Impact of Dry Hopping on Beer Flavor Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Beer Production and Dry Hopping
2.3. Iso-α-Acids
2.3.1. Isolation of Iso-α-Acids
2.3.2. RP-HPLC of Iso-α-Acids & Calculation of Trans/Cis Ratios
2.4. Polyphenols
2.4.1. Sample Preparation and Standard Addition
2.4.2. LC/MS/MS Analysis of Polyphenols
2.5. Descriptive Analysis
2.5.1. Panelists and Ballot Development
2.5.2. Evaluation of Experimental Beers
2.6. Data Analysis
3. Results
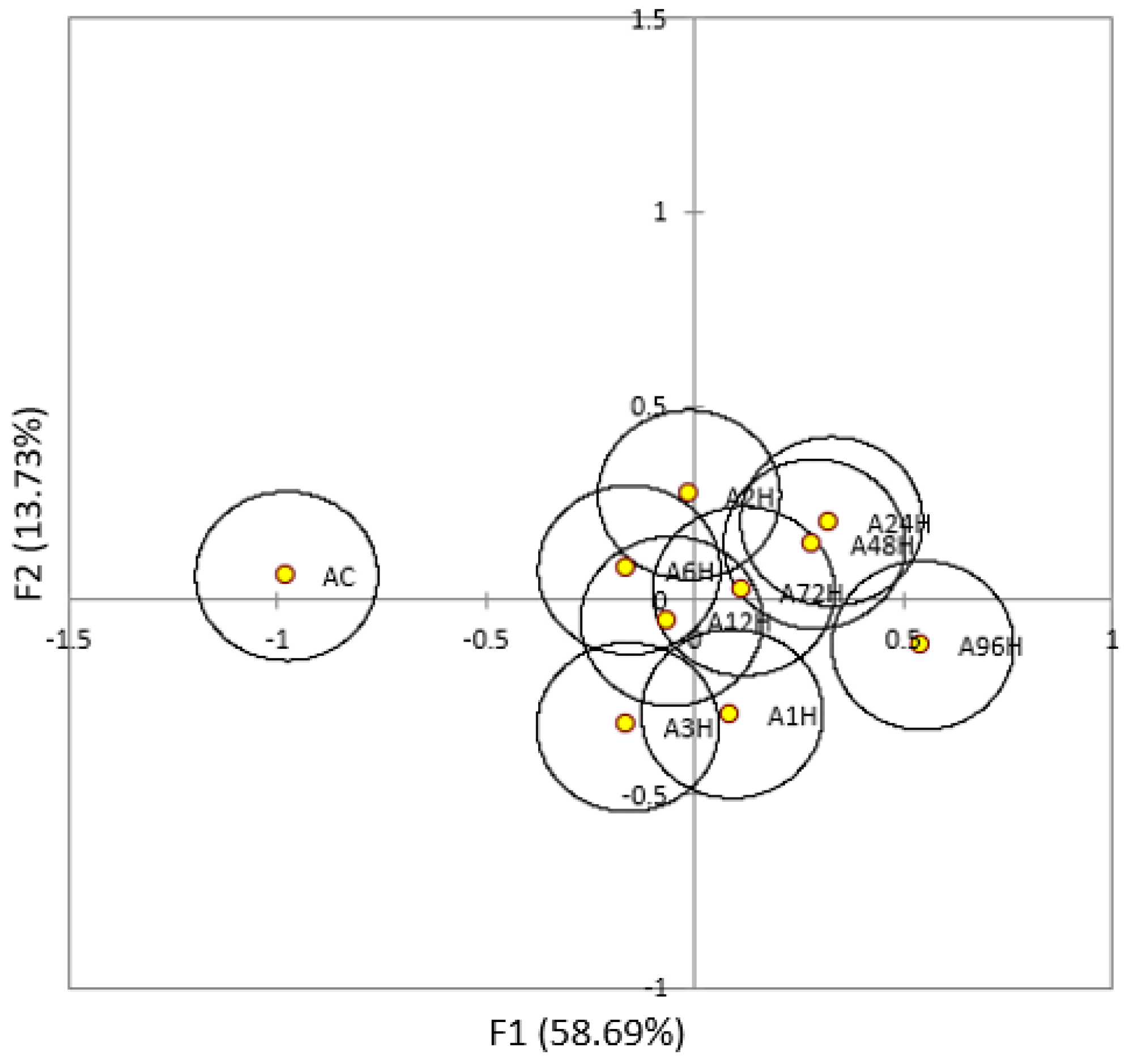
3.1. Iso-α-Acids
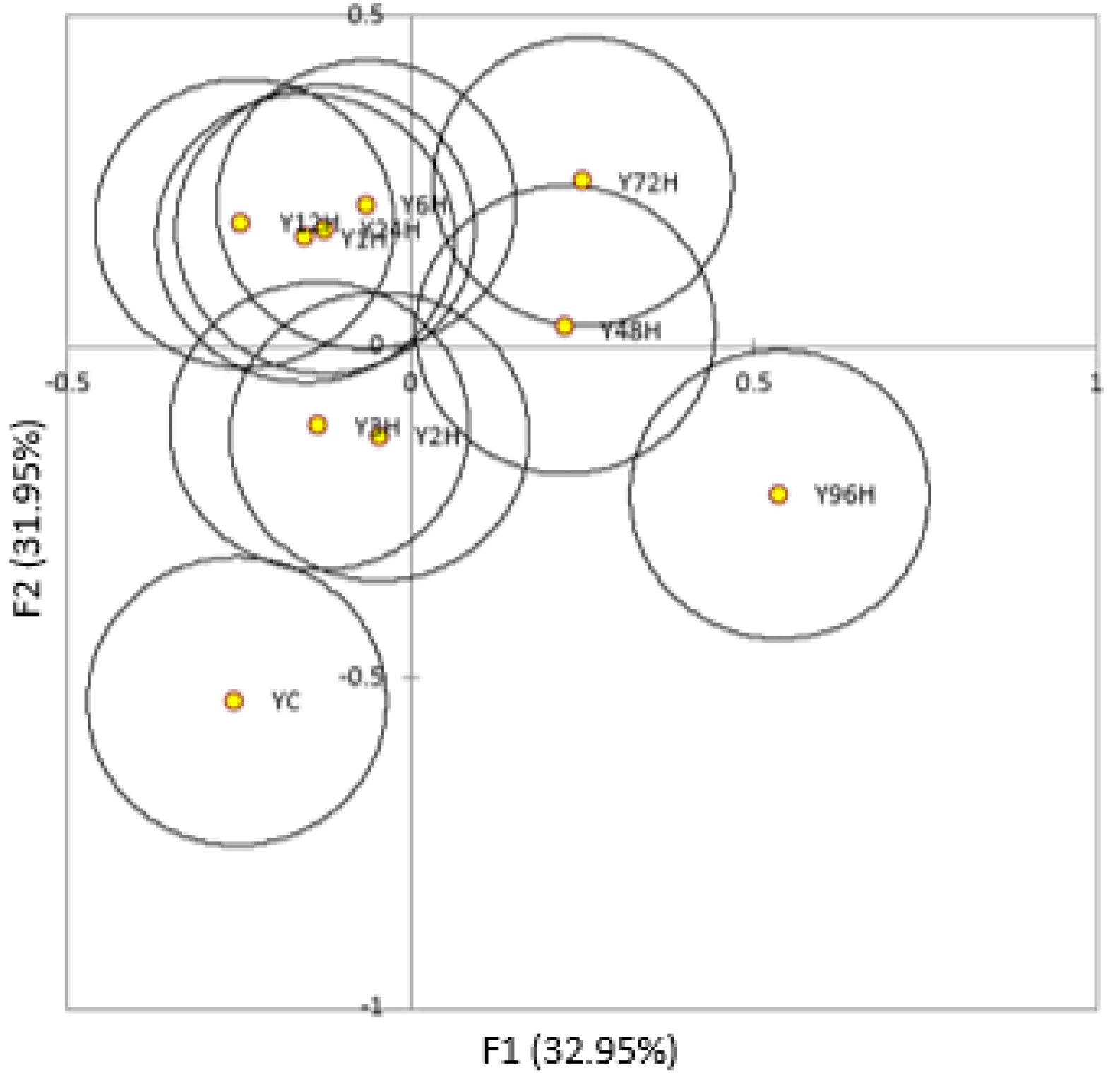
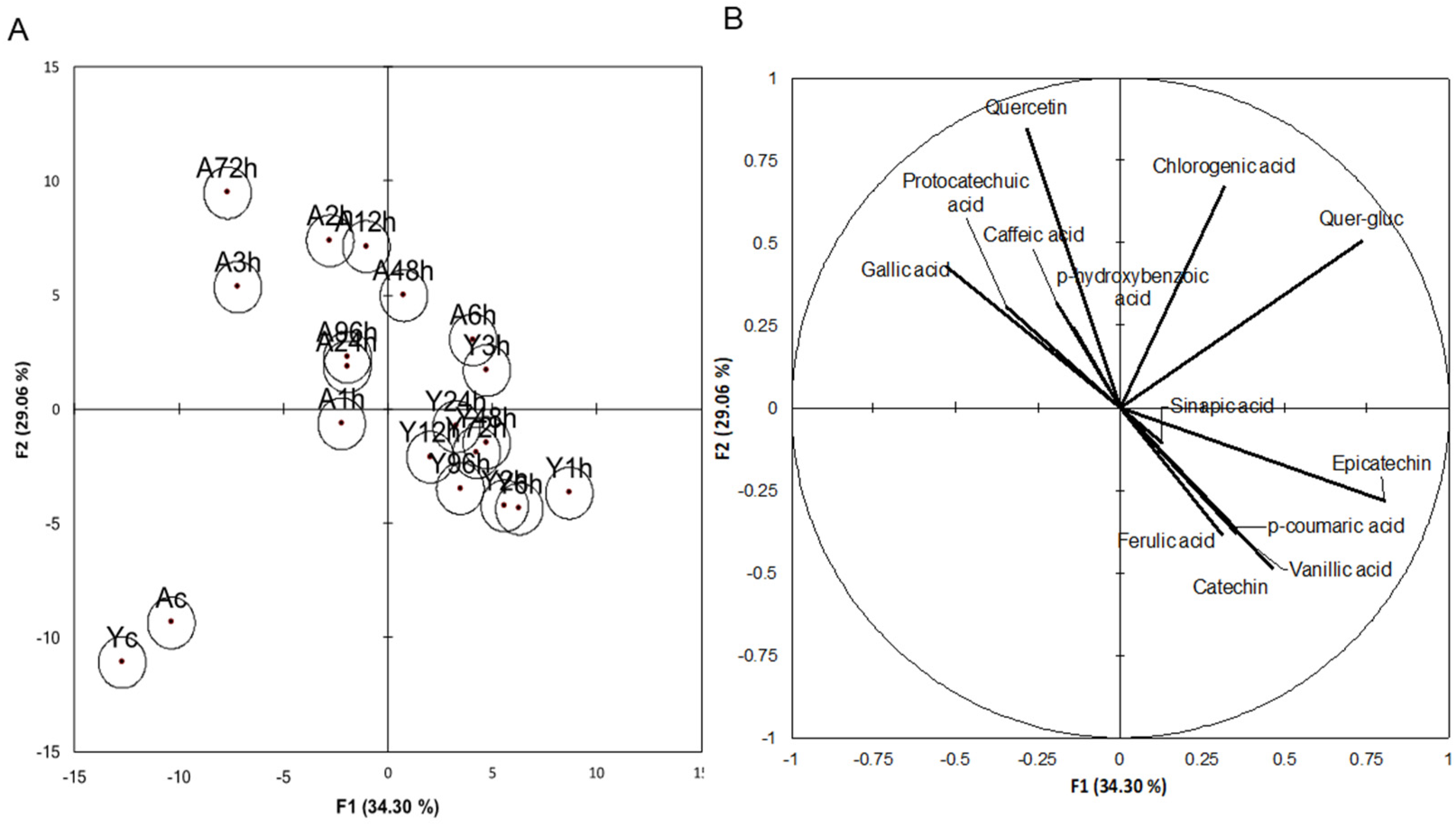
3.2. Phenolic Compounds
3.3. Descriptive Analysis
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Elution Order | Time Segment | Fragmentor Value | Collision Energy | Precursor Ion | Product Ion |
|---|---|---|---|---|---|---|
| Gallic acid | 1 | 1 | 80 | 12 | 169 | 125 |
| Protocatechuic acid | 2 | 2 | 80 | 15 | 153 | 109 |
| p-hydroxybenzoic acid | 3 | 3 | 80 | 15 | 137 | 93 |
| Catechin | 4 | 4 | 130 | 9 | 289 | 245 |
| Chlorogenic acid | 5 | 5 | 95 | 12 | 353 | 191 |
| Vanillic acid | 6 | 5 | 80 | 9 | 167 | 123 |
| Caffeic acid | 7 | 6 | 95 | 15 | 179 | 135 |
| Syringic acid | 8 | 7 | 80 | 9 | 197 | 153 |
| Epicatechin | 9 | 8 | 130 | 9 | 289 | 245 |
| p-coumaric acid | 10 | 9 | 80 | 15 | 163 | 119 |
| Ferulic acid | 11 | 10 | 80 | 15 | 193 | 134 |
| Sinapic acid | 12 | 11 | 95 | 6 | 223 | 208 |
| Cat gallate | 13 | 11 | 115 | 9 | 441 | 289 |
| Epi gallate | 14 | 12 | 115 | 9 | 441 | 289 |
