Saccharina latissima Cultivated in Northern Norway: Reduction of Potentially Toxic Elements during Processing in Relation to Cultivation Depth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sporeling Production
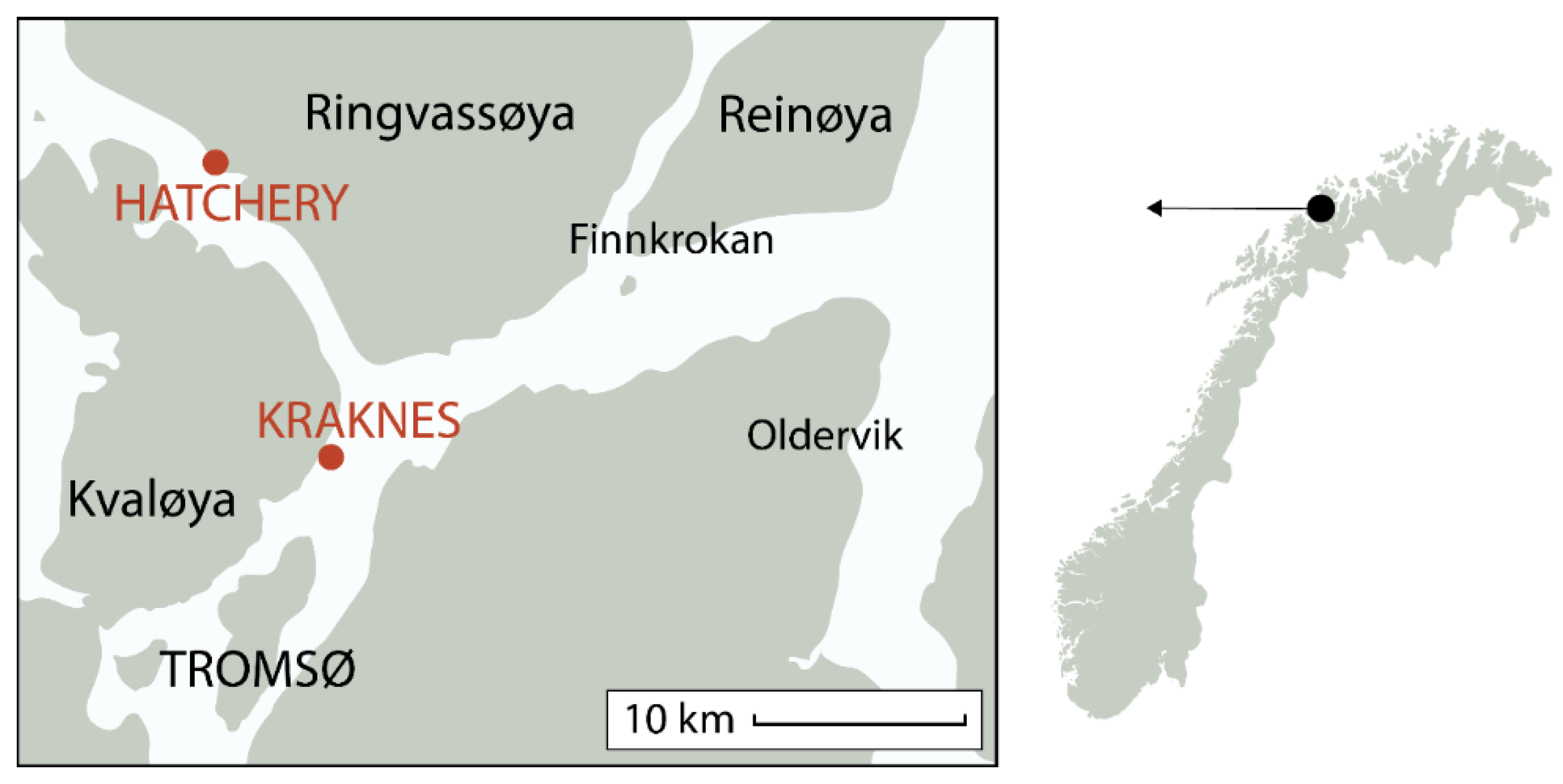
2.2. Deployment and Growout at Sea
2.3. Sampling
2.4. Cooking Experiment
2.5. Iodine Analysis
2.6. Analysis of Arsenic, Cadmium, Lead and Mercury
2.6.1. Sample Preparations
2.6.2. Instrumental Analysis
2.6.3. Quality Control and Quality Assurance Procedures
2.7. Dry Matter Analysis
2.8. Tolerable Daily Amounts of Saccharina latissima
2.9. Statistics
3. Results
3.1. Iodine
3.1.1. Effect of Cultivation Depth
3.1.2. Effect of Processing
3.2. Arsenic, Cadmium, Lead and Mercury
3.3. Tolerable Daily Amounts of Saccharina latissima
4. Discussion
4.1. Potentially Toxic Elements
4.1.1. Iodine
4.1.2. Arsenic
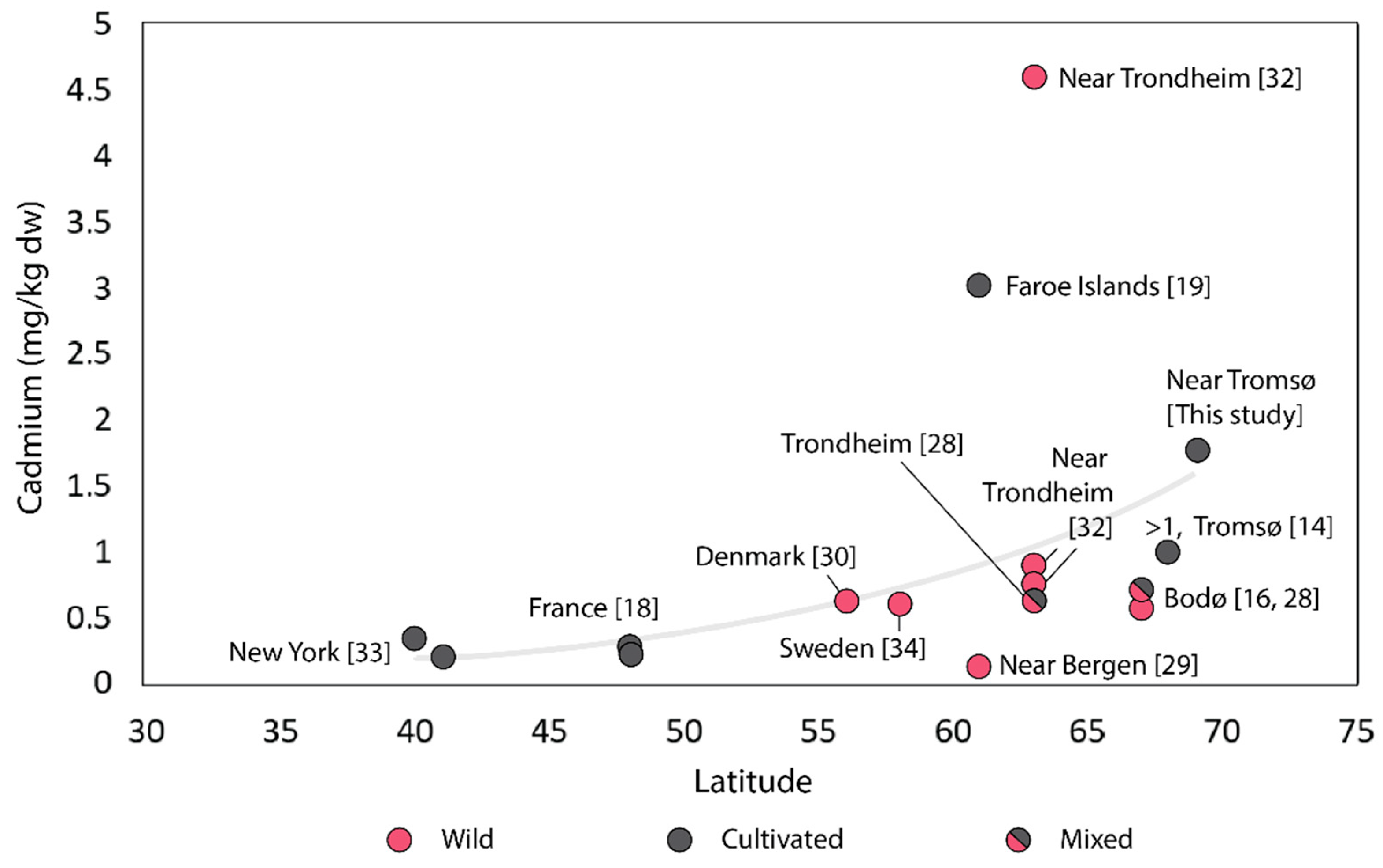
4.1.3. Cadmium
4.1.4. Lead
4.1.5. Mercury
4.2. Tolerable Daily Amounts of Saccharina latissima
4.3. Commercial Impact
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 4. [Google Scholar] [CrossRef]
- Palmieri, N.; Forleo, M.B. The potential of edible seaweed within the western diet. A segmentation of Italian consumers. Int. J. Gastron. Food Sci. 2020, 20, 100202. [Google Scholar] [CrossRef]
- Burg, S.W.K.V.D.; Dagevos, H.; Helmes, R.J.K. Towards sustainable European seaweed value chains: A triple P perspective. ICES J. Mar. Sci. 2019, 78, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Mouritsen, O.G.; Williams, L.; Bjerregaard, R.; Duelund, L. Seaweeds for umami flavour in the New Nordic Cuisine. Flavour 2012, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Holdt, S.L.; Edwards, M.D. Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. Environ. Boil. Fishes 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.M.; Allsopp, P.J.; Magee, P.; Gill, C.I.R.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Duinker, A.; Kleppe, M.; Fjære, E.; Biancarosa, I.; Heldal, H.E.; Dahl, L.; Lunestad, B.T. Knowledge Update on Macroalgae Food and Feed Safety-Based on Data Generated in the period 2014–2019 by the Institute of Marine Research, Norway; Report; National Institute of Nutrition and Seafood Research (NIFES): Bergen, Norway, 2020. [Google Scholar]
