Abstract
Growing interest in the development of innovative functional products as ideal carriers for synbiotics, e.g., nutrient bars, yogurt, chocolate, juice, ice cream, and cheese, to ensure the daily intake of probiotics and prebiotics, which are needed to maintain a healthy gut microbiota and overall well-being, is undeniable and inevitable. This review focuses on the modern approaches that are currently being developed to modulate the gut microbiota, with an emphasis on the health benefits mediated by co-encapsulated synbiotics and immobilized probiotics. The impact of processing, storage, and simulated gastrointestinal conditions on the viability and bioactivity of probiotics together with prebiotics such as omega-3 polyunsaturated fatty acids, phytochemicals, and dietary fibers using various delivery systems are considered. Despite the proven biological properties of synbiotics, research in this area needs to be focused on the proper selection of probiotic strains, their prebiotic counterparts, and delivery systems to avoid suppression of their synergistic or complementary effect on human health. Future directions should lead to the development of functional food products containing stable synbiotics tailored for different age groups or specifically designed to fulfill the needs of adjuvant therapy.
1. Introduction
The host’s microbiota is a complex ecosystem of bacteria, eukaryotic microbes, viruses, and archaea coexisting within the body and also on tissue surfaces. In these locations, the microbiota plays important roles in a variety of physiological activities, including digestion, metabolism, immune reactions, biosynthesis of numerous compounds, elimination of toxins, regulation of the gut-brain axis function, and even disease pathogenesis. The majority of these microbial communities reside within the gut and are influenced by the mode of birth, infant feeding, genetic background, and lifestyle, including diet, exercise, stress, medication, and overall health of the host. Generally, the sum of the unique microbial genes in the gut is called the gut microbiome [1,2,3,4]. The majority of symbiotic bacteria that colonize the human gut can be classified into several phyla, comprising Bacteroidetes and Firmicutes, followed by Proteobacteria, Actinobacteria, Fusobacteria, Verrucomicrobia, and Spirochaetes [2,5]. Gut microbial populations can vary significantly between individuals, even in healthy subjects. However, there is little doubt that basic physiological functions need to be maintained in the case of disruption of microbial composition, and this is achieved through a set of core microorganisms [5,6]. Unfavorable alterations in microbial composition and function are characteristics of many disease states and are known as dysbiosis. Although there is growing evidence that dysbiosis is associated with various human diseases, such as inflammatory bowel diseases, irritable bowel syndrome, allergies, asthma, metabolic syndrome, diabetes, obesity, cardiovascular diseases, cancer, and depression or anxiety, it is not well known if and how this contributes to pathogenesis [7,8,9,10,11,12,13]. Based on the increasing number of diverse disorders that are associated with dysbiosis, including antibiotic resistance, there is great interest in identifying ways to modulate the gut microbiota in order to find a preventive strategy for sustaining gut health. Well-known approaches to naturally modulate gut microbiota composition include a balanced diet that is rich in fresh fruits and vegetables, grains, and fermented products. Recent studies also suggest that regular exercise can alter gut microbial communities [14,15,16]. Modern approaches, such as the administration of probiotics and prebiotics—alone or mixed as synbiotics—in sufficient concentrations, as well as fecal microbiota transplantation (FMT) intervention in severe cases, have been intensively studied and may have health benefits, although protective mechanisms are not clearly understood [17,18,19]. Currently, there is growing interest in functional innovative products as ideal carriers, e.g., nutrient bars, yogurt, chocolate, juice, ice cream, cheese, or even sausage, for co-encapsulated synbiotics, to ensure the daily intake of probiotics and prebiotics needed to maintain a healthy microbiota and good mental health as well as boost immunity [20,21,22,23,24,25]. In connection with the COVID-19 outbreak, Zuo et al. [26] showed that the gut microbiome is perturbed after SARS-CoV-2 infection and outlined the existence of the gut-lung axis, in which the gut microbiota is metabolically able to affect lung function [27]. This possible link between SARS-Co-V-2 infection and gut microbiome or even lung microbiome alterations was reviewed elsewhere [28,29,30,31,32]. However, further research is needed to confirm whether probiotics or synbiotics positively interfere with COVID-19 disease severity or possess antiviral efficacy [33,34]. Further multi-center studies are also needed to establish that COVID-19-associated dysbiosis is not caused by medications used to treat the underlying illness.
This review focuses on modern approaches that are currently being developed to modulate the gut microbiota, with an emphasis on the health benefits mediated by co-encapsulated synbiotics and immobilized probiotics (Figure 1). The impact of processing, storage, and simulated gastrointestinal conditions (sGIC) on the viability and bioactivity of probiotics, with or without prebiotics such as omega-3 polyunsaturated fatty acids (PUFAs), phytochemicals, and dietary fibers, using various delivery systems are considered.
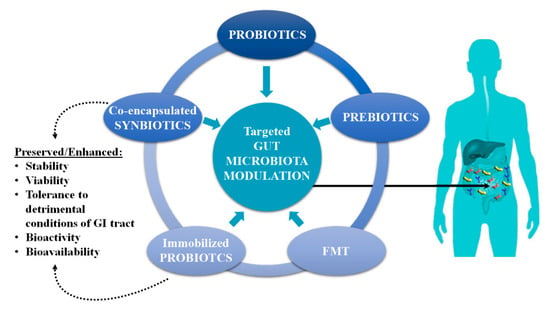
Figure 1.
Modern approaches used in the targeted gut microbiota modulation and benefits of co-encapsulated synbiotics and immobilized probiotics.
2. Gut Microbiota Modulation
2.1. Modulation by Probiotics
Probiotics are proposed as alternatives to antimicrobial drugs and adjuvant therapy in combating disease associated with gut dysbiosis. Probiotics are viable, non-pathogenic microorganisms that, when present in sufficient amounts, may confer health benefits on the host [35]. Various formulations, including capsules, tablets, powders, and food products containing probiotics, are commercially available today. Efficient delivery of probiotics to the intestine is crucial in achieving therapeutic efficiency because of the low bioavailability associated with the oral delivery of probiotics. To improve the health of the host through the beneficial action of bacterial species, it is widely accepted that the number of viable probiotic cells present in any type of formulation must attain a concentration equal to or greater than 106–107 CFU per gram or mL [36,37,38]. Lactobacillus and Bifidobacterium genera are the most frequently used bacteria for probiotic purposes, but other lactic-acid-producing bacteria, including Enterococcus, Streptococcus, and Lactococcus, are also widely used. In addition, next generation probiotic candidates, such as Akkermansia muciniphila, some Bacillus spp. and Propionibacterium freudenreichii—which belong to GRAS (Generally Recognized As Safe) microorganisms—and yeasts of the genus Saccharomyces exhibit probiotic characteristics [39,40,41]. For a strain to be considered probiotic, it needs to be resistant to host-induced stressors, where it should show an ability to adhere and/or proliferate at the site of action. It should also be safe to use and be deficient in any transferable antimicrobial resistant traits, though it may exhibit antimicrobial activity [42,43]. The beneficial effects of probiotics include sustaining a healthy microbiome, preventing pathogenic infections, and restoring microbial dysbiosis. Additional beneficial effects on the host are also favorable probiotic traits, including stabilizing and enhancing intestinal barrier function and producing anti-mutagenic, anti-carcinogenic, and other biologically important compounds such as short-chain fatty acids (SCFA), B-group vitamins, or vitamin K [44,45]. Moreover, probiotics are able to sense and regulate the action of secondary metabolites (e.g., bacteriocins, enzymes, and exopolysaccharides). Probiotic rich diets, adjunctive probiotic supplementation, and the prescription of personalized probiotics based on previous microbial analysis (targeted gut microbiota modulation) are linked with the prevention and potential treatment of several severe disorders, such as inflammatory bowel diseases, colorectal cancer, obesity, diabetes, and cardiovascular diseases as well as food allergies, depression, and brain function [17,46,47,48,49,50,51,52,53,54]. As the worldwide incidence of food allergies is increasing, there is an urgent need for well-controlled studies that demonstrate the positive outcomes of probiotics. For example, Tan-Lim et al. [55] determined the effectiveness of probiotics in a food allergy treatment for children. Lactobacillus rhamnosus GG administration likely helped infants to tolerate cow’s milk. Ma et al. [52] studied the protective effects of a lyophilized probiotic mixture (L. paracasei, L. reuteri, L. gasseri, L. salivarius, L. johnsonii, Bifidobacterium animalis) against food allergies. Ovalbumin-induced allergic responses were suppressed after treatment with probiotics, and this provided molecular insight into the probiotic mechanism of action. However, to ensure the long-term viability and efficacy of probiotics during processing, storage, and delivery to the site of action within the human body, advanced technologies such as microencapsulation or immobilization are recommended and have been extensively studied in the past decades [56,57,58,59].