| Quer-gluc | 15 | 13 | 130 | 21 | 463 | 301 |
| Quercetin | 16 | 14 | 115 | 18 | 301 | 151 |
| Compound | R2 | |||
|---|---|---|---|---|
| 1 µL Injection | 2.5 µL Injection | 5 µL Injection | 10 µL Injection | |
| Gallic acid | 0.9939 | 0.9970 | 0.8519 | 0.8831 |
| Protocatechuic acid | 0.9987 | 0.9959 | 0.8378 | 0.8699 |
| p-hydroxybenzoic acid | 0.9998 | 0.9849 | 0.8061 | 0.8406 |
| Catechin | 0.9996 | 0.9858 | 0.7128 | 0.7658 |
| Chlorogenic acid | 0.9990 | 0.9926 | 0.8135 | 0.8494 |
| Vanillic acid | 0.9987 | 0.9911 | 0.8296 | 0.8712 |
| Caffeic acid | 0.9999 | 0.9926 | 0.8099 | 0.8442 |
| Syringic acid | 0.9999 | 0.9888 | 0.8190 | 0.8560 |
| Epicatechin | 0.9997 | 0.9871 | 0.7943 | 0.8250 |
| p-coumaric acid | 0.9994 | 0.9843 | 0.7561 | 0.7827 |
| Ferulic acid | 0.9994 | 0.9892 | 0.7704 | 0.8043 |
| Sinapic acid | 0.9995 | 0.9855 | 0.8071 | 0.8367 |
| Cat gallate | 0.9998 | 0.9931 | 0.8437 | 0.8722 |
| Epi gallate | 0.9994 | 0.9951 | 0.8402 | 0.8661 |
| Quer-gluc | 0.9995 | 0.9869 | 0.7494 | 0.7795 |
| Quercetin | 0.9995 | 0.9901 | 0.8090 | 0.8271 |
| Time | Treatment | Gallic acid | Protocate-chuic Acid | p-Hydr-oxybenzoic Acid | Catechin | Vanillic Acid | Caffeic Acid | Epicate-chin | p-Coumaric Acid | Ferulic Acid | Sinapic Acid | Quercetin-Glucoside | Quercetin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Young | 0.325 (0.048) | 0.329 (0.033) | 0.302 (0.020) | 3.126 (0.266) | 0.393 (0.047) | 0.135 (0.01) | 0.243 (0.061) | 0.31 (0.005) | 0.439 (0.024) | 1.991 (0.141) | 0.422 (0.022) | 0.160 (0.163) |
