity (EC) and Emulsion Stability (ES)
2.8.3. Foaming Capacity (FC) and Foam Stability (FS)
2.9. Thermal Properties
2.10. Statistical Analysis
3. Results and Discussion
3.1. Protein Fractions
3.2. Amino Acid Analysis
3.3. SDS-PAGE
3.4. Morphology and Granule Size
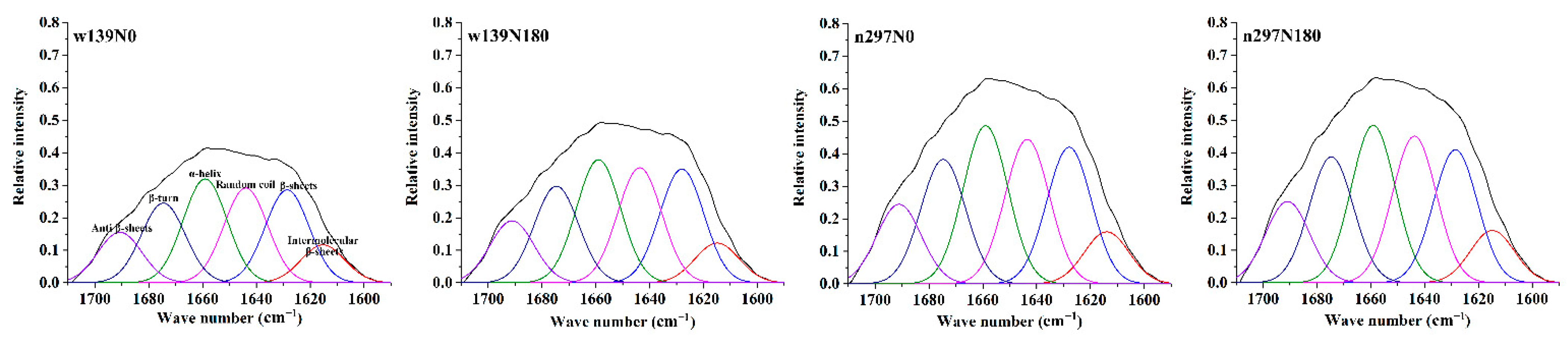
3.5. FTIR Spectroscopy
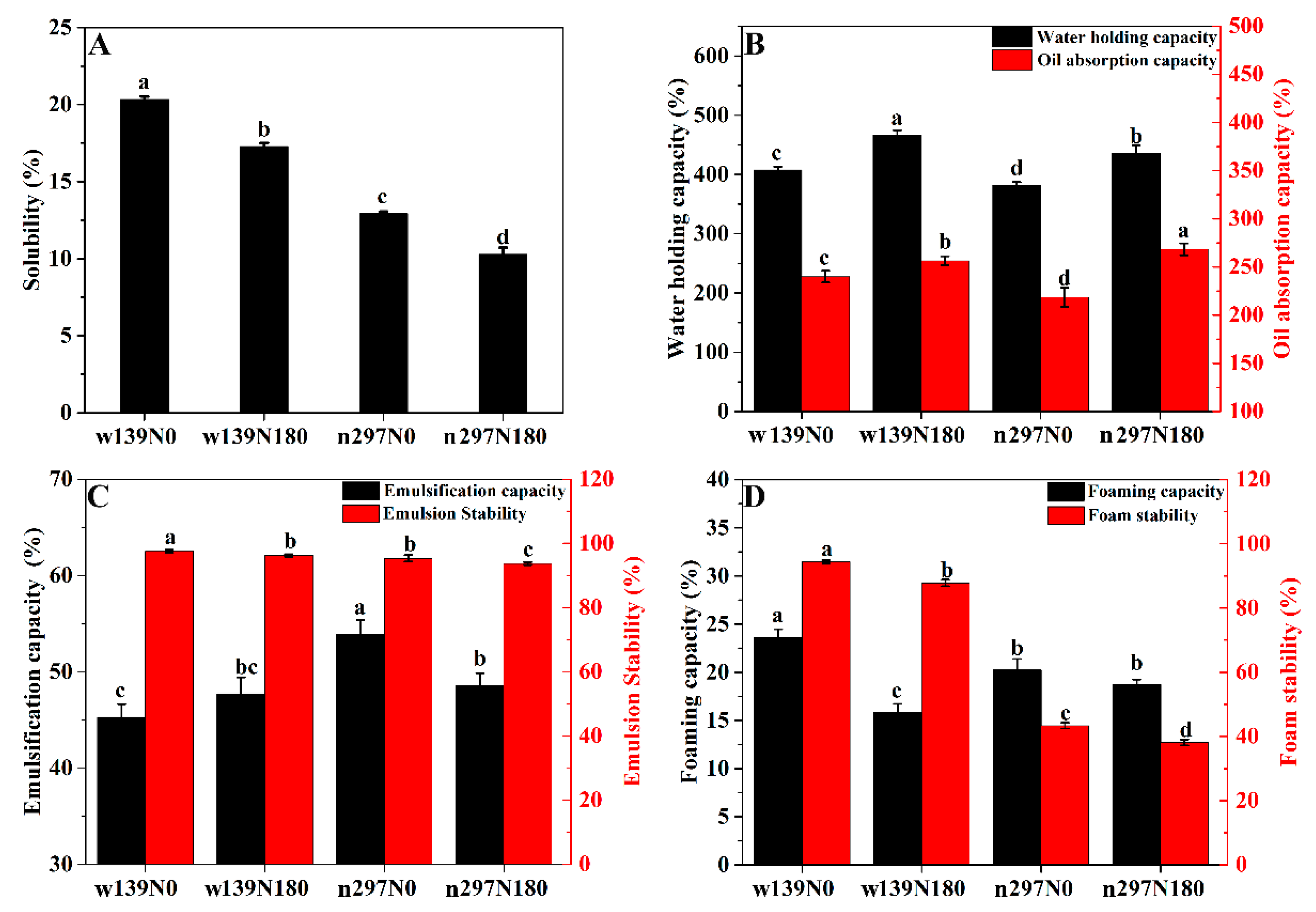
3.6. Protein Solubility (PS), Water Holding Capacity (WHC) and Oil Absorption Capacity (OAC)
3.7. Emulsification Capacity (EC) and Emulsion Stability (ES)
3.8. Foaming Capacity (FC) and Foam Stability (FS)
3.9. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| w139 | Waxy-139 |
| n297 | Non-waxy-297 |
| SEM | Scanning electron microscope |
| FTIR | Fourier transform infrared spectroscopy |
| PS | Protein solubility |
| WHC | Water holding capacity |
| OAC | Oil absorption capacity |
| EC | Emulsification capacity |
| ES | Emulsion stability |
| FC | Foaming capacity |
| FS | Foam stability |
| DSC | Differential scanning calorimetry |
| Td | Denaturation peak temperature |
| To | Onset temperature |
| Te | Endset temperature |
References
- Bilali, H.E.; Callenius, C.; Strassner, C.; Probst, L. Food and nutrition security and sustainability transitions in food systems. Food Energy Secur. 2018, 8, e00154. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Y.; Zhu, X.; Hua, X.; Shi, S.; Hu, J.; Peng, H.; Qiang, Z.; Sun, W. Physicochemical and functional properties of the protein isolate and major fractions prepared from Akebia trifoliata var. australis seed. Food Chem. 2012, 133, 923–929. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Maeve, H.; Maria, H.; Anne, M.; Mark, F.; Brijesh, T. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar]
- Yang, Q.; Zhang, P.; Qu, Y.; Gao, X.; Liang, J.; Yang, P.; Feng, B. Comparison of physicochemical properties and cooking edibility of waxy and non-waxy proso millet (Panicum miliaceum L.). Food Chem. 2018, 257, 271–278. [Google Scholar] [CrossRef]
- Nishizawa, N.; Sato, D.; Ito, Y.; Nagasawa, T.; Yi, M.W. Effects of dietary protein of proso millet on liver injury induced by D-galactosamine in rats. J. Agric. Chem. Soc. Jpn. 2002, 66, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Meng, Q.; Zhang, L.; Zhao, T.; Qiao, Z.; Wei, N.; Liu, G. Analysis of Protein Content in Panicum miliaceum L. Using Near Infrared Transmittance Spectroscopy. Agric. Sci. Technol. 2012, 13, 965–968. [Google Scholar]
- Kalinova, J.; Moudry, J. Content and Quality of Protein in Proso Millet (Panicum miliaceum L.) Varieties. Plant Foods Hum. Nutr. 2006, 61, 43–47. [Google Scholar] [CrossRef]