- Lüning, K.; Mortensen, L. European aquaculture of sugar kelp (Saccharina latissima) for food industries: Iodine content and epiphytic animals as major problems. Bot. Mar. 2015, 58, 449–455. [Google Scholar] [CrossRef]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbo, R.; Amlund, H.; Heesch, S.; Lock, E.-J. Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: Benefits of and limitations to their potential use in food and feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roleda, M.Y.; Skjermo, J.; Marfaing, H.; Jónsdóttir, R.; Rebours, C.; Gietl, A.; Stengel, D.B.; Nitschke, U. Iodine content in bulk biomass of wild-harvested and cultivated edible seaweeds: Inherent variations determine species-specific daily allowable consumption. Food Chem. 2018, 254, 333–339. [Google Scholar] [CrossRef]
- Stévant, P.; Marfaing, H.; Duinker, A.; Fleurence, J.; Rustad, T.; Sandbakken, I.; Chapman, A. Biomass soaking treatments to reduce potentially undesirable compounds in the edible seaweeds sugar kelp (Saccharina latissima) and winged kelp (Alaria esculenta) and health risk estimation for human consumption. Environ. Boil. Fishes 2018, 30, 2047–2060. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, A.; Brynning, G.; Johansen, A.; Lindegaard, M.S.; Sveigaard, H.H.; Aarup, B.; Fonager, L.; Andersen, L.L.; Rasmussen, M.B.; Larsen, M.M.; et al. Fermentation of sugar kelp (Saccharina latissima)—effects on sensory properties, and content of minerals and metals. Environ. Boil. Fishes 2019, 31, 3175–3187. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.W.; Holdt, S.L.; Sloth, J.J.; Marinho, G.S.; Sæther, M.; Funderud, J.; Rustad, T. Reducing the high iodine content of Saccharina latissima and improving the profile of other valuable compounds by water blanching. Foods 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- WHO. Iodine Deficiency in Europe: A Continuing Public Health Problem; Andersson, M., Benoist, B., Darnton-Hill, T., Delange, F., Eds.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Scientific Committee on Food (SCF); Scientific Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar]
- Scientific Committee on Food (SCF). Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Brussels, Belgium, 2006. [Google Scholar]
- Aakre, I.; Evensen, L.T.; Kjellevold, M.; Dahl, L.; Henjum, S.; Alexander, J.; Madsen, L.; Markhus, M.W. Iodine Status and Thyroid Function in a Group of Seaweed Consumers in Norway. Nutrients 2020, 12, 3483. [Google Scholar] [CrossRef] [PubMed]
- Henjum, S.; Brantsæter, A.L.; Holvik, K.; Lillegaard, I.T.; Mangschou, B.; Parr, C.L.; Starrfelt, J.; Stea, T.H.; Andersen, L.F.; Dahl, L.J.; et al. Benefit and Risk Assessment of Iodization of Household Salt and Salt Used in Bread and Bakery Products. Opinion of the Panel on Nutrition, Dietetic Products, Novel Food and Allergy of the Norwegian Scientific Committee for Food and Environment; Norwegian Scientific Committee for Food and Environment (VKM): Oslo, Norway, 2020. [Google Scholar]