| Aged | 0.2 (0.015) | 0.198 (0.021) | 0.284 (0.026) | 2.558 (0.114) | 0.309 (0.018) | 0.076 (0.016) | 0.235 (0.103) | 0.209 (0.024) | 0.383 (0.009) | 1.55 (0.054) | 0.238 (0.048) | 0.024 (0.042) | |
| 1H | Young | 0.183 (0.062) | 0.195 (0.048) | 0.225 (0.046) | 3.131 (0.279) | 0.333 (0.045) | 0.09 (0.035) | 0.21 (0.06) | 0.564 (0.054) | 0.424 (0.048) | 1.82 (0.251) | 0.33 (0.084) | 0.032 (0.028) |
| Aged | 0.174 (0.088) | 0.147 (0.084) | 0.330 (0.039) | 2.071 (0.125) | 0.316 (0.061) | 0.075 (0.033) | 0.193 (0.041) | 0.304 (0.013) | 0.344 (0.022) | 1.419 (0.06) | 0.246 (0.022) | 0.071 (0.106) | |
| 2H | Young | 0.102 (0.014) | 0.116 (0.046) | 0.186 (0.015) | 2.887 (0.512) | 0.258 (0.043) | 0.062 (0.01) | 0.15 (0.081) | 0.52 (0.078) | 0.379 (0.043) | 1.572 (0.116) | 0.232 (0.012) | 0.023 (0.041) |
| Aged | 0.263 (0.000) | 0.241 (0.001) | 0.283 (0.026) | 2.141 (0.252) | 0.219 (0.002) | 0.085 (0.012) | 0.08 (0.08) | 0.255 (0.012) | 0.303 (0.027) | 1.278 (0.068) | 0.223 (0.021) | 0.036 (0.036) | |
| 3H | Young | 0.297 (0.058) | 0.32 (0.061) | 0.295 (0.075) | 3.467 (0.229) | 0.430 (0.050) | 0.142 (0.025) | 0.195 (0.104) | 0.637 (0.091) | 0.491 (0.048) | 2.179 (0.203) | 0.421 (0.079) | 0.137 (0.075) |
| Aged | 0.316 (0.006) | 0.383 (0.104) | 0.470 (0.207) | 2.269 (0.22) | 0.299 (0.096) | 0.163 (0.09) | 0.111 (0.096) | 0.402 (0.164) | 0.452 (0.192) | 1.715 (0.375) | 0.316 (0.103) | 0.062 (0.056) | |