2.2. Modulation by Prebiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) offers expertise in microbiology, nutrition, and clinical research and recently updated the definition of prebiotics to “a substrate that is selectively utilized by host microorganisms conferring a health benefit”. This definition was expanded from the previous prebiotic definition of carbohydrate-based substances to non-carbohydrate ones, such as PUFAs, polyphenols, etc. [60]. Prebiotics naturally exist in diverse vegetables, fruits, and other sources, including asparagus, sugar beet, garlic, chicory, onion, Jerusalem artichoke, banana, honey, blueberry, barley, wheat, tomato, potato, rye, soybean, peas, and beans, and recently have been identified in tea, vegetable oils, seaweeds, and microalgae [61]. Prebiotics, a group of nutrients comprised mainly of non digestible oligosaccharides fructans and galactans and some PUFAs or polyphenols, rely on selective utilization by microorganisms, which results in shaping and modulating the host’s gut microbiota [18,62]. It was confirmed that prebiotics are utilized as selective substrates not only by groups of microorganisms present in the colon but also by microbes colonizing other body sites outside of the gastrointestinal tract (GI tract), which are associated with promoting host health [39,60]. The potential benefits related to prebiotics include shifting gut microbiota composition (e.g., the product of the fermentation process might stimulate or inhibit the growth of other microorganisms) along with the release of microbial metabolites such as SCFA [60,61,63]. Hiel et al. [64] evaluated the impact of daily consumption of vegetables rich in inulin-type fructans on the gut microbiota. An increase in the genus Bifidobacterium was observed, and the same effect was also demonstrated after consumption of Jerusalem artichokes processed in different forms [65,66]. Furthermore, the growth of Bifidobacterium longum subsp. longum and, to a lesser extent, B. pseudocatenulatum, B. bifidum, and B. adolescentis at the species level was observed. However, Bifidobacterium abundance returned to baseline levels three weeks after the end of the treatment [64]. Kjølbæk et al. [67] investigated the diet-induced effects of arabinoxylan-oligosaccharides and PUFAs on gut microbiota modulation. During arabinoxylan-oligosaccharides supplementation, increased abundance of the species B. adolescentis, B. longum, and members of the genera Faecalibacterium, Ruminococcus, Dorea, and Eubacterium was observed. Furthermore, an increased abundance in butyrate-producing species such as Roseburia, Coprococcus, and Anaerostipes and bacteria belonging to the Clostridia class, particularly Eubacterium rectale, Faecalibacterium prausnitzii, and Eubacterium hallii, was observed because of the cross-feeding process. Reduction in both Rikenellaceae and Porphyromonadaceae was also observed. However, fourweek PUFAs intake did not induce any significant shift in the gut microbiota composition. In the study by Vigsnæs et al. [68], Bifidobacterium spp. and Lactobacillus spp. were selectively increased, accompanied by a high production of volatile metabolite acetate, after fermentation of arabino-oligosaccharides or fructo-oligosaccharides (FOS) by fecal microbiota obtained from patients with ulcerative colitis. However, the relative abundance of the butyrate-producing species F. prausnitzii and the butyrate-producing bacterial groups Clostridium coccoides (cluster XIVa) and Clostridium leptum (cluster IV) was decreased after incubation with arabino-oligosaccharides as well as FOS [68]. Analyzed samples comprising potato starch showed rapid growth of Streptococcus and Prevotella during fermentation; however, in mixed samples with maize starch, these two genera decreased, whereas Ruminobacter, Succinivibrio and unclassified Lachnospiraceae gradually increased. The study also pointed out that structural properties of the substrate itself can shift microbiota community composition and function [69]. Combination of isomalto-oligosaccharides with green tea extract (GTE) rich in polyphenols selectively enhanced the abundance of Lactobacillus, Bifidobacterium, Akkermansia, Parabacteriodes, Roseburia, Rikenella, Ruminococcus, and Sutterella, while it decreased Butyricimonas, Desulfovibrio, Dorea, Mucispirillum, Neisseria, Odoribacter, Prevotella, Paraprevotella, and Streptococcus. It was also observed to restore the Firmicutes-to-Bacteriodetes ratio [70]. Monofloral honey from Prunella vulgaris is rich in a variety of polyphenolic compounds, which positively modulated the Firmicutes-to-Bacteroidetes ratio and restored Lactobacillus spp. populations in rats with induced colitis [71]. Polyphenols can also inhibit the growth and adhesion of pathogenic bacteria. For example, the green and black tea extracts, epigallocatechin-3-gallate (EGCG) and theaflavins, inhibit Fusobacterium nucleatum biofilm formation and adhesion to oral epithelial cells and matrix proteins [72]. Natural flavonoid isoorientin, with its antioxidant and anti-inflammatory properties, inhibited the growth of inflammation-induced pathogenic genera Alistipes, Helicobacter, and Oscillibacter [73]. More studies of different types of non-encapsulated and encapsulated polyphenols with an impact on gut microbiota modulation are summarized in the review article of Shi et al. [74]. Considering the safety and health benefits of prebiotics on the host microbiota, overall well-being, and long-term health, prebiotics should be consumed on a daily basis alone, mixed, or in association with probiotics, as a rational synbiotic strategy, since consumption from dietary sources is inevitable.
2.3. Modulation by FMT in Severe Dysbiotic States
FMT is an investigational therapy for administration of fecal microbiota from a healthy person (donor) to a patient with dysbiosis. The aim of FMT is to restore the composition and function of the patient’s microbial ecosystem to its healthy characteristics. However, healthy gut microbiota composition varies among different populations and depends on the lifestyle of an individual [19]. The European Medicines Agency (EMA) has left decision-making about the use of FMT in the hands of its member states, while in the United States, FMT is not FDA-approved, because its use has been associated with adverse outcomes in susceptible patients. Nevertheless, FMT is highly effective therapeutic alternative for Clostridioides difficile infection and could be a promising therapeutic approach in patients with inflammatory bowel diseases, irritable bowel syndrome, metabolic syndrome, and other dysbiotic diseases with potentially serious health consequences [75,76,77,78,79,80]. Several of these clinical trials demonstrated that FMT therapy induced positive changes in the composition of microbiome, making it more comparable to a healthy community. However, FMT is associated with the risk of transmission of pathogens, especially antibiotic-resistant strains, which pose a potential risk to recipients. Before application of FMT, a very careful selection and screening of potential donors is required to minimize the recipient health risks due to the transfer of infectious agents [81,82].
Another interesting future perspective of how to apply FMT, is to use human gut microbiota cultured anaerobically in vitro as a source of well-defined transplant material to avoid transmission of pathogens. Bioreactors such as Simulator of the Human Intestinal Microbial Ecosystem (SHIME) or mucosal SHIME (M-SHIME), Lacroix model, EnteroMix and TIM-2 dynamic computer-controlled in vitro model of the proximal colon enable a complex, well-defined, and stable microbiome community structure with good metabolic activity. However, the use of these in vitro models is facing significant limitations, such as the aforementioned stable microbiome community structure and lack of physiological host environment, e.g., stress factors, varied diet, and presence of antibodies or antimicrobial agents [83,84,85]. Furthermore, it is not clear how well these culture or bioreactor adapted communities will engraft in the host, and further studies that promote transfer and engraftment are needed.
3. Co-Encapsulated Synbiotics
3.1. Synbiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) updated the definition of synbiotics to “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host”. Furthermore, two subsets of synbiotics were specified. A “complementary synbiotic” is a synbiotic composed of a probiotic combined with a prebiotic, where both components work independently. A “synergistic synbiotic” is a synbiotic in which the substrate is designed to be selectively utilized by the co-administered microorganism(s) [86]. Evidence to suggest the synergistic and complementary effects of probiotics together with prebiotics on a gut microbial composition is strong. These studies showed that synbiotics can adjust the Firmicutes-to-Bacteroidetes ratio, inhibit harmful bacteria by direct antagonism (such as Klebsiella, Escherichia coli, and C. difficile) and, by competitive exclusion, accelerate the recovery of a healthy gut microbiome, e.g., by maintaining intestinal pH, producing important metabolites, and promoting recovery of the gut mucosal barrier. It should be noted that the positive effects of probiotics and prebiotics depend on their suitable combination, which requires consideration of strain specificity and antimicrobial activity. Synbiotics can help to balance the gut microbiota by regulating specific gut microorganisms, and this opens the door for the development of new types of functional foods with higher precision impact than nutritional supplements or other products rich in synbiotics. Furthermore, synbiotics have the potential to help combat multidrug-resistant microorganisms [87,88,89,90]. Several clinical trials were held to confirm or disprove the potential health benefits of synbiotics. Neyrinck et al. [91] confirmed that synbiotics administered to middle-aged subjects significantly decreased the number of days of abdominal discomfort and proinflammatory status that naturally is associated with aging. Middle-aged subjects were randomized to take synbiotics (Bifidobacterium animalis subsp. lactis and FOS) or a placebo for 30 days. Although 16S rRNA gene sequencing of DNA extracted from stool demonstrated that synbiotic treatment had no impact on gut microbiota composition, plasma pro-inflammatory cytokines (IL-6, IL-8, IL-17a and INF-γ) were significantly reduced after 30 days of synbiotic supplementation. This observation could reflect the inadequacy of 16S rRNA sequencing of fecal specimens to accurately detect probiotic strains or reflect compositional changes in the proximal intestine. Phavichitr et al. [92] studied the influence of synbiotics (Bifidobacterium breve M-16V and galacto-oligosacharides (GOS)/FOS (9:1)) at doses closer to the bacterial cells present in human milk on intestinal bifidobacteria relative abundance, reduction of potential pathogens, and gut physiological conditions of infants. This synbiotic mixture successfully created an infant-type gut environment rich in Bifidobacterium species and reduced the number of C. difficile, resulting in a gut microbiota composition closer to the breast-fed reference group. Effects of synbiotic supplementation were also studied in patients with chronic kidney disease [93], nonalcoholic fatty liver disease [94], autoimmune disease [95], diarrhea [96], and metabolic syndrome [97]. One recommended approach to maintain the gut microbiome is daily consumption of functional food. Consumption of synbiotically fortified yogurt (Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Bifidobacterium animalis subsp. lactis BB-12 enriched with whey protein, inulin, calcium, and vitamin D3) for ten weeks significantly reduced body fat mass and improved body composition, blood pressure, insulin sensitivity, and lipid profiles in obese patients with metabolic syndrome [98].
To further improve oral delivery of synbiotics and to secure their stability and viability as well as targeted release in the intestine involves co-encapsulation. The incorporation of probiotics and prebiotics such as omega-3 PUFAs, γ-aminobutyric acid (GABA), phytochemicals, dietary fibers, and micronutrients carried by single delivery matrix into functional foods or other products can confer health benefits via gut microbiota modulation [29,99,100].
3.2. Technologies and Carrier Materials Used in Fabrication of Co-Encapsulated Synbiotics
The most important consideration for ensuring that probiotics reach their target site after oral supplementation is survival following transit through the harsh acidic environment of the stomach, thereby permitting adequate colonization and proliferation [101]. It has been reported that the microencapsulation of probiotics into polymeric microcapsules successfully protects the probiotics from the harsh and changing conditions of the GI tract. Thus, microcapsules direct the delivery of living cargo without it losing its functionality to the target site [102,103]. Microencapsulation in general is a process in which not only the probiotic cells but also enzymes, natural bioactive substances, gaseous materials, etc. are incorporated into an encapsulating matrix or membrane [104,105]. Microcapsules can protect cargo from degrading factors contained within the ambient environment during the passage through the GI tract and promote its release at controlled rates under particular conditions, usually in the colon. Microcapsules also protect the cargo during the stabilization process and storage at a wide range of temperatures and can extend shelf-life considerably. In addition, microencapsulation of bioactive substances is designed to improve their low bioavailability in the host, mask their unpleasant flavor, expand the application range, and increase overall acceptability [106,107,108,109]. The biopolymer used for encapsulation should be permeable to nutrients and metabolites in order to maintain cell viability of the cargo. Biopolymer must also be non-cytotoxic, as well as non-antimicrobial to ensure that the host and its microbiota are not adversely affected [40,110,111,112]. The encapsulation efficiency and delivery of the cargo with the desired viability and bioactivity to the site of action depends on the composition and structure of the wall material and also on the proper selection of co-encapsulation technology. The desired delivery system should be able to release cargo under specific conditions, such as change of pH, enzymatic activity, ionic strength, or temperature [113,114]. The main biocompatible and food-grade carrier materials for the co-encapsulation purposes of synbiotics are alginate [115], chitosan [116], pectin [117], gelatin [118], starch [119], gum Arabic [120], whey protein [121], and lipid carriers [122] as well as various blends of these materials [42,123,124]. These encapsulation materials are also well-described in reports by Rodrigues et al. [39], Shori [101], Arslan et al. [125], and Sarao and Arora [126]. Recently, numerous studies have shown that incorporation of prebiotics like inulin, hi-maize, trehalose, resistant starch, etc. into the encapsulation wall material increases its resistance and the preserved viability of probiotics in extreme environments of the GI tract [127,128,129]. Selecting the right co-encapsulation technology is therefore important. This topic has been extensively reviewed [99,130,131,132,133,134], and so herein we only present a short review of the main techniques that are employed to co-encapsulate probiotics with bioactive substances in a single delivery format: spray drying [135], freeze drying [136], spray chilling [122], emulsification [137], extrusion [138], and coacervation [139].