- López, D.; Galante, M.; Robson, M.; Boeris, V.; Spelzini, D. Amaranth, quinoa and chia protein isolates: Physicochemical and structural properties. Int. J. Biol. Macromol. 2018, 109, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Dai, Q.; Wei, C.; Zhou, G.; Huo, Z. Effects of nitrogen level on structure and physicochemical properties of rice starch. Food Hydrocoll. 2017, 63, 525–532. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Dou, Z.; Zhou, Q.; Chen, W.; Wang, S.; Ding, Y. Nitrogen fertilizer at heading stage effectively compensates for the deterioration of rice quality by affecting the starch-related properties under elevated temperatures. Food Chem. 2019, 277, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Dalei, L.U.; Liquan, J.; Decheng, W.; Qing, H.; Huanfen, G.; Jiuran, Z.; Weiping, L.U. The starch pasting properties in different nitrogen topdressing treatments under spring and autumn season of waxy maize varieties. Acta Ecol. Sin. 2010, 20, 549–555. [Google Scholar]
- Shan, Y.L.; Zhang, G.Q.; Luo, Q.G.; Guo, S.W.; Cao, F. Effect of Nitrogen and Sulfur Fertilizer on Granule Size Distribution, Composition and Pasting Properties of Wheat Starch. J. Triticeae Crop. 2012, 32, 60–67. [Google Scholar]
- Zhang, Q.; Wang, H.; Wang, S.L.; Zhang, Y.H.; Wang, R.; Wang, X.L.; Li, J. Effects of tillage alternation pattern with subsoiling on soil physical and chemical properties and spring maize yield in the Loess Plateau. Chin. J. Appl. Ecol. 2020, 31, 459–466. [Google Scholar]
- Mao, X.; Hua, Y.; Chen, G. Amino Acid Composition, Molecular Weight Distribution and Gel Electrophoresis of Walnut (Juglans regia L.) Proteins and Protein Fractionations. Int. J. Mol. Sci. 2014, 15, 2003–2014. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Xu, M.; Ohm, J.B.; Chen, B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocoll. 2020, 106, 105889. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Q.; Ferdinand, U.; Gong, X.; Qu, Y.; Gao, W.; Ivanistau, A.; Feng, B.; Liu, M. Isolation and characterization of starch from light yellow, orange, and purple sweet potatoes. Int. J. Biol. Macromol. 2020, 160, 660–668. [Google Scholar] [CrossRef]
- Yu, L.; Guo, L.; Liu, Y.; Ma, Y.; Zhu, J.; Yang, Y.; Min, D.; Xie, Y.; Chen, M.; Tong, J.; et al. Novel parameters characterizing size distribution of A and B starch granules in the gluten network: Effects on dough stability in bread wheat. Carbohydr. Polym. 2021, 257, 117623. [Google Scholar] [CrossRef]
- Yang, L. Effect of Nitrogen Application on Glutamine Synthetase Activity and Protein Composition of Spring Corn. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2006. [Google Scholar]
- Wang, H.; Yang, Q.; Gao, L.; Gong, X.; Qu, Y.; Feng, B. Functional and physicochemical properties of flours and starches from different tuber crops. Int. J. Biol. Macromol. 2020, 148, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.; Santra, D.; Adedeji, A. Physicochemical and functional properties of proso millet storage protein fractions. Food Hydrocoll. 2020, 108, 105497. [Google Scholar] [CrossRef]
