Analytical Characterization of Water-Soluble Constituents in Olive-Derived By-Products
Abstract
1. Introduction
- OTP is the leftover biomass resulting from the pruning of olive trees during cultivation for an average production of 1.3 t/ha/y, and it is made up of approximately 30% leaves, 50% thin branches, and 20% olive wood [4]. It is a lignocellulosic material with a high content of carbohydrates (cellulose and hemicellulose, up to 55% of the total content) and thus holds great potential for use as a raw material within a biorefinery context for the production of biofuels and bioproducts. This by-product has attracted high research attention in recent years and has been investigated as a starting point for its conversion into bioethanol [5,6], cellulose nanofibers [7], xylitol, and antioxidants [4], or oligosaccharides with prebiotic potential [8], among many others;
- OL are originated in olive mills from cleaning operations of collected olives before entering the olive oil extraction process. They are typically disposed of through burning or used as animal feed; however, they are a great source of valuable organic substances like oleanoic acid, mannitol, and oleuropein [9]. The structural composition of OL is made up mostly of extractives (36%) and lignin (40%), with small contents of structural carbohydrates (6% cellulose and 4% hemicellulose) [10]. The high content of these extractable compounds in OL has focused the research on their extraction rather than on the use of OL as a substrate in biorefineries [11];
- OS are separated in olive mills or in olive pomace oil extracting industries before oil extraction and represent up to 15% of the total olive weight [9]. With a high calorific potential, OS is one of the most utilized residual biomasses for the self-generation of heat in agroindustries; up to 78% of all companies in Spain using solid biomass as fuel use OS [12]. Their structural composition consists of up to 50% sugar in the form of cellulose and hemicellulose and 25%–27% lignin [10]. This also grants them a potential use as a raw material in a biorefinery, as the research in this field focuses on its fractionation in order to maximize sugars liberation for further bioconversion [13];
- EOP is the leftover biomass obtained after the oil extraction of olive pomace, making up a 20% of the total dry mass of the pomace, which in turn constitutes 70%–80% of the total weight of the olive itself [14]. Pomace can be reused for further olive oil extraction by means of a solvent extraction process and a refining process and has been reported to be a source of high-value-added compounds such as phenols and polyphenols, vitamins, fatty acids, and other relevant antioxidants [10]. EOP is used as a solid biofuel for self-supply at small plants, though this application comes with high environmental impact due to the emission of hazardous particles and gases through combustion [14].
2. Materials and Methods
2.1. Olive By-Products Sample Preparation
2.2. Analytical Procedures for By-Products Chemical Composition and Extractives Quantification
2.3. Compositional Analysis of Aqueous Extracts
2.3.1. Sugars and Related Alditols
2.3.2. Carboxylic Acids
2.3.3. Inorganic Anions and Cations
2.3.4. Determination of Total Phenolic Content
2.3.5. Phenols by High-Performance Liquid Chromatography (HPLC)
2.3.6. Proteins
3. Results and Discussion
3.1. Structural Composition of Olive By-Products
3.2. Sugars and Alditols
3.3. Organic Acids
3.4. Inorganic Compounds
3.5. Phenolic Compounds
3.6. Overall Extractives Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spanish Ministry of Agriculture Fisheries and Food. Encuesta sobre Superficies y Rendimientos Cultivos (ESYRCE). Encuesta de Marco de Áreas de España. Available online: https://www.mapa.gob.es/en/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/ (accessed on 18 November 2020).
- Spanish Ministry of Agriculture Fisheries and Food. Superficies y producciones anuales de cultivo de acuerdo con el Reglamento (CE) 543/2009. Available online: https://www.mapa.gob.es/en/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 18 November 2020).