3.2.1. Co-Encapsulation with Omega-3 PUFAs and GABA
Consumption of prominent bioactive compounds such as omega-3 PUFAs in appropriate levels may trigger multiple health benefits, including prevention of cardiovascular disease, certain types of cancer, depression, non-alcoholic fatty liver disease, type-2 diabetes, obesity, and inflammation-mediated disorders [140,141,142,143].Omega-3 PUFAs are naturally occurring bioactive lipids, richly contained in fish products including oils (namely eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) and in plants and certain vegetable oils, such as flaxseed oil (alpha linolenic acid) [144,145,146]. Western-style diets do not meet the levels of omega-3 PUFAs required to fulfill the recommended daily intake for beneficial effects on human health. Therefore, incorporation of omega-3 PUFAs into various food products and their promotion as an important component of the human diet are needed [147,148,149].
The development of products comprising omega-3 PUFAs and probiotic strains together in a single microcapsule is an emerging area of research, because functional food products containing these particular bioactive ingredients separately have reported health benefits [150]. Microencapsulation of omega-3 PUFAs is one way to facilitate the incorporation of hydrophobic substances into functional food, thereby minimizing oxidative degradation, enhancing bioavailability, and allowing their use in stable and easy-to-handle formulations [114,147]. Studies combining both components show that PUFAs enhance the action of probiotics and vice versa, since probiotics can modulate the metabolism of dietary lipids. It was shown that PUFAs can affect the adhesion of lactobacilli in the gut, which is in line with studies suggesting that dietary PUFAs can affect the gut microbiota’s ability to adhere to the gut mucosa, possibly by modifying intestinal membrane fatty acid composition [151,152,153]. Indeed, PUFAs and probiotic supplements are being used as adjuvant therapy in inflammatory bowel diseases, allergies, rheumatoid arthritis, and obesity, with promising results. Both probiotics and PUFAs play an important role in modulating the intestinal immune system and are related to local and systemic inflammatory mechanisms [154,155]. In a randomized controlled trial, Kobyliak et al. [156] studied the intake efficiency of multi-probiotics enriched with omega-3 PUFAs as an adjuvant to the standard anti-diabetic therapy in individuals with type-2 diabetes. Supplementation once daily for eight weeks led to a significant reduction of insulin resistance, markers of chronic systemic inflammation, body weight, and body mass index as well as improved glycemic index profiles compared with placebo controls. Eratte et al. [139] reported that whey protein isolate–gum Arabic complex coacervates could successfully co-encapsulate tuna or coconut oil with Lactobacillus casei 431 and synergistically enhance the oxidative stability of omega-3-rich tuna oil. In addition, the viability and function of L. casei was observed in these spray-dried microcapsules during 90 days of storage. Free cells lost all viability within 1.5 h in the sGIC, but only a 1.5 log CFU/g loss of viability of probiotics was observed in co-encapsulated form. Moreover, the cell surface hydrophobicity and the ability of L. casei to adhere to the intestinal wall was significantly increased by co-encapsulation with omega-3 PUFAs [139,157,158]. Vaziri et al. [159] successfully co-encapsulated DHA-rich oil with Lactobacillus plantarum PTCC1058 by the extrusion-freeze-drying technique; the highest viability after L. plantarum co-encapsulation of 88.66% was seen when the carrier material was 0.39% gelatin, 0.55% pectin, and 1.06% alginate. Encapsulation efficiency of DHA-rich oil in microcapsules was 69.37%, and the survivability of L. plantarum under sGIC varied from 80.53 to 90.02%, depending on carrier material composition. Vega-Sagardia et al. [160] used vegetable oil to obtain information about the influence of the oil on bacterial viability. Lactobacillus fermentum UCO-979C counts in microcapsules with oil increased from 1.77 × 107 to 1.55 × 109 CFU/g. Alginate-Xantan gum-oil microcapsules containing bacteria biofilms released small quantities of probiotic bacteria when exposed to pH 3.0 for 90 min but also maintained their H. pylori inhibitory activity.
GABA is a naturally occurring amino acid, but it is non-proteinogenic in nature. GABA is a bioactive inhibitor of neurotransmission in the mammalian central nervous system. It is generally found in tea, vegetables, cereals, and fermented foods such as kimchi, miso, and tempeh, but only in small amounts. GABA is also sold as a dietary supplement in many countries, because it has relaxing, anti-anxiety, anti-cancer, and anti-diabetic effects, although there are doubts that it is able to cross the blood–brain barrier. On the other hand, low levels of GABA are linked to insomnia, anxiety, and weaker immune systems [161,162]. To enhance the nutritional potential of GABA, Pandey et al. [162] incorporated GABA together with probiotic Lactobacillus plantarum NCDC 414 in a single microcapsule composed of inulin, dextran, and maltodextrin using spray-drying. The optimal composition of microcapsules exhibited encapsulation efficiencies of 84.22% and 99.21% for GABA and L. plantarum, respectively. No significant differences in the viability of L. plantarum and GABA retention were found after 120 days of storage at 4 °C. Co-microencapsulation of these two substances has the potential for the development of a new kind of brain booster, although its impact on the peripheral nervous system function still needs to be evaluated.
3.2.2. Co-Encapsulation with Phytochemicals
Phytochemicals are bioactive compounds produced by plants, with ingestion linked to a reduction in the risk of major chronic diseases, including certain types of cancer as well as cardiovascular and neurodegenerative diseases [163]. They are commonly present in fruits, vegetables, grains, nuts, and legumes. Phytochemicals are classified into various groups, including carotenoids, cannabinoids, polyphenols (include flavonoids, stilbenes, tannins, lignans, and phenolic acids), alkaloids, curcuminoids, nitrogen-containing compounds, and organosulphur compounds. Flavonoids can be subdivided into flavonols, flavones, flavan-3-ols, flavanones, isoflavones, anthocyanins, and chalcones [106,108,164].
Polyphenols are common in the human diet, as they are abundantly present in a broad range of consumed fruits and vegetables as well as in products such as tea, coffee, wine, and chocolate. Thus, polyphenols are emerging as suitable prebiotic and synbiotic agents. The biological properties and possible beneficial effects of polyphenols are dependent on their biotransformation by gut microbiota and enterocyte enzymes into more bioavailable and simple forms in order to be easily absorbed by the GI tract [19,165,166]. This gives rise to numerous valuable benefits for the consumer, including a vast array of protective effects against viruses, bacteria, and protozoan parasites. Enzymatic transformations in the GI tract include elimination of glycosidic tailoring by gut microbiota of diverse genera (Lactobacillus, Eubacterium, and Bifidobacterium), resulting in the formation of aglycones [167]. A few articles deal with the possible pathways of microbial metabolism of polyphenols, with a particular emphasis on the finally absorbed compounds and their potential impact on human health [167,168]. There is evidence from animal and human studies that certain doses of selected polyphenols may modify gut microbial composition, and while some bacterial groups can be inhibited, other microorganisms benefit and expand [169]. For example, tea phenols and their derivatives have significantly reduced the growth of known pathogens such as C. difficile, C. perfringens, and Bacteroides spp., while commensal anaerobes such as Clostridium spp. and Bifidobacterium spp. and certain probiotics such as Lactobacillus spp. are less affected [170]. Song et al. [170] investigated the metabolic effect of red pitaya fruit (Hylocereus polyrhizus) β-cyanins on high-fat diet-fed mice and detected protective effects against diet-induced obesity and its related metabolic disorders. β-cyanins are also able to modulate gut microbiota, especially decreases in the ratio of Firmicutes and Bacteroidetes, with increases in the relative abundance of Akkermansia spp. Akkermansia muciniphila is a gram-negative anaerobic mucin-degrading bacterium and is reduced in several inflammatory and metabolic disorders, including obesity, type-2 diabetes, and inflammatory bowel diseases. A. muciniphila improves gut barrier function associated with the stimulation of mucins, increase in thickness of the colonic mucus layer, and improvement of the enterocyte monolayer integrity [171,172]. Furthermore, it sustains intestinal barrier integrity, regulates host inflammatory responses caused by a high-fat diet, reduces low-grade inflammation in obese animal models and in patients with metabolic syndrome, and positively affects metabolic responses such as the production of beneficial SCFA [173,174,175,176,177,178]. Chang et al. [179] successfully co-encapsulated Akkermansia muciniphila 139 in succinate-grafted alginate doped with EGCG by spray-drying. A. muciniphila encapsulated in modified alginate with EGCG was significantly protected compared with free cells and the unmodified alginate-coated probiotics from sGIC for 90 min. It was shown that EGCG filled the pores and cracks in the microcapsules during the encapsulation process, and thus loss of viability caused by oxygen was blocked effectively due to the antioxidant capacity of EGCG. Further studies focused on co-encapsulation of polyphenols from green tea and other sources with lactic-acid-producing bacteria are listed in Table 1.

Table 1.
Studies of synbiotics comprising different types of phytochemicals.