- Zhang, M.W. Response of Wheat Grain Quality with Different Protein Content to Nitrogen Fertilizer Levels and Its Physiological Mechanism. Ph.D. Thesis, Henan Agricultural University, Zhengzhou, China, 2015. [Google Scholar]
- Yue, J.; Gu, Z.; Zhu, Z.; Yi, J.; Ohm, J.-B.; Chen, B.; Rao, J. Impact of defatting treatment and oat varieties on structural, functional properties, and aromatic profile of oat protein. Food Hydrocoll. 2021, 112, 106368. [Google Scholar] [CrossRef]
- Wang, X.Y. Effect of Nitrogen Fertilizer Rate and Soil Moisture on Carbon and Nitrogen Metabolism and Grain Yield and Quality Formation in Wheat. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2005. [Google Scholar]
- Kohama, K.; Nagasawa, T.; Nishizawa, N. Polypeptide Compositions and NH2-terminal Amino Acid Sequences of Proteins in Foxtail and Proso Millets. Biosci. Biotechnol. Biochem. 1999, 63, 1921–1926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nwachukwu, I.D.; Aluko, R.E. Physicochemical and emulsification properties of flaxseed (Linum usitatissimum) albumin and globulin fractions. Food Chem. 2018, 255, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Yao, L.; Qin, F.; Hou, G.; Chen, B.; Jin, L.; Deng, J.; Shen, Y. Characterisation, physicochemical and functional properties of protein isolates from Amygdalus pedunculata Pall seeds. Food Chem. 2020, 311, 125888. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and functional properties of Chinese quince seed protein isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Zuber, H. Temperature adaptation of lactate dehydrogenase Structural, functional and genetic aspects. Biophys. Chem. 1988, 29, 171–179. [Google Scholar] [CrossRef]
- Qiang, Z.; Xiong, H.; Selomulya, C.; Xiao, D.C. Effects of Spray Drying and Freeze Drying on the Properties of Protein Isolate from Rice Dreg Protein. Food Bioprocess Technol. 2013, 6, 1759–1769. [Google Scholar]
- Mendoza, E.; Adachi, M.; Bernardo, A.; Utsumi, S. Mungbean [Vigna radiata (L.) Wilczek] globulins: Purification and characterization. J. Agric. Food Chem. 2001, 49, 1552–1558. [Google Scholar] [CrossRef]
- Steffolani, M.E.; Villacorta, P.; Morales-Soriano, E.R.; Repo-Carrasco, R.; León, A.E.; Pérez, G.T. Physicochemical and Functional Characterization of Protein Isolated from Different Quinoa Varieties (Chenopodium quinoa Willd.). Cereal Chem. 2016, 93, 275–281. [Google Scholar] [CrossRef]