| 6H | Young | 0.188 (0.031) | 0.198 (0.035) | 0.258 (0.054) | 3.292 (0.2) | 0.378 (0.065) | 0.09 (0.003) | 0.188 (0.051) | 0.542 (0.007) | 0.458 (0.052) | 1.873 (0.266) | 0.310 (0.065) | 0.007 (0.012) |
| Aged | 0.25 (0.004) | 0.255 (0.011) | 0.436 (0.031) | 3.005 (0.117) | 0.404 (0.005) | 0.133 (0.008) | 0.291 (0.047) | 0.398 (0.013) | 0.448 (0.047) | 1.853 (0.183) | 0.420 (0.086) | 0.262 (0.168) | |
| 12H | Young | 0.259 (0.037) | 0.277 (0.005) | 0.264 (0.003) | 2.915 (0.047) | 0.316 (0.013) | 0.111 (0.004) | 0.255 (0.037) | 0.489 (0.01) | 0.398 (0.006) | 1.602 (0.06) | 0.299 (0.048) | 0.057 (0.005) |
| Aged | 0.317 (0.028) | 0.276 (0.055) | 0.328 (0.079) | 2.412 (0.38) | 0.302 (0.041) | 0.128 (0.024) | 0.224 (0.106) | 0.321 (0.039) | 0.336 (0.066) | 1.46 (0.231) | 0.322 (0.055) | 0.099 (0.051) | |
| 24H | Young | 0.231 (0.034) | 0.226 (0.037) | 0.216 (0.033) | 2.212 (0.321) | 0.308 (0.046) | 0.068 (0.01) | 0.159 (0.044) | 0.369 (0.046) | 0.315 (0.028) | 1.295 (0.128) | 0.175 (0.020) | ND |
| Aged | 0.284 (0.014) | 0.25 (0.051) | 0.323 (0.051) | 2.054 (0.27) | 0.317 (0.049) | 0.092 (0.004) | 0.17 (0.06) | 0.284 (0.062) | 0.302 (0.027) | 1.29 (0.138) | 0.202 (0.078) | 0.012 (0.021) | |
| 48H | Young | 0.249 (0.01) | 0.227 (0.006) | 0.306 (0.028) | 3.064 (0.17) | 0.347 (0.027) | 0.09 (0.01) | 0.203 (0.04) | 0.497 (0.014) | 0.449 (0.037) | 1.874 (0.176) | 0.313 (0.074) | 0.029 (0.05) |
| Aged | 0.334 (0.06) | 0.374 (0.01) | 0.458 (0.068) | 2.624 (0.149) | 0.377 (0.058) | 0.14 (0.019) | 0.221 (0.06) | 0.33 (0.009) | 0.428 (0.061) | 1.797 (0.32) | 0.362 (0.073) | 0.075 (0.088) | |