Resveratrol, curcumin, and quercetin belong to biologically important natural phenols, widely known for their antioxidant, anti-carcinogenic, anti-inflammatory, and cardio-protective properties. Apart from their individual benefits, probiotics together with natural phenols have been demonstrated to perform a synergistic effect on host digestive health, such as the recovery of GI tract homeostasis. Although curcumin has individual benefits, it suffers from poor bioavailability and rapid degradation because it is sensitive to environmental conditions [19,180,181]. Therefore, Su et al. [182] co-encapsulated Lactobacillus rhamnosus GG (ATCC 53103) and curcumin within a propylene glycol alginate-based hydrogel delivery system (PGA-β-lgNPs-Cur). PGA-β-lgNPs-Cur composite hydrogel helped to reduce the chemical degradation of curcumin and increased the survival of L. rhamnosus GG during UV light exposure and long-term storage. Over four weeks of storage, up to 91.3% of curcumin remained chemically stable and 9.7 log CFU/g cells survived. PGA-β-lgNPs-Cur composite was also able to impede the release of prebiotic curcumin in the first 60 min of exposure to sGIC activity. Up to 8.9 log CFU/mL of viable L. rhamnosus GG could be detected when trapped in the composite hydrogel matrix after incubation in sGIC for 180 min [182]. Resveratrol, a scavenger of reactive oxygen species (ROS) and other free radicals, is metabolized by hepatic and gut microbiota enzymes, the result of which can impact gut microbiota diversity and composition, including inhibiting Enterococcus faecalis, increasing the Bacteroidetes-to-Firmicutes ratio, and increasing the Lactobacillus and Bifidobacterium populations [183]. Vázquez-Maldonado et al. [184] co-encapsulated Bacillus clausii and resveratrol in inulin and lactose by spray-drying. Co-microencapsulation of Bacillus clausii with resveratrol showing good efficacy: 8.52 ± 0.10 log CFU/g for inulin and 8.62 ± 0.06 log CFU/g for lactose capsules. Resveratrol carried alone in inulin capsules showed the highest antioxidant activity (26%), and in co-encapsulated forms with bacteria showed similar activity against free radicals: 21% in inulin and 23% in lactose. However, the detrimental effects of quercetin co-encapsulated with probiotics on bacterial viability was observed by Chávarri et al. [185]. Cell viability and encapsulation yields were low after co-encapsulating Bifidobacterium bifidum and Lactobacillus gasseri with quercetin.
3.2.3. Co-Encapsulation with Dietary Fibers
Soluble and insoluble dietary fibers are defined as non-digestible carbohydrate polymers of three or more monomeric units that resist digestion in the small intestine and are selectively utilized by host microorganisms in the large intestine, with beneficial effect on human health, including:
- non-starch polysaccharides: cellulose, hemicelluloses, pectins, hydrocolloids;
- resistant oligosaccharides: FOS, GOS, inulin (which can selectively promote the growth of Bifidobacterium spp. and Lactobacillus spp.), and other resistant oligosaccharides;
- resistant starch: consisting of physically enclosed starch, chemically and/or physically modified starches, retrograded amylose, and some types of raw starch granules;
- lignin associated with the DF polysaccharides;
- chemically synthesized fibers [17,189,190,191].
In everyday life, whole grains, fruits, nuts, pulses, and other kinds of vegetables represent the main food sources of dietary fibers. As powerful energy sources for most gut microbes, dietary fibers can directly alter species composition and colony size and prevent pathogen adhesion. In addition, the fermentation process can be altered by dietary fibers, which normally leads to production of key physiological metabolites such as SCFA (namely acetate, propionate, and butyrate). Thus, dietary fibers affect the supply of important metabolites and by-products for other microorganisms in a cross-feeding process. Healthy colonic microbiota is characterized by SCFA production, of which butyrate is utilized as the main energy source for colonocytes, stimulating their growth and also the production of cytokines, which maintain barrier integrity and function [83,192,193,194,195]. Increasing levels of SCFA in the gut helps to reduce the luminal pH, creating a desirable environment for beneficial bacteria, inhibiting the growth of pathogenic agents, and enhancing mineral absorption, vitamin bioavailability, and barrier function [63,192]. For example, luminal pH alteration can change the bacterial profile of acid-sensitive species and stimulate production of microbiota-derived butyrate by Faecalibacterium prausnitzii and Eubacterium as well as Anaerostipes and Roseburia species. Although, Bifidobacterium spp. are not able to produce butyrate, they are associated with a butyrogenic effect through cross-feeding between Bifidobacterium spp. and butyrate producing bacteria [61,196,197]. Emerging research is heavily focused on microbiota–gut–brain communication, the so-called “gut-brain axis”, which conceptually provides bidirectional signaling between the gut microbiota and the central nervous system (CNS) [17]. Dalile et al. [198] clearly reviewed the role of SCFA in microbiota-gut-brain cross-talk. Dietary fiber substrates for SCFA-producing bacteria are highlighted, and the effects of SCFA on signaling pathways, including neural, humoral, immune, and endocrine routes, are identified. For example, inulin and FOS are substrates for Bacteroides and Faecalibacterium fermenting species, whereas GOS are utilized by Bifidobacterium, and resistant starch by Ruminococcus and Bacteroides, etc.
In several studies, probiotics have been successfully incorporated together with different types of dietary fibers into microcapsules, enhancing their storage stability, protection during processing, and passage through the GI tract [199,200]. One of the most extensively studied dietary fibers is inulin, a thermally stable and poorly soluble form of fructan. Inulin confers protection from oxidative stress (e.g., indirectly scavenging ROS by enhancing SCFA production and preventing lipid peroxidation in the stomach). It has also been used as a building material for microcapsules in order to protect probiotic cargo. Therefore, inulin has a multifunctional character; in addition to serving as a coating material, it serves as a prebiotic substrate [201,202]. Xavier et al. [202] confirmed that 10% inulin is a suitable coating agent to protect microencapsulated L. acidophilus La-5 during the spray-drying process and sGIC. Atia et al. [128] studied the effect of inulin addition to alginate microcapsules and reported its ability to protect probiotic strains Pediocuccus acidilactici UL5, L. reuteri, and L. salivarius. Microcapsules with different inulin concentrations of 0%, 5%, 10%, 15%, and 20% (w/v) in 2% (w/v) alginate solution were prepared, and the most effective was the alginate matrix with 5% inulin. Antimicrobial and probiotic properties of bacterial strains were not affected by co-encapsulation, and protection against low pH was increased by the addition of inulin. Kumherová et al. [203] co-encapsulated B. animalis subsp. lactis BB-12 with inulin and/or ascorbic acid by an extrusion method in alginate or by emulsion in milk protein. Co-encapsulation in a protein matrix enriched with 1% (w/w) inulin and 0.5% (w/w) antioxidant ascorbic acid showed a higher survival rate of probiotic bacteria during sGIC when compared with free cells or bacteria encapsulated in alginate. Inulin was also successfully co-encapsulated with Bifidobacterium mixed cultures, L. plantarum CCTCC M 2,014,170 and L. rhamnosus GG, and bacterial survival and resistance to sGIC was enhanced [204,205,206]. Table 2 summarizes studies where inulin and other dietary fibers, including FOS, GOS, polydextrose, trehalose, hi-maize, rice bran, resistant starch, and lacticol, were studied to assess the protection of bacterial cargo and overall improved efficacy of synbiotic activity.

Table 2.
Probiotics co-encapsulated with dietary fibers.
FOS and GOS from natural sources or enzymatically synthetized are popular compounds utilized by various food and medical industries because they are effective in combatting pathogens and are easily fermented by beneficial gut microbiota into SCFA [207]. Sathyabama et al. [208] co-encapsulated natural carbohydrate sources, namely sugar beet (rich in FOS) and chicory (rich in inulin and FOS), with probiotic strains Enterococcus faecium and Staphylococcus succinus in alginate by emulsification. This study reported that chicory beads were more stable while exposed to sGIC, but sugar beads resulted in a higher survival rate of probiotic strains under the action of bile. These are important considerations when designing microcapsules, since artificial food additives are also linked with the emergence of new epidemic pathogens, such as the trehalose microcapsule expansion of C. difficile.
4. Gut Microbiota Modulation by Immobilized Probiotics
Immobilization and encapsulation are two different processes, the terms of which are used interchangeably. Immobilization refers to the trapping of material within or throughout a carrier’s matrix; a small percentage of immobilized cargo is exposed to the environment at the carrier’s surface, and thus the immobilization process is not efficient in protecting the whole cargo. On the other hand, encapsulated cargo is contained within the coating material, which is formed continuously around an inner core matrix, as detailed in Section 3.2 (Figure 2). It is well established that the immobilization of probiotics enhances the viability of cultures and reduces the impact of environmental inactivating factors such as physicochemical changes during processing, storage, functional food production, and passage through the GI tract. Similarly to encapsulation, the biocompatible matrix used for immobilization should allow the bidirectional transport of nutrients and grow factors, as these are essential for cell metabolism and also for elimination of waste products [219,220,221,222]. The effectiveness of cell immobilization strategies depends mainly on the correct choice of the matrix used, which can be obtained from natural sources or manufactured. Various biocompatible supports have been used for the immobilization of lactic-acid-producing bacteria. Wheat grains, with their prebiotic character, provide the proteins, starch, dietary fibers, carbohydrates, minerals, and vitamins required for the development and preservation of bacteria and also promote human health. These were used as support for lactic-acid-producing bacteria by Sidira et al. [25,223,224] and Bosnea et al. [225]. Soybean grains, as a new type of support for the immobilization of L. casei CSL3, were used by Vitola et al. [226]. Milk proteins, such as whey protein and casein, can be used as natural carriers for microorganisms in functional food products, including yogurt or cheese, due to their structural and physicochemical properties. They were efficiently used as supports for immobilization of lactic-acid-producing bacteria [38,227,228] and kefir co-cultures [229,230]. However, the challenge in preparation of such dairy products is ensuring that sufficient numbers of viable probiotics are maintained until the product is consumed as well as during passage through the GI tract to its site of action [21,231]. Fruit pieces were previously also used as immobilization supports of lactic-acid-producing bacteria [221,222,232]. Fruit pieces contain natural prebiotic cellulose, which may contribute to cell survival and proliferation in the colon, thus enhancing the beneficial effects of the probiotics. Other types of fruit matrices are listed in Table 3. Jayani et al. [40] studied bacterial cellulose nanofiber as a delivery vehicle for the immobilization of L. acidophilus 016 through the adsorption-incubation technique. The viable cell count after 24 days of storage was 7.63 log CFU/g, compared with 10.72 log CFU/g immediately after immobilization. Bacterial cellulose exhibits exceptional properties, including high purity, high water retention capacity, a comprehensive crystalline network structure, good chemical stability, biocompatibility, and biodegradability, all of which are highly desired traits in many applications [40,233,234]. Furthermore, Nwagu et al. [235] used probiotic Bacillus sp. spores as immobilization support for bioactive agent bromelain. The immobilized bromelain showed significantly greater storage and thermal stability than the free bromelain. In follow-up research, Ugwuodo et al. [236] showed that the immobilized bromelain exhibited approximately 0.9-fold anti-inflammatory activity compared to free bromelain. Recent studies, summarized in Table 3, are focused on the viability of immobilized probiotics after exposure to sGIC and the potential use as a component of functional food products.
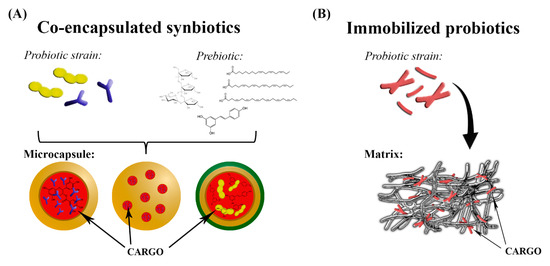
Figure 2.