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef]
- Susmozas, A.; Moreno, A.D.; Romero-García, J.M.; Manzanares, P.; Ballesteros, M. Designing an olive tree pruning biorefinery for the production of bioethanol, xylitol and antioxidants: A techno-economic assessment. Holzforschung 2019, 73, 15–23. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Susmozas, A.; Ruiz, E.; Castro, E.; Cardona-Alzate, C.A. Techno-economic feasibility of bioethanol production via biorefinery of olive tree prunings (OTP): Optimization of the pretreatment stage. Holzforschung 2019, 73, 3–13. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Álvarez, C.; Manzanares, P.; Moreno, A.D. Fermentation strategies for the efficient use of olive tree pruning biomass from a flexible biorefinery approach. Fuel 2020, 277. [Google Scholar] [CrossRef]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef]
- Ruiz, E.; Gullón, B.; Moura, P.; Carvalheiro, F.; Eibes, G.; Cara, C.; Castro, E. Bifidobacterial growth stimulation by oligosaccharides generated from olive tree pruning biomass. Carbohydr. Polym. 2017, 169, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuels Bioprod. Biorefin. 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Moya, M.; Ruiz, E.; Fernández-Bolaños, J.; Castro, E. Obtaining sugars and natural antioxidants from olive leaves by steam-explosion. Food Chem. 2016, 210, 457–465. [Google Scholar] [CrossRef]
- Avebiom. El Hueso de Aceituna, rey de las Agrobiomasas en España. Available online: https://www.avebiom.org/biomasanews/pellets-y-otros-biocombustibles/el-hueso-de-aceituna-rey-de-las-agrobiomasas-en-espana (accessed on 21 January 2021).
- Doménech, P.; Duque, A.; Higueras, I.; Iglesias, R.; Manzanares, P. Biorefinery of the olive tree-production of sugars from enzymatic hydrolysis of olive stone pretreated by alkaline extrusion. Energies 2020, 13, 4517. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gómez-Cruz, I.; Romero, I.; Gullón, B.; Ruiz, E.; Brnčićc, M.; Castro, E. Ultrasound-assisted extraction as a first step in a biorefinery strategy for valorisation of extracted olive pomace. Energies 2019, 12, 2679. [Google Scholar] [CrossRef]
- Berbel, J.; Posadillo, A. Review and analysis of alternatives for the valorisation of agro-industrial olive oil by-products. Sustainability 2018, 10, 237. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html (accessed on 21 January 2021).
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2005. Available online: https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html (accessed on 21 January 2021).