- Brinegar, C.; Sine, B.; Nwokocha, L. High-Cysteine 2S Seed Storage Proteins from Quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1996, 44, 1621–1623. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Xie, C.; Zhang, X.; Yan, S.; Xu, J.; et al. Effect of Spray-Drying and Freeze-Drying on the Properties of Soybean Hydrolysates. J. Chem. 2020, 2020, 9201457. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Arzeni, C.; Martínez, K.; Zema, P.; Arias, A.; Pérez, O.E.; Pilosof, A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012, 108, 463–472. [Google Scholar] [CrossRef]
- Withana-Gamage, T.S.; Wanasundara, J.P.D.; Pietrasik, Z.; Shand, P.J. Physicochemical, thermal and functional characterisation of protein isolates from Kabuli and Desi chickpea (Cicer arietinum L.): A comparative study with soy (Glycine max) and pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef]
- Kudre, T.; Benjakul, S.; Kishimura, H. Effects of protein isolates from black bean and mungbean on proteolysis and gel properties of surimi from sardine (Sardinella albella). LWT 2013, 50, 511–518. [Google Scholar] [CrossRef]
- Mune, M.; Sogi, D.S.; Minka, S.R. Response surface methodology for investigating structure–function relationship of grain legume proteins. J. Food Process. Preserv. 2018, 42, e13524. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, Q.; Selomulya, C.; Xiong, H. The effect of deamidation on the structural, functional, and rheological properties of glutelin prepared from Akebia trifoliata var. australis seed. Food Chem. 2015, 178, 96–105. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, L.; Tao, X.; Su, J.; Meeren, P. Adjustment of the structural and functional properties of okara protein by acid precipitation. Food Biosci. 2020, 37, 100677. [Google Scholar] [CrossRef]
- Jitngarmkusol, S.; Hongsuwankul, J.; Tananuwong, K. Chemical compositions, functional properties, and microstructure of defatted macadamia flours. Food Chem. 2008, 110, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Macarulla, M.T.; Barrio, A.; Martínez, J. Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–341. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.; Li, Y. Drying methods affect physicochemical and functional properties of quinoa protein isolate. Food Chem. 2021, 339, 127823. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Ragab, D.; Babiker, E.E.; Eltinay, A.H. Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Kudre, T.G.; Benjakul, S.; Kishimura, H. Comparative study on chemical compositions and properties of protein isolates from mung bean, black bean and bambara groundnut. J. Sci. Food Agric. 2013, 93, 2429–2436. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, B.; Rao, J. Pea protein isolate–high methoxyl pectin soluble complexes for improving pea protein functionality: Effect of pH, biopolymer ratio and concentrations. Food Hydrocoll. 2018, 80, 245–253. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.B.; Chen, B.; Rao, J. Physicochemical properties and aroma profiles of flaxseed proteins extracted from whole flaxseed and flaxseed meal. Food Hydrocoll. 2020, 104, 105731. [Google Scholar] [CrossRef]