Schematic illustration of co-encapsulation (A) and immobilization (B) technologies.

Table 3.
Recent reports about viability of immobilized probiotics in simulated gastrointestinal conditions.
5. “Side Effects” of Gut Microbiota Functional Redundancy
It is also important to consider the contribution of stability, resistance, resilience, and redundancy features as it relates to the functional status of the native microbiota after any kind of intervention. If the gut microbiota is not resistant to disturbance, it alters its composition of species, genes, proteins, and functions. One way that microbiota is able to recover from such a functional disturbance is to promote growth and incorporate unrelated microbial species into the initial community; these unrelated taxa possess genes and proteins that are functionally redundant, promoting microbiota core functions despite compositional changes. For example, microbiota that are different at a compositional level show functional degeneracy by maintaining uniform profiles of proteins and metabolites [245,246,247,248]. Functional redundancy represents a natural ability of microbiota communities to restore core functions, emphasizing the need to closely monitor functional changes at the molecular level of host-microbe interactions. This becomes especially important when considering modulation of microbiota communities through microbial treatments that use co-microencapsulated synbiotics or immobilized probiotics.
6. Conclusions and Future Directions
Today, there is intense demand for the industrial production of multiple bioactive ingredients for co-encapsulation into microcapsules to enhance the stability and efficiency of probiotics as well as to decrease cost of the final product. Co-encapsulation of synbiotics (probiotic and prebiotic products) into single delivery systems has future profit-making potential, because numerous studies support their daily consumption to help to combat disease and maintain gut health and overall consumer well-being. However, several conditions need to be fulfilled in order to reduce losses during production of microcapsules and their subsequent application. First, encapsulation techniques and carrier materials need to be carefully selected. Suitable probiotic and prebiotic candidates with or without interdependency should be strategically chosen with precision microbial therapy in mind. The search for new prebiotic compounds and for the right combinations of prebiotics and probiotics with a synergistic effect on human health should be relentless, leaving no stone unturned. Despite the well-known extraordinary properties of synbiotics, additional in vivo and clinical trials are essential to demonstrate efficacy, and they need to be sufficiently powered with a randomized placebo control study design. To date, only few animal studies have been done to evaluate the effectiveness of co-encapsulated synbiotics in vivo [249,250,251]. This is particularly important when using microcapsules containing multiple bioactive ingredients, as the positive and negative interactions of synbiotics together with the encapsulation material become paramount to investigate. The mechanism of action of various co-encapsulated synbiotics in the host also need to be elucidated.
Author Contributions
Conceptualization, M.K.; writing—original draft preparation, M.K. and I.B.; writing—review and editing, T.C.S. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education of Slovak Republic and Academy of Sciences VEGA no. 1/0393/20; the Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic for the Structural Funds of EU, under project ITMS 313011V336; the grant of the Pavol Jozef Safarik University in Kosice, Slovakia, under contract no. VVGS-2020-1506.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors thank Anna Kamlarova, Department of Experimental Medicine, Faculty of Medicine, and Terezia Kiskova, Department of Animal Physiology, Institute of Biology and Ecology, Faculty of Science, both of Pavol Jozef Safarik University in Kosice, Slovakia, for the help with nomenclature of microorganisms and consultations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peluzio, M.D.C.G.; Martinez, J.A.; Milagro, F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021, 108, 11–26. [Google Scholar] [CrossRef]
- Wu, S.; Bekhit, A.E.-D.A.; Wu, Q.; Chen, M.; Liao, X.; Wang, J.; Ding, Y. Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci. Technol. 2021, 108, 164–176. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Saha, S.; Das, S. Metagenomic Surveys of Gut Microbiota. Genom. Proteom. Bioinform. 2015, 13, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Shreiner, A.B.; Gillilland, M.G.; Kitamoto, S.; Ishii, C.; Hirayama, A.; Kuffa, P.; El-Zaatari, M.; Grasberger, H.; Seekatz, A.M.; et al. Functional Characterization of Inflammatory Bowel Disease–Associated Gut Dysbiosis in Gnotobiotic Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 468–481. [Google Scholar] [CrossRef]
- Eid, H.M.; Wright, M.L.; Kumar, N.V.A.; Qawasmeh, A.; Hassan, S.T.S.; Mocan, A.; Nabavi, S.M.; Rastrelli, L.; Atanasov, A.G.; Haddad, P.S. Significance of Microbiota in Obesity and Metabolic Diseases and the Modulatory Potential by Medicinal Plant and Food Ingredients. Front. Pharmacol. 2017, 8, 387. [Google Scholar] [CrossRef]
- Sharma, V.R.; Singh, M.; Kumar, V.; Yadav, M.; Sehrawat, N.; Sharma, D.K.; Sharma, A.K. Microbiome dysbiosis in cancer: Exploring therapeutic strategies to counter the disease. Semin. Cancer Biol. 2021, 70, 61–70. [Google Scholar] [CrossRef]
- Wu, W.; Kong, Q.; Tian, P.; Zhai, Q.; Wang, G.; Liu, X.; Zhao, J.; Zhang, H.; Lee, Y.K.; Chen, W. Targeting Gut Microbiota Dysbiosis: Potential Intervention Strategies for Neurological Disorders. Engineering 2020, 6, 415–423. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Berin, M.C. Food allergy and the microbiome: Current understandings and future directions. J. Allergy Clin. Immunol. 2019, 144, 1468–1477. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The intervention of unique plant polysaccharides—Dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. LWT 2019, 116, 108501. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Bosnea, L.; Kanellaki, M.; Ganatsios, V.; Koutinas, A.A. Wheat bran as prebiotic cell immobilisation carrier for industrial functional Feta-type cheese making: Chemical, microbial and sensory evaluation. Biocatal. Agric. Biotechnol. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Bélanger, G. Effects of storage conditions, microencapsulation and inclusion in chocolate particles on the stability of probiotic bacteria in ice cream. Int. Dairy J. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Tursi, A.; Papa, A. Intestinal microbiome modulation during covid-19: Another chance to manage the disease? Gastroenterology 2020. [Google Scholar] [CrossRef]
- Janda, L.; Mihalčin, M.; Šťastná, M. Is a healthy microbiome responsible for lower mortality in COVID-19? Biologia 2021, 76, 819–829. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021, 108, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zha, Y.; Chong, H.; Zhong, C.; Ning, K. Utilizing microbiome approaches to assist source tracking, treatment and prevention of COVID-19: Review and assessment. Comput. Struct. Biotechnol. J. 2020, 18, 3615–3622. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum. Microbiome J. 2020, 17, 100073. [Google Scholar] [CrossRef]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef]
- Din, A.U.; Mazhar, M.; Waseem, M.; Ahmad, W.; Bibi, A.; Hassan, A.; Ali, N.; Gang, W.; Qian, G.; Ullah, R.; et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021, 133, 110947. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol. 2020, 102, 178–192. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. 2006. Available online: www.fao.org (accessed on 18 February 2021).
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.D.C.O.; Pitangui, N.D.S.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Sidira, M.; Santarmaki, V.; Kiourtzidis, M.; Argyri, A.A.; Papadopoulou, O.; Chorianopoulos, N.; Tassou, C.; Kaloutsas, S.; Galanis, A.; Kourkoutas, Y. Evaluation of immobilized Lactobacillus plantarum 2035 on whey protein as adjunct probiotic culture in yoghurt production. LWT 2017, 75, 137–146. [Google Scholar] [CrossRef]
- Rodrigues, F.; Cedran, M.; Bicas, J.; Sato, H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial Cellulose Nano Fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Kosciow, K.; Deppenmeier, U. Characterization of three novel β-galactosidases from Akkermansia muciniphila involved in mucin degradation. Int. J. Biol. Macromol. 2020, 149, 331–340. [Google Scholar] [CrossRef]
- Poletto, G.; Raddatz, G.C.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Wagner, R.; de Menezes, C.R. Study of viability and storage stability of Lactobacillus acidophillus when encapsulated with the prebiotics rice bran, inulin and Hi-maize. Food Hydrocoll. 2019, 95, 238–244. [Google Scholar] [CrossRef]
- Pereira, G.V.D.M.; Coelho, B.D.O.; Júnior, A.I.M.; Soccol, V.T.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut microbiota-derived vitamins—Underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.; Laiño, J.E.; Del Valle, M.J.; Vannini, V.; Van Sinderen, D.; Taranto, M.; De Valdez, G.F.; De Giori, G.S.; Sesma, F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Xue, F.; Yu, S.-F.; Li, X.-S.; Liu, L.; Jia, Y.-Y.; Yan, W.-J.; Tan, Q.-R.; Wang, H.-N.; Peng, Z.-W. Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. J. Affect. Disord. 2021, 282, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jin, H.; Kwok, L.-Y.; Sun, Z.; Liong, M.-T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef] [PubMed]
- Chahwan, B.; Kwan, S.; Isik, A.; van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Wu, Y.-J.; Hu, C.-Y. Monosaccharide composition influence and immunomodulatory effects of probiotic exopolysaccharides. Int. J. Biol. Macromol. 2019, 133, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.-Y. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef]
- Valdovinos-García, L.; Abreu, A.; Valdovinos-Díaz, M. Probiotic use in clinical practice: Results of a national survey of gastroenterologists and nutritionists. Rev. Gastroenterol. Mex. 2019, 84, 303–309. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Li, Q.; Shi, Z.; Wu, H.; Zhang, H.; Tang, L.; Yi, R.; Su, H.; Sun, X. Oral administration of a mixture of probiotics protects against food allergy via induction of CD103+ dendritic cells and modulates the intestinal microbiota. J. Funct. Foods 2019, 55, 65–75. [Google Scholar] [CrossRef]
- Abdrabou, A.M.; Osman, E.Y.; Aboubakr, O.A. Comparative therapeutic efficacy study of Lactobacilli probiotics and citalopram in treatment of acute stress-induced depression in lab murine models. Hum. Microbiome J. 2018, 10, 33–36. [Google Scholar] [CrossRef]