- Miyai, K.; Tokushige, T.; Kondo, M. Suppression of thyroid function during ingestion of seaweed “kombu” (Laminaria japonica) in normal Japanese adults. Endocr. J. 2008, 55, 1103–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Li, Q.; Cui, W.; Wang, X.; Gao, Q.; Zhong, C.; Sun, G.; Chen, X.; Xiong, G.; Yang, X.; et al. Maternal iodine insufficiency and excess are associated with adverse effects on fetal growth: A prospective cohort study in Wuhan, China. J. Nutr. 2018, 148, 1814–1820. [Google Scholar] [CrossRef] [Green Version]
- Henjum, S.; Brantsæter, A.L.; Kurniasari, A.; Dahl, L.; Aadland, E.K.; Gjengedal, E.L.F.; Birkeland, S.; Aakre, I. Suboptimal iodine status and low iodine knowledge in young Norwegian women. Nutrients 2018, 10, 941. [Google Scholar] [CrossRef] [Green Version]
- Lightowler, H.J.; Davies, G.J. Iodine intake and iodine deficiency in vegans as assessed by the duplicate-portion technique and urinary iodine excretion. Br. J. Nutr. 1998, 80, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matvaretabellen [English: The Norwegian Food Composition Database]. Available online: www.matvaretabellen.no (accessed on 2 March 2021).
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control. 2019, 95, 121–134. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Anacleto, P.; Barbosa, V.; Sloth, J.J.; Rasmussen, R.R.; Tediosi, A.; Fernandez-Tejedor, M.; Heuvel, F.H.V.D.; Kotterman, M.; Marques, A. Toxic elements and speciation in seafood samples from different contaminated sites in Europe. Environ. Res. 2015, 143, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.M.; Manns, D.; D’Este, M.; Krause-Jensen, D.; Rasmussen, M.B.; Larsen, M.M.; Alvarado-Morales, M.; Angelidaki, I.; Bruhn, A. Variation in biochemical composition of Saccharina latissima and Laminaria digitata along an estuarine salinity gradient in inner Danish waters. Algal Res. 2016, 13, 235–245. [Google Scholar] [CrossRef]
- Schiener, P.; Zhao, S.; Theodoridou, K.; Carey, M.; Mooney-McAuley, K.; Greenwell, C. The nutritional aspects of biorefined Saccharina latissima, Ascophyllum nodosum and Palmaria palmata. Biomass Convers. Biorefin. 2017, 7, 221–235. [Google Scholar] [CrossRef] [Green Version]
- Ometto, F.; Steinhovden, K.B.; Kuci, H.; Lunnbäck, J.; Berg, A.; Karlsson, A.; Handå, A.; Wollan, H.; Ejlertsson, J. Seasonal variation of elements composition and biomethane in brown macroalgae. Biomass Bioenergy 2018, 109, 31–38. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.; Yarish, C. Evaluation of the metal content of farm grown Gracilaria tikvahiae and Saccharina latissima from Long Island Sound and New York Estuaries. Algal Res. 2019, 40, 101484. [Google Scholar] [CrossRef] [Green Version]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. Environ. Boil. Fishes 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- EFSA Panel of Contaminants in the Food Chain. Scientific opinion on arsenic in food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- EFSA Panel of Contaminants in the Food Chain. Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- EFSA Panel of Contaminants in the Food Chain. Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.P.; Dogliotti, E.; Di Domenico, A.; Férnandez-Cruz, M.L.; Fürst, P.; Fink-Gremmels, J.; Galli, C.L.; et al. Scientific opinion of the panel on contaminants in the food chain—Cadmium in food. EFSA J. 2009, 980, 1–139. [Google Scholar]
- Almela, C.; Laparra, J.M.; Vélez, D.; Barberá, R.; Farré, R.; Montoro, R. Arsenosugars in raw and cooked edible seaweed: Characterization and bioaccessibility. J. Agric. Food Chem. 2005, 53, 7344–7351. [Google Scholar] [CrossRef]
- Laparra, J.M.; Velez, D.; Montoro, R.; Barbera, R.; Farre, R. Bioaccessibility of inorganic arsenic species in raw and cooked Hizikia fusiforme seaweed. Appl. Organomet. Chem. 2004, 18, 662–669. [Google Scholar] [CrossRef]
- Ownsworth, E.; Selby, D.; Ottley, C.J.; Unsworth, E.; Raab, A.; Feldmann, J.; Sproson, A.D.; Kuroda, J.; Faidutti, C.; Bücker, P. Tracing the natural and anthropogenic influence on the trace elemental chemistry of estuarine macroalgae and the implications for human consumption. Sci. Total. Environ. 2019, 685, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsson, S.; Christie, H.; Fieler, R. Variation in biomass and biofouling of kelp, Saccharina latissima, cultivated in the Arctic, Norway. Aquaculture 2019, 506, 445–452. [Google Scholar] [CrossRef]
- Forbord, S.; Steinhovden, K.B.; Rød, K.K.; Handå, A.; Skjermo, J. Cultivation protocol for Saccharina latissima. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 37–59. [Google Scholar]
- Rodushkin, I.; Engström, E.; Baxter, D.C. Sources of contamination and remedial strategies in the multi-elemental trace analysis laboratory. Anal. Bioanal. Chem. 2009, 396, 365–377. [Google Scholar] [CrossRef]
- Rodushkin, I.; Nordlund, P.; Engström, E.; Baxter, D.C. Improved multi-elemental analyses by inductively coupled plasma-sector field mass spectrometry through methane addition to the plasma. J. Anal. At. Spectrom. 2005, 20, 1250–1255. [Google Scholar] [CrossRef]
- Engström, E.; Stenberg, A.; Senioukh, S.; Edelbro, R.; Baxter, D.C.; Rodushkin, I. Multi-elemental characterization of soft biological tissues by inductively coupled plasma–sector field mass spectrometry. Anal. Chim. Acta 2004, 521, 123–135. [Google Scholar] [CrossRef]