| 72H | Young | 0.206 (0.056) | 0.23 (0.029) | 0.289 (0.019) | 3.066 (0.427) | 0.392 (0.061) | 0.095 (0.015) | 0.188 (0.033) | 0.487 (0.078) | 0.428 (0.051) | 1.834 (0.299) | 0.266 (0.041) | ND |
| Aged | 0.338 (0.027) | 0.296 (0.05) | 0.334 (0.081) | 2.057 (0.259) | 0.293 (0.123) | 0.121 (0.033) | 0.206 (0.052) | 0.262 (0.041) | 0.318 (0.046) | 1.351 (0.269) | 0.273 (0.054) | ND | |
| 96H | Young | 0.228 (0.019) | 0.25 (0.019) | 0.327 (0.031) | 3.416 (0.197) | 0.399 (0.029) | 0.135 (0.013) | 0.255 (0.055) | 0.542 (0.011) | 0.494 (0.014) | 2.238 (0.082) | 0.447 (0.014) | 0.048 (0.023) |
| Aged | 0.24 (0.017) | 0.24 (0.043) | 0.417 (0.028) | 2.453 (0.365) | 0.329 (0.035) | 0.112 (0.026) | 0.238 (0.002) | 0.296 (0.071) | 0.424 (0.051) | 1.801 (0.099) | 0.322 (0.018) | 0.03 (0.029) |
References
- Praet, T.; Opstaele, F.; Causmaecker, B.; Bellaio, G.; Rouck, G.; Aerts, G.; Cooman, L. De novo formation of sequiterpene Oxidation Products during wort boiling and impacts of the Kettle Hopping Regime on Sensory Characteristics of Pilot Scale Lager Beers. Brew. Sci. 2015, 68, 130–145. [Google Scholar]
- Bamforth, C. Tap into the Art and Science of Brewing, 3rd ed.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Haley, J.; Peppard, T.L. Differences in utilization of the essential oils of hops during the production of dry-hopped and late-hopped beers. J. Inst. Brew. 1983, 89, 87–91. [Google Scholar] [CrossRef]