- Chen, S.-F.; Mowery, R.A.; Scarlata, C.J.; Chambliss, C.K. Compositional Analysis of Water-Soluble Materials in Corn Stover. J. Agric. Food Chem. 2007, 55, 5912–5918. [Google Scholar] [CrossRef]
- Chen, S.-F.; Mowery, R.A.; Sevcik, R.S.; Scarlata, C.J.; Chambliss, C.K. Compositional Analysis of Water-Soluble Materials in Switchgrass. J. Agric. Food Chem. 2010, 58, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- García-Diego, C.; Sánchez, M. Procedimiento de Trabajo: Determinación de Cationes Alcalinos, Alcalinotérreos y Amonio en Aguas Mediante Cromatografía Iónica (EM-PT-IE06); CIEMAT Laboratory Procedures: Madrid, Spain, 2008. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A Simple 96-Well Microplate Method for Estimation of Total Polyphenol Content in Seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Hernández, V.; Romero-García, J.M.; Dávila, J.A.; Castro, E.; Cardona, C.A. Techno-economic and environmental assessment of an olive stone based biorefinery. Resour. Conserv. Recycl. 2014, 92, 145–150. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; del Mar Contreras, M.; Romero, I.; Castro, E. A biorefinery approach to obtain antioxidants, lignin and sugars from exhausted olive pomace. J. Ind. Eng. Chem. 2021, 96, 356–363. [Google Scholar] [CrossRef]
- Heredia-Moreno, A.; Guillén-Bejarano, R.; Fernández-Bolaños, J.; Rivas-Moreno, M. Olive stones as a source of fermentable sugars. Biomass 1987, 14, 143–148. [Google Scholar] [CrossRef]
- Martín García, A.I.; Moumen, A.; Yáñez Ruiz, D.R.; Molina Alcaide, E. Chemical composition and nutrients availability for goats and sheep of two-stage olive cake and olive leaves. Anim. Feed Sci. Technol. 2003, 107, 61–74. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Baker, P.W.; Charlton, A. A comparison in protein extraction from four major crop residues in Europe using chemical and enzymatic processes-a review. Innov. Food Sci. Emerg. Technol. 2020, 59, 102239. [Google Scholar] [CrossRef]
- Del Contreras, M.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Olive Pomace-Derived Biomasses Fractionation through a Two-Step Extraction Based on the Use of Ultrasounds: Chemical Characteristics. Foods 2021, 10, 111. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Integrated process for sequential extraction of bioactive phenolic compounds and proteins from mill and field olive leaves and effects on the lignocellulosic profile. Foods 2019, 8, 531. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef]
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Saha, B.C.; Racine, F.M. Biotechnological production of mannitol and its applications. Appl. Microbiol. Biotechnol. 2011, 89, 879–891. [Google Scholar] [CrossRef]