- Evankow, A.; Christie, H.; Hancke, K.; Brysting, A.K.; Junge, C.; Fredriksen, S.; Thaulow, J. Genetic heterogeneity of two bioeconomically important kelp species along the Norwegian coast. Conserv. Genet. 2019, 20, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Chai, C.; Qian, Q.; Yan, X.; Fan, X. Determination of chemical species of iodine in some seaweeds (I). Sci. Total. Environ. 1997, 204, 215–221. [Google Scholar] [CrossRef]
- Shah, M.; Wuilloud, R.G.; Kannamkumarath, S.S.; Caruso, J.A. Iodine speciation studies in commercially available seaweed by coupling different chromatographic techniques with UV and ICP-MS detection. J. Anal. At. Spectrom. 2005, 20, 176–182. [Google Scholar] [CrossRef]
- Martinelango, P.K.; Tian, K.; Dasgupta, P.K. Perchlorate in seawater: Bioconcentration of iodide and perchlorate by various seaweed species. Anal. Chim. Acta 2006, 567, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Pedersen, K.M.; Iversen, F.; Terpling, S.; Gustenhoff, P.; Petersen, S.B.; Laurberg, P. Naturally occurring iodine in humic substances in drinking water in Denmark is bioavailable and determines population iodine intake. Br. J. Nutr. 2008, 99, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Nitschke, U.; Stengel, D.B. A new HPLC method for the detection of iodine applied to natural samples of edible seaweeds and commercial seaweed food products. Food Chem. 2015, 172, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kreissig, K.J.; Hansen, L.T.; Jensen, P.E.; Wegeberg, S.; Geertz-Hansen, O.; Sloth, J.J. Characterisation and chemometric evaluation of 17 elements in ten seaweed species from Greenland. PLoS ONE 2021, 16, e0243672. [Google Scholar] [CrossRef]
- Feldmann, J.; Krupp, E.M. Critical review or scientific opinion paper: Arsenosugars—a class of benign arsenic species or justification for developing partly speciated arsenic fractionation in foodstuffs? Anal. Bioanal. Chem. 2011, 399, 1735–1741. [Google Scholar] [CrossRef]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total. Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef] [Green Version]
- García-Sartal, C.; Barciela-Alonso, M.D.C.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Study of cooking on the bioavailability of As, Co, Cr, Cu, Fe, Ni, Se and Zn from edible seaweed. Microchem. J. 2013, 108, 92–99. [Google Scholar] [CrossRef]