- Feyzi, S.; Varidi, M.; Zare, F.; Varidi, M.J. A comparison of chemical, structural and functional properties offenugreek (Trigonella foenum graecum) protein isolates producedusing different defatting solvents. Int. J. Biol. Macromol. 2017, 105, 27–35. [Google Scholar] [CrossRef]
- Tang, C.-H. Thermal properties of buckwheat proteins as related to their lipid contents. Food Res. Int. 2007, 40, 381–387. [Google Scholar] [CrossRef]

| Varieties | Flour | Isolated Proso Millet Protein | ||||
|---|---|---|---|---|---|---|
| Protein (%) | Albumin (%) | Prolamin (%) | Glutelin (%) | Globulin (%) | Protein (%) | |
| w139N0 | 15.89 ± 0.07c | 1.45 ± 0.05b | 4.05 ± 0.02b | 0.29 ± 0.01c | 2.32 ± 0.08c | 61.80 ± 0.17c |
| w139N180 | 17.18 ± 0.22b | 1.65 ± 0.05a | 4.36 ± 0.05a | 0.48 ± 0.01a | 2.67 ± 0.02a | 64.05 ± 1.35c |
| n297N0 | 15.03 ± 0.42d | 1.39 ± 0.07b | 4.06 ± 0.03b | 0.35 ± 0.05ab | 2.44 ± 0.07b | 73.27 ± 1.65b |
| n297N180 | 18.16 ± 0.27a | 1.60 ± 0.02a | 4.25 ± 0.23ab | 0.42 ± 0.03a | 2.43 ± 0.03b | 76.57 ± 1.24a |
| Amino Acids | Proso Millet Protein Isolate (g/100 g Protein) | FAO/WHO a | |||
|---|---|---|---|---|---|
| w139N0 | w139N180 | n297N0 | n297N180 | Adult | |
| Asp | 2.14 ± 0.16a | 2.22 ± 0.20a | 2.31 ± 0.03a | 2.32 ± 0.07a | |
| Ser | 1.63 ± 0.06c | 1.81 ± 0.08b | 1.89 ± 0.03b | 2.03 ± 0.04a | |
| Glu | 2.58 ± 0.15a | 2.98 ± 0.23b | 3.42 ± 0.05a | 3.40 ± 0.06a | |
| Gly | 2.17 ± 0.06d | 2.40 ± 0.06c | 2.52 ± 0.03b | 2.70 ± 0.01a | |
| Ala | 1.95 ± 0.09b | 2.15 ± 0.12a | 2.15 ± 0.02a | 2.27 ± 0.05a | |
| Gys | 0.24 ± 0.01c | 0.33 ± 0.01b | 0.34 ± 0.01b | 0.44 ± 0.01a | |
| Tyr | 1.31 ± 0.05d | 1.41 ± 0.04c | 1.57 ± 0.01b | 1.74 ± 0.02a | |
| Arg | 3.18 ± 0.09d | 3.83 ± 0.06c | 4.47 ± 0.03b | 4.83 ± 0.02a | |
| Pro | 1.98 ± 0.08c | 2.22 ± 0.10b | 2.30 ± 0.02b | 2.50 ± 0.04a | |
| Trp | - | 0.01 ± 0.00 | - | - | 0.50 |
| Thr | 1.24 ± 0.06c | 1.38 ± 0.07b | 1.34 ± 0.02b | 1.48 ± 0.03a | 0.90 |
| Val | 1.99 ± 0.09c | 2.13 ± 0.09b | 2.17 ± 0.04b | 2.33 ± 0.05a | 1.30 |
| Met | 0.65 ± 0.02c | 0.73 ± 0.02b | 0.73 ± 0.02b | 0.84 ± 0.01a | 1.70 |
| Ile | 1.27 ± 0.05b | 1.30 ± 0.06b | 1.29 ± 0.02b | 1.42 ± 0.06a | 1.30 |
| Leu | 2.60 ± 0.09c | 2.84 ± 0.14b | 2.78 ± 0.06bc | 3.03 ± 0.09a | 1.90 |
| Phe | 2.06 ± 0.07c | 2.11 ± 0.04c | 2.37 ± 0.04b | 2.49 ± 0.03a | 1.90 |
| His | 1.22 ± 0.06d | 1.39 ± 0.05c | 1.47 ± 0.03b | 1.63 ± 0.01a | 1.60 |
| Lys | 1.90 ± 0.12a | 2.13 ± 0.26a | 1.98 ± 0.04a | 2.08 ± 0.05a | 1.60 |
| ARM b | 3.36d | 3.52c | 3.94b | 4.23a | 1.90 |