- Sidira, M.; Galanis, A.; Ypsilantis, P.; Karapetsas, A.; Progaki, Z.; Simopoulos, C.; Kourkoutas, Y. Effect of Probiotic-Fermented Milk Administration onGastrointestinal Survival of Lactobacillus casei ATCC 393 and Modulation of Intestinal Microbial Flora. Microb. Physiol. 2010, 19, 224–230. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Probiotics as treatment for food allergies among pediatric patients: A meta-analysis. World Allergy Organ. J. 2018, 11, 25. [Google Scholar] [CrossRef]
- Tripathi, M.; Giri, S. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Kailasapathy, K. Encapsulation technologies for functional foods and nutraceutical product development. CAB Rev. 2009, 4, 1–19. [Google Scholar] [CrossRef]
- Doleyres, Y.; Lacroix, C. Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int. Dairy J. 2005, 15, 973–988. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of Probiotics and Prebiotics with the Gut Microbiota. In Progress in Molecular Biology and Translational Science; Academic Press: Amsterdam, The Netherlands, 2020; Volume 171, pp. 265–300. [Google Scholar]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.I.; Kambashi, B.; Rodriguez, J.; Cani, P.D.; et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Ramnani, P.; Gaudier, E.; Bingham, M.; Van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: A human intervention study. Br. J. Nutr. 2010, 104, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kleessen, B.; Schwarz, S.; Boehm, A.; Fuhrmann, H.; Richter, A.; Henle, T.; Krueger, M. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br. J. Nutr. 2007, 98, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kjølbæk, L.; Benítez-Páez, A.; del Pulgar, E.M.G.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Vigsnæs, L.K.; Holck, J.; Meyer, A.S.; Licht, T.R. In VitroFermentation of Sugar Beet Arabino-Oligosaccharides by Fecal Microbiota Obtained from Patients with Ulcerative Colitis To Selectively Stimulate the Growth of Bifidobacterium spp. and Lactobacillus spp. Appl. Environ. Microbiol. 2011, 77, 8336–8344. [Google Scholar] [CrossRef]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Cuiv, P.O.; Morrison, M.; Gidley, M.J. Food Starch Structure Impacts Gut Microbiome Composition. mSphere 2018, 3, e00086-18. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.; Miguel, M.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro- and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wan, Z.; Ou, A.; Liang, X.; Guo, X.; Zhang, Z.; Wu, L.; Xue, X. Monofloral honey from a medical plant, Prunella Vulgaris, protected against dextran sulfate sodium-induced ulcerative colitisviamodulating gut microbial populations in rats. Food Funct. 2019, 10, 3828–3838. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; He, S.; Gao, C.; Wang, C.; Shao, Y. Effects of Natural Flavonoid Isoorientin on Growth Performance and Gut Microbiota of Mice. J. Agric. Food Chem. 2018, 66, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, S.; Fan, S.; Ma, Y.; Li, D.; Tao, Y.; Han, Y. Encapsulation of bioactive polyphenols by starch and their impacts on gut microbiota. Curr. Opin. Food Sci. 2021, 38, 102–111. [Google Scholar] [CrossRef]
- Scheeler, A. Where Stool is a Drug: International Approaches to Regulating the use of Fecal Microbiota for Transplantation. J. Law Med. Ethics 2019, 47, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Haziri, D.; Brzozowski, T.; Hess, T.; Heyman, S.; Kwiecien, S.; Konturek, S.J.; Koziel, J. Emerging role of fe-cal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 2015, 66, 483–491. [Google Scholar]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.; Bogaerde, J.V.D.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kakiyama, G.; Savidge, T.; Takei, H.; Kassam, Z.A.; Fagan, A.; Gavis, E.A.; Pandak, W.M.; Nittono, H.; Hylemon, P.B.; et al. Antibiotic-Associated Disruption of Microbiota Composition and Function in Cirrhosis Is Restored by Fecal Transplant. Hepatology 2018, 68, 1549–1558. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation inClostridium difficileinfection is associated with treatment outcome. Gut 2017, 67, 634–643. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, T.; El-Nezami, H.; Savidge, T.C. Food ingredients in human health: Ecological and metabolic perspectives implicating gut microbiota function. Trends Food Sci. Technol. 2020, 100, 103–117. [Google Scholar] [CrossRef]
- Venema, K.; Abbeele, P.V.D. Experimental models of the gut microbiome. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Abbeele, P.V.D.; Roos, S.; Eeckhaut, V.; MacKenzie, D.A.; Derde, M.; Verstraete, W.; Marzorati, M.; Possemiers, S.; Vanhoecke, B.; Van Immerseel, F.; et al. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb. Biotechnol. 2011, 5, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Spinler, J.K.; Auchtung, J.; Brown, A.; Boonma, P.; Oezguen, N.; Ross, C.L.; Luna, R.A.; Runge, J.; Versalovic, J.; Peniche, A.; et al. Next-Generation Probiotics Targeting Clostridium difficile through Precursor-Directed Antimicrobial Biosynthesis. Infect. Immun. 2017, 85, e00303-17. [Google Scholar] [CrossRef]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J. Dairy Sci. 2020, 103, 5816–5829. [Google Scholar] [CrossRef]
- Newman, A.M.; Arshad, M. The Role of Probiotics, Prebiotics and Synbiotics in Combating Multidrug-Resistant Organisms. Clin. Ther. 2020, 42, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Santiago-López, L.; Hernández-Mendoza, A.; Garcia, H.S.; Mata-Haro, V.; Vallejo-Cordoba, B.; González-Córdova, A.F. The effects of consuming probiotic-fermented milk on the immune system: A review of scientific evidence. Int. J. Dairy Technol. 2015, 68, 153–165. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Rodriguez, J.; Taminiau, B.; Amadieu, C.; Herpin, F.; Allaert, F.-A.; Cani, P.D.; Daube, G.; Bindels, L.B.; Delzenne, N.M. Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: A double-blind randomized placebo-controlled trial. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Phavichitr, N.; COLOR Study Group; Wang, S.; Chomto, S.; Tantibhaedhyangkul, R.; Kakourou, A.; Intarakhao, S.; Jongpiputvanich, S.; Roeselers, G.; Knol, J. Impact of synbiotics on gut microbiota during early life: A randomized, double-blind study. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, M.; Morvaridzadeh, M.; Agah, S.; Rahimlou, M.; Christopher, E.; Zadro, J.R.; Heshmati, J. Effect of Probiotic, Prebiotic, and Synbiotic Supplementation on Cardiometabolic and Oxidative Stress Parameters in Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Clin. Ther. 2021, 43, e71–e96. [Google Scholar] [CrossRef]
- Scorletti, E.; Afolabi, P.R.; Miles, E.A.; Smith, D.E.; Almehmadi, A.; AlShathry, A.; Childs, C.E.; Del Fabbro, S.; Bilson, J.; Moyses, H.E.; et al. Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1597–1610.e7. [Google Scholar] [CrossRef]
- Askari, G.; Ghavami, A.; Shahdadian, F.; Moravejolahkami, A.R. Effect of synbiotics and probiotics supplementation on autoimmune diseases: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2021, 40, 3221–3234. [Google Scholar] [CrossRef]
- Kambale, R.M.; Nancy, F.I.; Ngaboyeka, G.A.; Kasengi, J.B.; Bindels, L.B.; Van der Linden, D. Effects of probiotics and synbiotics on diarrhea in undernourished children: Systematic review with meta-analysis. Clin. Nutr. 2021, 40, 3158–3169. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sánchez, M.A.; Herisson, F.M.; Cluzel, G.L.; Caplice, N.M. Metabolic syndrome and synbiotic targeting of the gut microbiome. Curr. Opin. Food Sci. 2021, 41, 60–69. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Bellissimo, N.; de Zepetnek, J.O.T.; Brett, N.R.; Mazloomi, S.M.; Fararouie, M.; Bedeltavana, A.; Famouri, M.; Mazloom, Z. The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: A 10-week randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 565–574. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2020, 1–25. [Google Scholar] [CrossRef]
- Román, G.; Jackson, R.; Gadhia, R.; Román, A.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Cui, L.-H.; Yan, C.-G.; Li, H.-S.; Kim, W.-S.; Hong, L.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. A New Method of Producing a Natural Antibacterial Peptide by Encapsulated Probiotics Internalized with Inulin Nanoparticles as Prebiotics. J. Microbiol. Biotechnol. 2018, 28, 510–519. [Google Scholar] [CrossRef]
- García-Díaz, M.; Birch, D.; Wan, F.; Nielsen, H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv. Drug Deliv. Rev. 2018, 124, 107–124. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: An overview. J. Funct. Foods 2018, 48, 65–84. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.-M.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Co-Microencapsulation of Flavonoids from Yellow Onion Skins and Lactic Acid Bacteria Lead to Multifunctional Ingredient for Nutraceutical and Pharmaceutics Applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [CrossRef]
- Annunziata, G.; Jiménez-García, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Yus, C.; Gracia, R.; Larrea, A.; Andreu, V.; Irusta, S.; Sebastian, V.; Mendoza, G.; Arruebo, M. Targeted Release of Probiotics from Enteric Microparticulated Formulations. Polymer 2019, 11, 1668. [Google Scholar] [CrossRef]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Sánchez, M.T.; Ruiz, M.A.; Lasserrot, A.; Hormigo, M.; Morales, M.E. An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: Protection, survival and stability study. Food Hydrocoll. 2017, 69, 67–75. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Forghani, Z.; Jafari, S.M. A systematic review and meta-analysis of fish oil encapsulation within different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fan, G.-Q.; Zhang, Z.; Zhang, R.; Deng, Z.-Y.; McClements, D.J. Encapsulation of omega-3 fatty acids in nanoemulsions and microgels: Impact of delivery system type and protein addition on gastrointestinal fate. Food Res. Int. 2017, 100, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Lovillo, A.; Beristain, C.; Pascual, L.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT 2016, 73, 191–196. [Google Scholar] [CrossRef]
- El-Abd, M.M.; Abdel-Hamid, M.; El-Sayed, S.H.; El Metwaly, A.H.; El-Demerdash, M.E.; Zeinab, F.A.M. Viability of Micro-encapsulated Probiotics Combined with Plant Extracts in Fermented Camel Milk under Simulated Gastrointestinal Condi-tions. Middle East J. Appl. Sci. 2018, 8, 837–850. [Google Scholar]
- Zhang, Y.; Lin, J.; Zhong, Q. S/O/W emulsions prepared with sugar beet pectin to enhance the viability of probiotic Lactobacillus salivarius NRRL B-30514. Food Hydrocoll. 2016, 52, 804–810. [Google Scholar] [CrossRef]
- Alehosseini, A.; del Pulgar, E.-M.G.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Fabra, M.J.; Sanz, Y.; Sarabi-Jamab, M.; Ghorani, B.; Lopez-Rubio, A. Unpurified Gelidium-extracted carbohydrate-rich fractions improve probiotic protection during storage. LWT 2018, 96, 694–703. [Google Scholar] [CrossRef]