- Pétursdóttir, Á.H.; Blagden, J.; Gunnarsson, K.; Raab, A.; Stengel, D.B.; Feldmann, J.; Gunnlaugsdóttir, H. Arsenolipids are not uniformly distributed within two brown macroalgal species Saccharina latissima and Alaria esculenta. Anal. Bioanal. Chem. 2019, 411, 4973–4985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duinker, A. Alger: Mat—Forskning—Formidling: Mineraler og Tungmetaller i Alger fra Lindesnes. 2014, Report; NIFES: Bergen, Norway, 2014. [Google Scholar]
- Kleppe, M. Kadmium, Uorganisk Arsen og Jod i Dyrket og Viltvoksende Butare (Alaria esculenta) og Sukkertare (Saccharina latissima). Master’s Thesis, University of Bergen, Bergen, Norway, 2017. [Google Scholar]
- Carro, L.; Barriada, J.L.; Herrero, R.; De Vicente, M.E.S. Interaction of heavy metals with Ca-pretreated Sargassum muticum algal biomass: Characterization as a cation exchange process. Chem. Eng. J. 2015, 264, 181–187. [Google Scholar] [CrossRef]
- Salgado, L.T.; Andrade, L.R.; Filho, G.M.A. Localization of specific monosaccharides in cells of the brown alga Padina gymnospora and the relation to heavy-metal accumulation. Protoplasma 2005, 225, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chiocchetti, G.D.M.E.; Jadán-Piedra, C.; Vélez, D.; Devesa, V. Metal(loid) contamination in seafood products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3715–3728. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, I.; Guarda, I.; Mourato, M.; Martins, L.L.; Gomes, R.; Matos, J.; Gomes-Bispo, A.; Bandarra, N.M.; Cardoso, C.; Afonso, C. Undervalued Atlantic brown seaweed species (Cystoseira abies-marina and Zonaria tournefortii): Influence of treatment on their nutritional and bioactive potential and bioaccessibility. Eur. Food Res. Technol. 2021, 247, 221–232. [Google Scholar] [CrossRef]
- Jinadasa, K.K.; Herbello-Hermelo, P.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Mercury speciation in edible seaweed by liquid chromatography—Inductively coupled plasma mass spectrometry after ionic imprinted polymer-solid phase extraction. Talanta 2021, 224, 121841. [Google Scholar] [CrossRef]
- Blikra, M.J.; Løvdal, T.; Vaka, M.R.; Roiha, I.S.; Lunestad, B.T.; Lindseth, C.; Skipnes, D. Assessment of food quality and microbial safety of brown macroalgae (Alaria esculenta and Saccharina latissima). J. Sci. Food Agric. 2019, 99, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
| Element | Processing Step | All Depths | 0–2 m | 5–7 m | 8–10 m |
|---|---|---|---|---|---|
| Iodine (I) | Raw | 4100 ± 700 A | 3300 ± 0 Aa | 3600 ± 100 Ab | 4700 ± 100 Ac |
| Rinsed | 3600 ± 400 A | 3200 ± 100 Aa | 3400 ± 200 Ba | 4100 ± 200 Bb | |
| Boiled | 600 ± 100 B | 600 ± 100 Ba | 600 ± 100 Ca | 600 ± 100 Ca | |
| Arsenic (As) | Raw | 62.7 ± 4.3 A | 57.7 | 64.9 | 65.4 |
| Rinsed | 50.8 ± 3.8 B | 49.8 ± 6.4 a | 50.2 ± 2.5 a | 52.5 ± 3.9 a | |