- Del Río, P.G.; Gomes-Dias, J.S.; Rocha, C.M.R.; Romaní, A.; Garrote, G.; Domingues, L. Recent trends on seaweed fractionation for liquid biofuels production. Bioresour. Technol. 2020, 299, 122613. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Novak, K.; Pflügl, S. Towards biobased industry: Acetate as a promising feedstock to enhance the potential of microbial cell factories. FEMS Microbiol. Lett. 2018, 365, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of lignocellulosic sugars to acetic acid by Moorella thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Arienzo, M.; Capasso, R. Analysis of metal cations and inorganic anions in olive oil mill waste waters by atomic absorption spectroscopy and ion chromatography. Detection of metals bound mainly to the organic polymeric fraction. J. Agric. Food Chem. 2000, 48, 1405–1410. [Google Scholar] [CrossRef]
- Ameziane, H.; Nounah, A.; Khamar, M.; Zouahri, A. Use of olive pomace as an amendment to improve physico-chemical parameters of soil fertility. Agron. Res. 2019, 17, 2158–2171. [Google Scholar] [CrossRef]
- Dugo, G.; Pellicanò, T.M.; Pera, L.L.; Turco, V.L.; Tamborrino, A.; Clodoveo, M.L. Determination of inorganic anions in commercial seed oils and in virgin olive oils produced from de-stoned olives and traditional extraction methods, using suppressed ion exchange chromatography (IEC). Food Chem. 2007, 102, 599–605. [Google Scholar] [CrossRef]
- Roche, M.; Dufour, C.; Mora, N.; Dangles, O. Antioxidant activity of olive phenols: Mechanistic investigation and characterization of oxidation products by mass spectrometry. Org. Biomol. Chem. 2005, 3, 423. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Prior, Á.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Synergistic effect of 3,4-dihydroxyphenylglycol with hydroxytyrosol and α-tocopherol on the Rancimat oxidative stability of vegetable oils. Innov. Food Sci. Emerg. Technol. 2019, 51, 100–106. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar] [CrossRef]
- Arando, A.; Delgado, J.V.; Fernández-Prior, A.; León, J.M.; Bermúdez-Oria, A.; Nogales, S.; Pérez-Marín, C.C. Effect of different olive oil-derived antioxidants (hydroxytyrosol and 3,4-dihydroxyphenylglycol) on the quality of frozen-thawed ram sperm. Cryobiology 2019, 86, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally Occurring Hydroxytyrosol Derivatives: Hydroxytyrosyl Acetate and 3,4-Dihydroxyphenylglycol Modulate Inflammatory Response in Murine Peritoneal Macrophages. Potential Utility as New Dietary Supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioprocess Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of pulsed electric fields and high pressure on improved recovery of high-added-value compounds from olive pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef]