| Hydrophobic c | 14.67c | 15.87b | 16.31b | 17.58a | |
| Hydrophilic d | 4.42c | 4.93b | 5.14b | 5.69a | |
| Basic e | 6.30d | 7.52c | 7.92b | 8.54a | |
| Acidic f | 4.72b | 5.2ab | 5.73a | 5.72a | |
| Up g | 6.59c | 7.33b | 7.66b | 8.39a | |
| EAA h | 12.93c | 14.02b | 14.13b | 15.30a | |
| Varieties | d (0.1) b | d (0.5) | d (0.9) | D [3,2] | D [4,3] |
|---|---|---|---|---|---|
| w139N0 | 3.52 ± 0.06d | 27.02 ± 0.42d | 116.65 ± 1.84d | 8.34 ± 0.09d | 45.85 ± 0.45d |
| w139N180 | 4.58 ± 0.09b | 36.79 ± 0.31c | 126.99 ± 0.32c | 10.39 ± 0.11b | 53.39 ± 0.49c |
| n297N0 | 3.91 ± 0.06c | 43.44 ± 0.39b | 160.42 ± 0.80b | 9.44 ± 0.15c | 65.16 ± 0.27b |
| n297N180 | 4.88 ± 0.06a | 52.97 ± 0.16a | 174.77 ± 0.33a | 11.18 ± 0.25a | 73.38 ± 0.24a |
| Varieties | Secondary Structure Composition | Thermal Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intermolecular β-Sheets (%) | β-Sheets (%) | Random Coils (%) | α-Helices (%) | β-Turns (%) | Antiparallel β-Sheets (%) | To (°C) | Td (°C) | Te (°C) | |
| w139N0 | 5.56 ± 0.18a | 13.04 ± 0.09b | 13.76 ± 0.07a | 14.73 ± 0.02b | 11.23 ± 0.08d | 7.12 ± 0.11c | 100.36 ± 0.48b | 106.99 ± 0.43d | 138.57 ± 0.67c |
| w139N180 | 4.71 ± 0.02c | 13.61 ± 0.08a | 13.38 ± 0.03c | 14.61 ± 0.02c | 11.49 ± 0.02c | 7.39 ± 0.02b | 101.86 ± 0.21a | 110.04 ± 0.43bc | 141.87 ± 0.15b |
| n297N0 | 4.95 ± 0.05b | 12.35 ± 0.20c | 13.52 ± 0.04b | 14.90 ± 0.05a | 11.61 ± 0.07b | 7.51 ± 0.03ab | 99.81 ± 0.10b | 107.89 ± 1.42cd | 141.69 ± 2.03b |
| n297N180 | 4.79 ± 0.02bc | 12.94 ± 0.06b | 13.43 ± 0.02c | 14.40 ± 0.03d | 11.72 ± 0.04a | 7.71 ± 0.23a | 102.27 ± 0.82a | 111.08 ± 2.54a | 147.32 ± 0.25a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, D.; Wan, C.; Luo, Y.; Yang, Q.; Gao, X.; Feng, B. Improving the Functionality of Proso Millet Protein and Its Potential as a Functional Food Ingredient by Applying Nitrogen Fertiliser. Foods 2021, 10, 1332. https://doi.org/10.3390/foods10061332
Wang H, Li D, Wan C, Luo Y, Yang Q, Gao X, Feng B. Improving the Functionality of Proso Millet Protein and Its Potential as a Functional Food Ingredient by Applying Nitrogen Fertiliser. Foods. 2021; 10(6):1332. https://doi.org/10.3390/foods10061332
Chicago/Turabian StyleWang, Honglu, Dongmei Li, Chenxi Wan, Yan Luo, Qinghua Yang, Xiaoli Gao, and Baili Feng. 2021. "Improving the Functionality of Proso Millet Protein and Its Potential as a Functional Food Ingredient by Applying Nitrogen Fertiliser" Foods 10, no. 6: 1332. https://doi.org/10.3390/foods10061332
APA StyleWang, H., Li, D., Wan, C., Luo, Y., Yang, Q., Gao, X., & Feng, B. (2021). Improving the Functionality of Proso Millet Protein and Its Potential as a Functional Food Ingredient by Applying Nitrogen Fertiliser. Foods, 10(6), 1332. https://doi.org/10.3390/foods10061332