| Boiled | 36.0 ± 3.1 B | 34.2 ± 0.8 a | 39.5 ± 1.1 a | 34.4 ± 3.1 a | |
| Cadmium (Cd) | Raw | 1.77 ± 0.50 A | 1.23 | 1.89 | 2.20 |
| Rinsed | 2.05 ± 0.39 A | 1.83 ± 0.16 a | 1.83 ± 0.37 a | 2.50 ± 0.04 a | |
| Boiled | 2.19 ± 0.38 A | 1.76 ± 0.00 a | 2.28 ± 0.33 a | 2.52 ± 0.17 a | |
| Mercury (Hg) | Raw | 0.0323 ± 0.0022 A | 0.0298 | 0.0328 | 0.0342 |
| Rinsed | 0.0424 ± 0.0037 B | 0.0441 ± 0.0072 a | 0.0416 ± 0.0016 a | 0.0414 ± 0.0023 a | |
| Boiled | 0.0280 ± 0.0032 A | 0.0278 ± 0.0006 a | 0.0284 ± 0.0052 a | 0.0277 ± 0.0047 a | |
| Lead (Pb) | Raw | 0.446 ± 0.178 A | 0.695 | 0.351 | 0.292 |
| Rinsed | 0.946 ± 0.731 A | 0.695 ± 0.202 a | 1.48 ± 1.46 a | 0.665 ± 0.439 a | |
| Boiled | 1.16 ± 1.14 A | 0.727 ± 0.135 a | 1.93 ± 2.02 a | 0.83 ± 0.72 a | |
| Dry matter | Rinsed | 89.1 ± 0.3 | 89.2 ± 0.2 | 89.1 ± 0.2 | 88.8 ± 0.3 |
| Boiled | 87.2 ± 0.3 | 87.0 ± 0.1 | 87.3 ± 0.4 | 87.2 ± 0.3 |
| Processing Step | 150 μg Iodine | 600 μg Iodine |
|---|---|---|
| Raw | 0.037 | 0.15 |
| Rinsed | 0.042 | 0.17 |
| Boiled | 0.25 | 1.0 |
| Element | Chemical Form | Content | Tolerable Amounts | TDI (%): 1 g kelp/70 kg bw | ||||
|---|---|---|---|---|---|---|---|---|
| μg/kg bw | μg/70 kg | |||||||
| μg/g | μg/0.25 g | TWI | TDI | TWI | TDI | |||
| Cd | Total | 2.19 | 0.55 | 2.5 | 0.4 | 175 | 25 | 8.7 |
| Hg | Total | 0.03 | 0.01 | - | - | - | - | - |
| MeHg | n.a. | n.a. | 1.3 | 0.2 | 91 | 13 | 0.2 | |
| iHg | n.a. | n.a. | 4 | 0.6 | 280 | 40 | 0.1 | |
| Pb | Total | 1.16 | 0.29 | 3.5 | 0.5 | 245 | 35 | 3.3 |
| As | Total | 36.0 | 9.00 | - | - | - | - | - |
| iAs 1 | 0.63 | 0.16 | 2.1 | 0.3 | 147 | 21 | 3.0 | |
| Origin | Initial State | Processing | Reduction (%) | Final Content (mg/kg dw) | Water-to-Kelp Ratio | Reference |
|---|---|---|---|---|---|---|
| Faroe Island | Frozen, cut, refrozen | Boiling (15 min) | 38.4 | 1620 | 3.8 | [15] |
| Norway | Frozen-thawed | Boiling (15 min) | 85.6 | 600 | 10 | This study |
| France | Fresh, <2 h post-harvest | Soaking (32 °C) w/air bubbling (1–22 h) | 84.8 | 1000 | 20 | [14] |
| Norway | Fresh, whole thallus | Blanching (30 °C, 300 s) | 78.0 | 1014 | 33 | [16] |
| Blanching (45 °C, 300 s) | 91.6 | 388 | 33 | |||
| Blanching (60 °C, 300 s) | 93.0 | 321 | 33 | |||
| Blanching (80 °C, 120 s) | 93.6 | 293 | 33 |
| Processing | Species | Location | Raw State | Reduction (%) | Final Concentration (mg/kg dw) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| tAs | iAs | tAs | iAs | |||||
| Blanching (10–20 s) | L. digitata | Scotland | Washed and dried | 29 | 5 | 32 | 19 | [41] |
| L. digitata | Scotland | Dried | 39 | 33 | 28 | 12 | [41] | |