- Vogel, P.; Machado, I.K.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Dal Bosco, S.M. Polyphenols benefits of olive leaf (Olea europaea L.) to human health. Nutr. Hosp. 2015, 31, 1427–1433. [Google Scholar] [CrossRef]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]

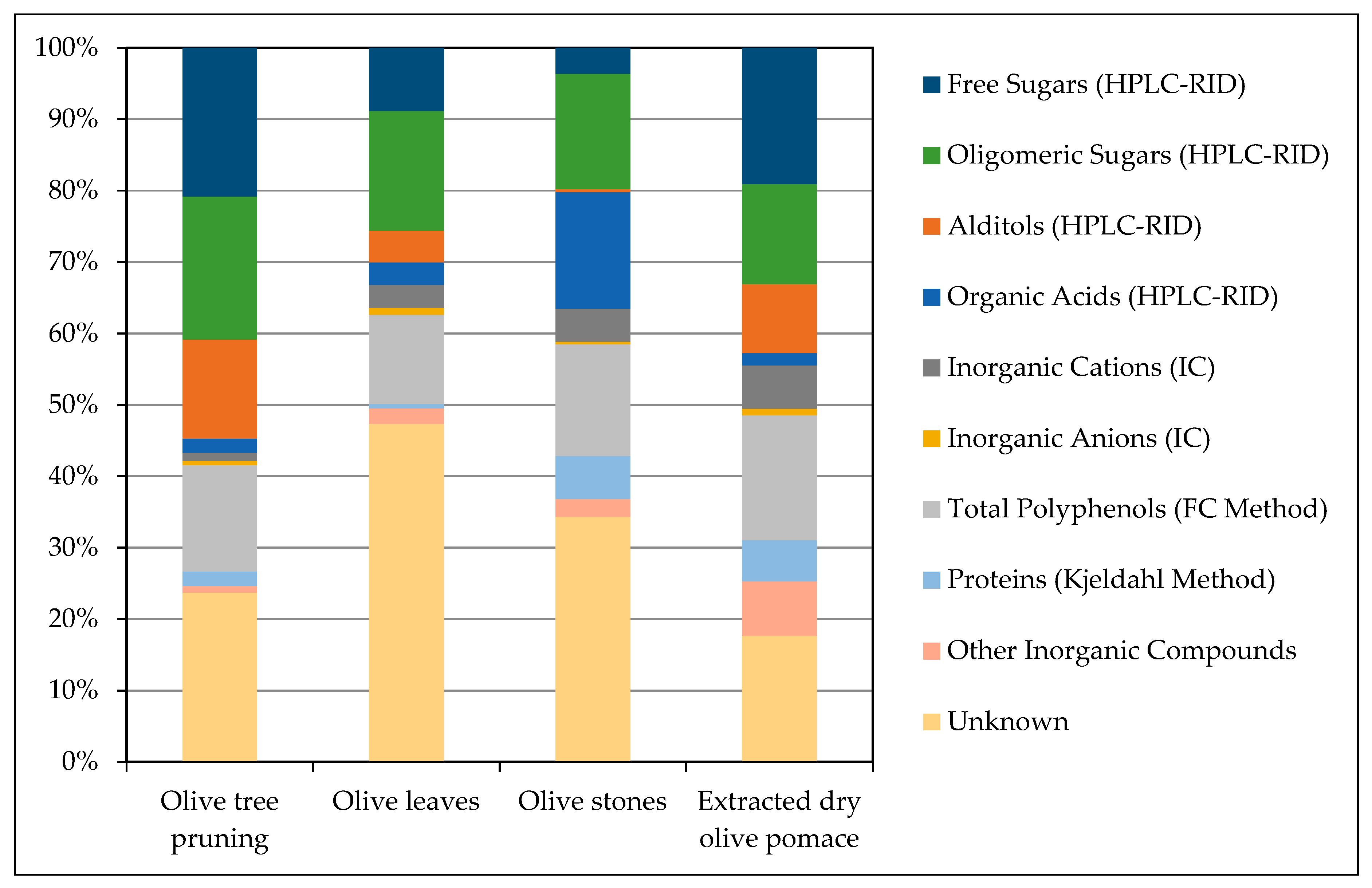
| Component | OTP (%dwb) | OL (%dwb) | OS (%dwb) | EOP (%dwb) |
|---|---|---|---|---|
| Total extractives | 27.8 ± 0.0 | 35.0 ± 0.0 | 6.3 ± 0.5 | 42.0 ± 1.2 |
| Aqueous | 23.7 ± 0.0 | 21.9 ± 0.2 | 3.9 ± 0.4 | 37.5 ± 1.5 |
| Organic | 4.1 ± 0.2 | 13.2 ± 0.6 | 2.4 ± 0.2 | 4.5 ± 0.4 |
| Cellulose | 20.8 ± 0.9 | 9.7 ± 0.1 | 20.9 ± 0.22 | 10.9 ± 1.0 |
| Hemicellulose | 14.5 ± 0.4 | 8.4 ± 0.1 | 26.0 ± 0.1 | 11.7 ± 0.6 |
| Xylan | 9.2 ± 0.4 | 3.3 ± 0.1 | 23.5 ± 0.1 | 9.4 ± 0.4 |
| Galactan | 1.9 ± 0.0 | 1.9 ± 0.0 | 1.2 ± 0.0 | 1.0 ± 0.1 |
| Arabinan | 2.6 ± 0.1 | 2.8 ± 0.0 | 1.2 ± 0.0 | 1.0 ± 0.0 |
| Mannan | 0.8 ± 0.1 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 |
| Lignin | 22.6 ± 0.5 | 25.7 ± 0.0 | 35.6 ± 0.6 | 23.1 ± 0.5 |
| Acid-insoluble | 19.7 ± 0.5 | 23.2 ± 0.0 | 33.9 ± 0.4 | 21.5 ± 0.4 |
| Acid-soluble | 2.9 ± 0.1 | 2.5 ± 0.0 | 1.7 ± 0.1 | 1.6 ± 0.1 |
| Ash | 2.7 ± 0.2 | 9.1 ± 0.1 | 0.6 ± 0.0 | 9.0 ± 0.4 |
| Acetyl groups | 3.3 ± 0.1 | 1.6 ± 0.0 | 5.9 ± 0.1 | 2.1 ± 0.0 |
| Proteins | 3.4 ± 0.2 | 7.8 ± 0.0 | 0.7 ± 0.0 | 9.1 ± 0.2 |
| Component in Aqueous Extractives | OTP (% w/w) | OL (% w/w) | OS (% w/w) | EOP (% w/w) |
|---|---|---|---|---|
| Sugars (monomers) | 20.8 | 8.8 | 3.6 | 19.1 |
| Glucose | 13.4 ± 0.8 | 5.5 ± 0.5 | 0.8 ± 0.2 | 10.4 ± 1.2 |
| Xylose | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.45 ± 0.0 |
| Galactose | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | 1.0 ± 0.2 |
| Arabinose | 0.5 ± 0.1 | 0.6 ± 0.2 | 1.1 ± 0.2 | 0.5 ± 0.1 |
| Mannose | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.1 ± 0.1 | 1.6 ± 0.1 |
| Fructose | 5.6 ± 0.5 | 1.4 ± 0.2 | 0.5 ± 0.0 | 5.2 ± 1.0 |
| Sugars (oligomers determined as) | 20.1 | 16.8 | 16.2 | 14.1 |
| Glucose | 15.6 ± 3.3 | 10.4 ± 1.5 | 1.3 ± 0.2 | 7.6 ± 1.8 |
| Xylose | 0.2 ± 0.1 | 0.8 ± 0.4 | 10.1 ± 1.8 | 0.2 ± 0.1 |
| Galactose | 1.8 ± 0.5 | 2.7 ± 0.7 | 1.9 ± 0.1 | 2.4 ± 0.4 |
| Arabinose | 2.4 ± 0.6 | 2.5 ± 0.6 | 2.6 ± 0.3 | 3.6 ± 0.6 |
| Mannose | n.d. | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.3 |
| Alditols | ||||
| Mannitol | 13.9 ± 0.4 | 4.4 ± 0.4 | 0.4 ± 0.2 | 9.6 ± 1.6 |
| Component in Aqueous Extractive | OTP (% w/w) | OL (% w/w) | OS (% w/w) | EOP (% w/w) |
|---|---|---|---|---|