- Alfaro-Galarza, O.; Villegas, E.O.L.; Rivero-Perez, N.; Maruri, D.T.-; Jiménez-Aparicio, A.; Palma-Rodríguez, H.; Vargas-Torres, A. Protective effects of the use of taro and rice starch as wall material on the viability of encapsulated Lactobacillus paracasei subsp. Paracasei. LWT 2020, 117, 108686. [Google Scholar] [CrossRef]
- Colín-Cruz, M.; Pimentel-González, D.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Pinto, S.S.; Fritzen-Freire, C.B.; Benedetti, S.; Murakami, F.S.; Petrus, J.C.C.; Prudêncio, E.S.; Amboni, R.D. Potential use of whey concentrate and prebiotics as carrier agents to protect Bifidobacterium-BB-12 microencapsulated by spray drying. Food Res. Int. 2015, 67, 400–408. [Google Scholar] [CrossRef]
- Okuro, P.K.; Thomazini, M.; Balieiro, J.C.; Liberal, R.D.; Favarotrindade, C.S. Co- encapsulation of Lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Res. Int. 2013, 53, 96–103. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Valente, A.J.; Miguel, M.G.; Lindman, B. Exploring the prebiotic effect of cyclodextrins on probiotic bacteria entrapped in carboxymetyl cellulose-chitosan particles. Colloids Surf. B Biointerfaces 2018, 168, 156–162. [Google Scholar] [CrossRef]
- Damodharan, K.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Co-encapsulation of lactic acid bacteria and prebiotic with alginate-fenugreek gum-locust bean gum matrix: Viability of encapsulated bacteria under simulated gastrointestinal condition and during storage time. Biotechnol. Bioprocess Eng. 2017, 22, 265–271. [Google Scholar] [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT 2015, 63, 685–690. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. LWT 2019, 110, 102–109. [Google Scholar] [CrossRef]
- Atia, A.; Gomaa, A.; Fernandez, B.; Subirade, M.; Fliss, I. Study and Understanding Behavior of Alginate-Inulin Synbiotics Beads for Protection and Delivery of Antimicrobial-Producing Probiotics in Colonic Simulated Conditions. Probiotics Antimicrob. Proteins 2018, 10, 157–167. [Google Scholar] [CrossRef]
- Serrano-Casas, V.; Pérez-Chabela, M.L.; Cortés-Barberena, E.; Totosaus, A. Improvement of lactic acid bacteria viability in acid conditions employing agroindustrial co-products as prebiotic on alginate ionotropic gel matrix co-encapsulation. J. Funct. Foods 2017, 38, 293–297. [Google Scholar] [CrossRef]
- Seifert, A.; Kashi, Y.; Livney, Y.D. Delivery to the gut microbiota: A rapidly proliferating research field. Adv. Colloid Interface Sci. 2019, 274, 102038. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Ding, Z.; Hou, D.; Prakash, S.; Zhao, Y.; Fan, Z.; Zhang, D.; Wang, Z.; Liu, M.; Han, J. Influence of polysaccharide as co-encapsulant on powder characteristics, survival and viability of microencapsulated Lactobacillus paracasei Lpc-37 by spray drying. J. Food Eng. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Black Currant Extract and Lactic Acid Bacteria in Biopolymeric Matrices. Molecules 2020, 25, 1700. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Gomaa, A.; Subirade, M. Co-encapsulation of Lactobacillus helveticus cells and green tea extract: Influence on cell survival in simulated gastrointestinal conditions. J. Funct. Foods 2016, 26, 451–459. [Google Scholar] [CrossRef]
- Phoem, A.N.; Mayiding, A.; Saedeh, F.; Permpoonpattana, P. Evaluation of Lactobacillus plantarum encapsulated with Eleutherine americana oligosaccharide extract as food additive in yoghurt. Braz. J. Microbiol. 2019, 50, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’Ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Zhang, S.; Zhang, W.; Barrow, C.J. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012, 131, 639–644. [Google Scholar] [CrossRef]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Andrejčáková, Z.; Sopková, D.; Vlčková, R.; Hertelyová, Z.; Gancarčíková, S.; Nemcová, R. The Application of Lactobacillus reuteri CCM 8617 and Flaxseed Positively Improved the Health of Mice Challenged with Enterotoxigenic E. coli O149:F4. Probiotics Antimicrob. Proteins 2019, 12, 937–951. [Google Scholar] [CrossRef]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Ruiz, J.C.R.; Ortiz-Vázquez, E.; Campos, M.R.S. Encapsulation of vegetable oils as source of omega-3 fatty acids for enriched functional foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liang, L.; Zhang, Z.; Deng, Z.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation in nanoemulsions and filled microgels fortified with omega-3 fatty acids using casein as a natural antioxidant. Food Hydrocoll. 2017, 63, 240–248. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Barrow, C.J.; Nolan, C.; Holub, B.J. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J. Funct. Foods 2009, 1, 38–43. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Kankaanpää, P.E.; Salminen, S.J.; Isolauri, E.; Lee, Y.K. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef]
- Nemcova, R.; Borovská, D.; Koščová, J.; Gancarcikova, S.; Mudroňová, D.; Buleca, V.; Pistl, J. The effect of supplementation of flax-seed oil on interaction of Lactobacillus plantarum—Biocenol™ LP96 and Escherichia coli O8:K88ab:H9 in the gut of germ-free piglets. Res. Vet. Sci. 2012, 93, 39–41. [Google Scholar] [CrossRef]
- Bomba, A.; Nemcová, R.; Gancarcíková, S.; Herich, R.; Pistl, J.; Révajová, V.; Jonecová, Z.; Bugarský, A.; Levkut, M.; Kasteĺ, R.; et al. The influence of omega-3 polyunsaturated fatty acids (omega-3 pufa) on lactobacilli adhesion to the intestinal mucosa and on immunity in gnotobiotic piglets. Berl. Munch. Tierarztl. Wochenschr. 2003, 116, 312–316. [Google Scholar]
- Durkin, L.; Childs, C.; Calder, P. Omega-3 Polyunsaturated Fatty Acids and the Intestinal Epithelium—A Review. Foods 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Boyko, N.; Tsyryuk, O.; Beregova, T.; Ostapchenko, L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res. Clin. Pract. 2018, 141, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Mykhalchyshyn, G.; Molochek, N.; Savchuk, O.; Kyriienko, D.; Komisarenko, I. Probiotic and omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance, improves glycemia and obesity parameters in individuals with type 2 diabetes: A randomised controlled trial. Obes. Med. 2020, 19, 100248. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.; Adhikari, B.P. In-vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chem. 2017, 227, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Eratte, D.; Wang, B.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival and fermentation activity of probiotic bacteria and oxidative stability of omega-3 oil in co-microcapsules during storage. J. Funct. Foods 2016, 23, 485–496. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Vega-Sagardía, M.; Rocha, J.; Sáez, K.; Smith, C.T.; Gutierrez-Zamorano, C.; García-Cancino, A. Encapsulation, with and without oil, of biofilm forming Lactobacillus fermentum UCO-979C strain in alginate-xanthan gum and its anti- Helicobacter pylori effect. J. Funct. Foods 2018, 46, 504–513. [Google Scholar] [CrossRef]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) Administration on Stress and Sleep in Humans: A Systematic Review. Front. Neurosci. 2020, 14, 14. [Google Scholar] [CrossRef]
- Pandey, P.; Mishra, H.N. Co-microencapsulation of γ-Aminobutyric acid (GABA) and Probiotic Bacteria in Thermostable and Biocompatible Exopolysaccharides Matrix. LWT 2021, 136, 110293. [Google Scholar] [CrossRef]
- Shrishrimal, N.; Das, D. Retraction Notice: Phytochemicals from Plants to Combat Cardiovascular Disease. Curr. Med. Chem. 2012, 19, 2242–2251. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Plant-polyphenols based second-generation synbiotics: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Van Der Lugt, B.; Van Beek, A.A.; Aalvink, S.; Meijer, B.; Sovran, B.; Vermeij, W.; Brandt, R.M.C.; De Vos, W.M.; Savelkoul, H.F.J.; Steegenga, W.T.; et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1−/Δ7 mice. Immun. Ageing 2019, 16, 1–17. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; De Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, J.; Sun, L.; Lyu, X.; Chang, X.-Y.; Mi, X.; Hu, M.-G.; Wu, C.; Chen, X. Akkermansia muciniphila: A potential novel mechanism of nuciferine to improve hyperlipidemia. Biomed. Pharmacother. 2021, 133, 111014. [Google Scholar] [CrossRef]
- Deng, L.; Ou, Z.; Huang, D.; Li, C.; Lu, Z.; Liu, W.; Wu, F.; Nong, C.; Gao, J.; Peng, Y. Diverse effects of different Akkermansia muciniphila genotypes on Brown adipose tissue inflammation and whitening in a high-fat-diet murine model. Microb. Pathog. 2020, 147, 104353. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Almeida, D.; Seabra, C.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Uncovering Akkermansia muciniphila resilience or susceptibility to different temperatures, atmospheres and gastrointestinal conditions. Anaerobe 2020, 61, 102135. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; De Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved viability of Akkermansia muciniphila by encapsulation in spray dried succinate-grafted alginate doped with epigallocatechin-3-gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.S.; Berrones, A.J.; Krishnakumar, I.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Responsiveness to curcumin intervention is associated with reduced aortic stiffness in young, obese men with higher initial stiffness. J. Funct. Foods 2017, 29, 154–160. [Google Scholar] [CrossRef]
- Su, J.; Cai, Y.; Zhi, Z.; Guo, Q.; Mao, L.; Gao, Y.; Yuan, F.; Van der Meeren, P. Assembly of propylene glycol alginate/β-lactoglobulin composite hydrogels induced by ethanol for co-delivery of probiotics and curcumin. Carbohydr. Polym. 2021, 254, 117446. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. BioMed Res. Int. 2019, 2019, 5403761–5403769. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, D.V.; Espinosa-Solis, V.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Martinez-Gutierrez, F.; Román-Aguirre, M.; Saavedra-Leos, M.Z. Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol. Processes 2020, 8, 849. [Google Scholar] [CrossRef]