| S. japonica | Japan | Dry, commercial samples | 22 | 61 | 58 | 0.059 | [41] | |
| Boiling (15 min) | S. latissima | Norway | Frozen-thawed | 43 | n.a. | 36 | n.a. | This study |
| Faroe Islands | −8.2 | n.a. | 42 | n.a. | [15] | |||
| Boiling (20 min) | H. fusiforme | Unknown | Dry, commercial samples | 46 | n.a. | 56 | n.a. | [39] |
| H. fusiforme | 30 | 46 | 91 | 43 | [40] | |||
| H. fusiforme | 43 | 50 | 75 | 43 | [40] | |||
| H. fusiforme | 33 | 49 | 84 | 45 | [40] | |||
| U. pinnatifida | −0.34 | n.a. | 50 | n.a. | [39] | |||
| U. pinnatifida | 49 | n.a. | 26 | n.a. | [56] | |||
| Boiling (60 min) | S. japonica | Unknown | Dry, commercial samples | 65 | n.a. | 27 | n.a. | [56] |
| Soaking (15 min) | H. fusiforme | Unknown | Dry, commercial samples | 28 | n.a. | 94 | n.a. | [39] |
| Soaking (30 min) | L. digitata | Scotland | Dried | 69 | 64 | 14 | 6.4 | [41] |
| S. japonica | Japan | Dry, commercial samples | 45 | 67 | 41 | 0.050 | [41] | |
| Soaking (15 min), followed by boiling (2 min) | U. pinnatifida | Unknown | Dry, commercial samples | 28 | n.a. | 34 | n.a. | [39] |
| Soaking (30 min), followed by bringing to boil | L. digitata | Scotland | Washed and dried | 87 | 95 | 5.9 | 1.0 | [41] |
| L. digitata | Scotland | Dried | 91 | 96 | 4.0 | 0.72 | [41] | |
| S. japonica | Japan | Dry, commercial samples | 87 | 75 | 9.4 | 0.038 | [41] | |
| Boiling (15 min), lactic acid fermentation (48 h) | S. latissima | Faroe Islands | Frozen-thawed | 5.9 | n.a. | 37 | n.a. | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordbrekk Blikra, M.; Wang, X.; James, P.; Skipnes, D. Saccharina latissima Cultivated in Northern Norway: Reduction of Potentially Toxic Elements during Processing in Relation to Cultivation Depth. Foods 2021, 10, 1290. https://doi.org/10.3390/foods10061290
Jordbrekk Blikra M, Wang X, James P, Skipnes D. Saccharina latissima Cultivated in Northern Norway: Reduction of Potentially Toxic Elements during Processing in Relation to Cultivation Depth. Foods. 2021; 10(6):1290. https://doi.org/10.3390/foods10061290
Chicago/Turabian StyleJordbrekk Blikra, Marthe, Xinxin Wang, Philip James, and Dagbjørn Skipnes. 2021. "Saccharina latissima Cultivated in Northern Norway: Reduction of Potentially Toxic Elements during Processing in Relation to Cultivation Depth" Foods 10, no. 6: 1290. https://doi.org/10.3390/foods10061290
APA StyleJordbrekk Blikra, M., Wang, X., James, P., & Skipnes, D. (2021). Saccharina latissima Cultivated in Northern Norway: Reduction of Potentially Toxic Elements during Processing in Relation to Cultivation Depth. Foods, 10(6), 1290. https://doi.org/10.3390/foods10061290