| Organic acids | 2.0 | 3.2 | 16.3 | 1.7 |
| Acetic acid | 0.3 ± 0.1 | 1.0 ± 0.2 | 8.4 ± 0.5 | 1.0 ± 0.0 |
| Other acids | 1.6 ± 0.1 | 2.2 ± 0.7 | 7.9 ± 1.2 | 0.7 ± 0.0 |
| Component in Aqueous Extractives | OTP (% w/w) | OL (% w/w) | OS (% w/w) | EOP (% w/w) |
|---|---|---|---|---|
| Total Cations | 1.16 | 3.24 | 4.60 | 6.07 |
| K+ | 0.71 ± 0.02 | 1.58 ± 0.07 | 2.96 ± 0.09 | 5.36 ± 0.10 |
| Ca2+ | 0.12 ± 0.01 | 0.70 ± 0.01 | 0.22 ± 0.01 | 0.34 ± 0.01 |
| Na+ | 0.25 ± 0.01 | 0.49 ± 0.01 | 0.65 ± 0.01 | 0.16 ± 0.00 |
| Mg2+ | 0.08 ± 0.00 | 0.46 ± 0.01 | 0.09 ± 0.00 | 0.16 ± 0.01 |
| NH4+ | n.d. | 0.01 ± 0.00 | 0.69 ± 0.01 | 0.04 ± 0.00 |
| Total Anions | 0.62 | 0.95 | 0.39 | 0.93 |
| Cl− | 0.08 ± 0.01 | 0.13 ± 0.01 | 0.32 ± 0.01 | 0.52 ± 0.03 |
| NO3− | 0.01 ± 0.00 | n.d. | 0.01 ± 0.00 | n.d. |
| SO42− | 0.53 ± 0.06 | 0.81 ± 0.02 | 0.06 ± 0.00 | 0.41 ± 0.02 |
| Other Inorganic Compounds | 0.91 | 2.19 | 2.49 | 7.64 |
| Component in Aqueous Extractives | OTP (% w/w) | OL (% w/w) | OS (% w/w) | EOP (% w/w) |
|---|---|---|---|---|
| Total Phenolic Alcohols | 2.02 | 1.33 | 1.58 | 1.31 |
| Tyr | 0.06 ± 0.01 | n.d. | 1.10 ± 0.10 | 0.21 ± 0.08 |
| OH-Tyr | 0.80 ± 0.11 | 0.28 ± 0.03 | 0.31 ± 0.01 | 1.10 ± 0.32 |
| DHPG | 1.14 ± 0.03 | 1.04 ± 0.07 | n.d. | n.d. |
| Vanillin | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.17 ± 0.17 | n.d. |
| Total Phenolic Acids | 0.01 | n.d. | 0.31 | n.d. |
| Vanillic acid | n.d. | n.d. | 0.21 ± 0.01 | n.d. |
| Syringaldehyde | 0.01 ± 0.00 | n.d. | 0.10 ± 0.01 | n.d. |
| Other Phenols | 0.71 | 0.83 | 2.70 | 1.93 |
| Total Phenols (HPLC) * | 2.74 | 2.17 | 4.59 | 3.24 |
| Total Polyphenols (FC) | 14.9 ± 0.4 | 12.5 ± 0.2 | 15.6 ± 1.6 | 17.5 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doménech, P.; Duque, A.; Higueras, I.; Fernández, J.L.; Manzanares, P. Analytical Characterization of Water-Soluble Constituents in Olive-Derived By-Products. Foods 2021, 10, 1299. https://doi.org/10.3390/foods10061299
Doménech P, Duque A, Higueras I, Fernández JL, Manzanares P. Analytical Characterization of Water-Soluble Constituents in Olive-Derived By-Products. Foods. 2021; 10(6):1299. https://doi.org/10.3390/foods10061299
Chicago/Turabian StyleDoménech, Pablo, Aleta Duque, Isabel Higueras, José Luis Fernández, and Paloma Manzanares. 2021. "Analytical Characterization of Water-Soluble Constituents in Olive-Derived By-Products" Foods 10, no. 6: 1299. https://doi.org/10.3390/foods10061299
APA StyleDoménech, P., Duque, A., Higueras, I., Fernández, J. L., & Manzanares, P. (2021). Analytical Characterization of Water-Soluble Constituents in Olive-Derived By-Products. Foods, 10(6), 1299. https://doi.org/10.3390/foods10061299