- Chavarri, M.; Maranon, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M.D.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Hernández-Barrueta, T.; Martínez-Bustos, F.; Castaño-Tostado, E.; Lee, Y.; Miller, M.J.; Amaya-Llano, S.L. Encapsulation of probiotics in whey protein isolate and modified huauzontle’s starch: An approach to avoid fermentation and stabilize polyphenol compounds in a ready-to-drink probiotic green tea. LWT 2020, 124, 109131. [Google Scholar] [CrossRef]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-extrusion Encapsulation of Probiotic Lactobacillus acidophilus Alone or Together with Apple Skin Polyphenols: An Aqueous and Value-Added Delivery System Using Alginate. Food Bioproc. Technol. 2013, 7, 1581–1596. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S. Potential of solid lipid microparticles covered by the protein-polysaccharide complex for protection of probiotics and proanthocyanidin-rich cinnamon extract. Food Res. Int. 2020, 136, 109520. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E.; Bertková, I.; Štofilová, J. Dietary fibre as prebiotics in nutrition. Central Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Prado, S.B.R.D.; Minguzzi, B.T.; Hoffmann, C.; Fabi, J.P. Modulation of human gut microbiota by dietary fibers from unripe and ripe papayas: Distinct polysaccharide degradation using a colonic in vitro fermentation model. Food Chem. 2021, 348, 129071. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Ayua, E.O.; Kazem, A.E.; Hamaker, B.R. Whole grain cereal fibers and their support of the gut commensal Clostridia for health. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100245. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Livingston, K.A.; Obin, M.; Roberts, S.B.; Chung, M.; McKeown, N.M. Dietary Fiber and the Human Gut Microbiota: Application of Evidence Mapping Methodology. Nutrients 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Million, M.; Raoult, D. The Butyrogenic and Lactic Bacteria of the Gut Microbiota Determine the Outcome of Allogenic Hematopoietic Cell Transplant. Front. Microbiol. 2020, 11, 1642. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.; Sanguansri, L.; Weerakkody, R.; Bull, M.; Singh, T.K.; Augustin, M.A. Effect of encapsulant matrix on stability of microencapsulated probiotics. J. Funct. Foods 2016, 25, 447–458. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- dos Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Kumherová, M.; Veselá, K.; Jokešová, K.; Klojdová, I.; Horáčková, Š. Influence of co-encapsulation of Bifidobacterium animalis subsp. lactis Bb12 with inulin and ascorbic acid on its viability. Czech J. Food Sci. 2020, 38, 57–62. [Google Scholar] [CrossRef]
- Fayed, B.; Abood, A.; El-Sayed, H.S.; Hashem, A.M.; Mehanna, N.S. A synbiotic multiparticulate microcapsule for enhancing inulin intestinal release and Bifidobacterium gastro-intestinal survivability. Carbohydr. Polym. 2018, 193, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Xu, H.; Aguilar, Z.P.; Wei, H. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT 2016, 68, 8–13. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Khangwal, I.; Shukla, P. Potential prebiotics and their transmission mechanisms: Recent approaches. J. Food Drug Anal. 2019, 27, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sathyabama, S.; Kumar, M.R.; Devi, P.B.; Vijayabharathi, R.; Priyadharisini, V.B. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. Int. J. Pharm. 2014, 466, 400–408. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Li, C.; Zheng, S.; Li, H.; Yu, J. Development of a microencapsulated synbiotic product and its application in yoghurt. LWT 2020, 122, 109033. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Pinto, S.S.; Verruck, S.; Vieira, C.R.; Prudêncio, E.S.; Amante, E.R.; Amboni, R.D. Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium-BB-12 under simulated gastrointestinal conditions and heat treatments. LWT 2015, 64, 1004–1009. [Google Scholar] [CrossRef]
- Darjani, P.; Hosseininezhad, M.; Kadkhodaee, R.; Milani, E. Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Nunes, G.L.; Etchepare, M.D.A.; Cichoski, A.J.; Zepka, L.Q.; Lopes, E.J.; Barin, J.S.; Flores, É.M.D.M.; Silva, C.D.B.D.; De Menezes, C.R. Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Rosolen, M.D.; Bordini, F.W.; de Oliveira, P.D.; Conceição, F.R.; Pohndorf, R.S.; Fiorentini, Â.M.; da Silva, W.P.; Pieniz, S. Symbiotic microencapsulation of Lactococcus lactis subsp. lactis R7 using whey and inulin by spray drying. LWT 2019, 115, 108411. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Fonseca, B.D.S.D.; Poletto, G.; Jacob-Lopes, E.; Cichoski, A.J.; Muller, E.I.; Flores, E.M.M.; Silva, C.D.B.D.; de Menezes, C.R. Influence of the prebiotics hi-maize, inulin and rice bran on the viability of pectin microparticles containing Lactobacillus acidophilus LA-5 obtained by internal gelation/emulsification. Powder Technol. 2020, 362, 409–415. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Poletto, G.; de Deus, C.; Codevilla, C.F.; Cichoski, A.J.; Jacob-Lopes, E.; Muller, E.I.; Flores, E.M.M.; Esmerino, E.A.; de Menezes, C.R. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2020, 130, 108902. [Google Scholar] [CrossRef] [PubMed]
- Maleki, O.; Khaledabad, M.A.; Amiri, S.; Asl, A.K.; Makouie, S. Microencapsulation of Lactobacillus rhamnosus ATCC 7469 in whey protein isolate-crystalline nanocellulose-inulin composite enhanced gastrointestinal survivability. LWT 2020, 126, 109224. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization Technologies in Probiotic Food Production. J. Nutr. Metab. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Ramaiyan, B.; Subhasree, R. Encapsulation “The Future of Probiotics”—A Review. Adv. Biol. Res. 2009, 3, 96–103. [Google Scholar]
- Kourkoutas, Y.; Xolias, V.; Kallis, M.; Bezirtzoglou, E.; Kanellaki, M. Lactobacillus casei cell immobilization on fruit pieces for probiotic additive, fermented milk and lactic acid production. Process. Biochem. 2005, 40, 411–416. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bosnea, L.; Taboukos, S.; Baras, C.; Lambrou, D.; Kanellaki, M. Probiotic Cheese Production Using Lactobacillus casei Cells Immobilized on Fruit Pieces. J. Dairy Sci. 2006, 89, 1439–1451. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem. 2015, 178, 201–207. [Google Scholar] [CrossRef]
- Sidira, M.; Kourkoutas, Y.; Kanellaki, M.; Charalampopoulos, D. In vitro study on the cell adhesion ability of immobilized lactobacilli on natural supports. Food Res. Int. 2015, 76, 532–539. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Kourkoutas, Y.; Albantaki, N.; Tzia, C.; Koutinas, A.A.; Kanellaki, M. Functionality of freeze-dried L. casei cells immobilized on wheat grains. LWT 2009, 42, 1696–1702. [Google Scholar] [CrossRef]
- Vitola, H.R.S.; Dannenberg, G.; Marques, J.D.L.; Lopes, G.V.; Da Silva, W.P.; Fiorentini, Â.M. Probiotic potential of Lactobacillus casei CSL3 isolated from bovine colostrum silage and its viability capacity immobilized in soybean. Process. Biochem. 2018, 75, 22–30. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y.; Kanellaki, M. Novel probiotic whey cheese with immobilized lactobacilli on casein. LWT 2017, 86, 627–634. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Sidira, M.; Koutinas, A.A.; Kourkoutas, Y. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. J. Dairy Sci. 2014, 97, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kourkoutas, Y.; Koutinas, A.; Kanellaki, M.; Kourkoutas, I. Thermally-dried immobilized kefir on casein as starter culture in dried whey cheese production. Food Microbiol. 2009, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Tsaousi, K.; Kourkoutas, Y.; Panas, P.; Kanellaki, M.; Koutinas, A.A. Fermentation efficiency of thermally dried immobilized kefir on casein as starter culture. Process. Biochem. 2008, 43, 1323–1329. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Panas, P.; Kourkoutas, Y.; Koutinas, A.A. Evaluation of the Thermally Dried Immobilized Cells of Lactobacillus delbrueckii subsp.bulgaricuson Apple Pieces as a Potent Starter Culture. J. Agric. Food Chem. 2007, 55, 9829–9836. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Khorasani, A.C.; Shojaosadati, S.A. Bacterial nanocellulose-pectin bionanocomposites as prebiotics against drying and gastrointestinal condition. Int. J. Biol. Macromol. 2016, 83, 9–18. [Google Scholar] [CrossRef]
- Nwagu, T.N.; Ugwuodo, C.J. Stabilizing bromelain for therapeutic applications by adsorption immobilization on spores of probiotic Bacillus. Int. J. Biol. Macromol. 2019, 127, 406–414. [Google Scholar] [CrossRef]
- Ugwuodo, C.J.; Nwagu, T.N.T.; Ugwu, T.T.; Onwosi, C.O. Enhancement of the Anti-inflammatory Effect of Bromelain by Its Immobilization on Probiotic Spore of Bacillus cereus. Probiotics Antimicrob. Proteins 2020, 1–15. [Google Scholar] [CrossRef]
- Ester, B.; Noelia, B.; Laura, C.-J.; Francesca, P.; Cristina, B.; Rosalba, L.; Marco, D.R. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. LWT 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Vitola, H.R.S.; Cruxen, C.; da Silva, F.T.; Thiel, P.R.; Marques, J.D.L.; da Silva, W.P.; Fiorentini, Â.M. Lactobacillus casei CSL3: Evaluation of supports for cell immobilization, viability during storage in Petit Suisse cheese and passage through gastrointestinal transit in vitro. LWT 2020, 127, 109381. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Bhat, A.; Irorere, V.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving survival of probiotic bacteria using bacterial poly-γ-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Żywicka, A. Survival of probiotic lactic acid bacteria immobilized in different forms of bacterial cellulose in simulated gastric juices and bile salt solution. LWT 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Chaikham, P.; Rattanasena, P. Survival of immobilized probiotics in chocolate during storage and with an in vitro gastrointestinal model. Food Biosci. 2016, 16, 37–43. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process. Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Kyereh, E.; Sathivel, S. Viability of Lactobacillus plantarum NCIMB 8826 immobilized in a cereal-legume complementary food “weanimix” with simulated gastrointestinal conditions. Food Biosci. 2021, 40, 100848. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef]
- Ha, C.W.Y.; Lam, Y.Y.; Holmes, A.J. Mechanistic links between gut microbial community dynamics, microbial functions and metabolic health. World J. Gastroenterol. 2014, 20, 16498–16517. [Google Scholar] [CrossRef]
- Pérez-Cobas, A.E.; Gosalbes, M.J.; Friedrichs, A.; Knecht, H.; Artacho, A.; Eismann, K.; Otto, W.; Rojo, D.; Bargiela, R.; Von Bergen, M.; et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut 2013, 62, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Ivanovska, T.P.; Mladenovska, K.; Zhivikj, Z.; Pavlova, M.J.; Gjurovski, I.; Ristoski, T.; Petrushevska-Tozi, L. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int. J. Pharm. 2017, 527, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, A.; Yuhana, M. Widanarni Encapsulated Synbiotic Dietary Supplementation at Different Dosages to Prevent Vibriosis in White Shrimp, Litopenaeus vannamei. HAYATI J. Biosci. 2015, 22, 163–168. [Google Scholar] [CrossRef]
- Singh, P.K.; Kaur, I.P. Synbiotic (probiotic and ginger extract) loaded floating beads: A novel therapeutic option in an experimental paradigm of gastric ulcer. J. Pharm. Pharmacol. 2012, 64, 207–217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).