- Foster, T. Pale Ale: History, Brewing Technique, Recipes, 2nd ed.; Brewers Publication: Boulder, CO, USA, 1999; p. 26. [Google Scholar]
- Baert, J.J.; De Clippeleer, J.; Hughes, P.S.; De Cooman, L.; Aerts, G. On the Origin of Free and Bound Staling Aldehydes in Beer. J. Agric. Food Chem. 2012, 60, 11449–11472. [Google Scholar] [CrossRef] [PubMed]
- De Clippeleer, J.; De Rouck, G.; De Cooman, L.; Aerts, G. Influence of the hopping technology on the storage-induced appearance of staling aldehydes in beer. J. Inst. Brew. 2010, 116, 381–398. [Google Scholar] [CrossRef]
- Hashimoto, N.; Kuroiwa, Y. Proposed pathways for the formation of volatile aldehydes during storage of bottled beer. Proceedings. Annu. Meet. Am. Soc. Brew. Chem. 1975, 33, 104–111. [Google Scholar] [CrossRef]
- De Keukeleire, D.; De Cooman, L.; Rong, H.; Heyerick, A.; Kalita, J.; Milligan, S.R. Functional properties of hop polyphenols. Basic Life Sci. 1999, 66, 739–760. [Google Scholar]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Quifer-Rada, P.; Vallverdu-Queralt, A.; Martinex-Huelamo, M.; Chiva-Blanch, G.; Jauregui, O.; Estruch, R.; Lamuela-Raventos, R. A comprehensive characterization of beer polyphenols by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of phenolic content and antioxidant activity of different beer types. J. Food Agric. Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Callemien, D.; Collins, S. Structure, organoleptic properties, quantification methods, and stability of phenolic compounds in beer—A review. Food Rev. Int. 2009, 26, 1–84. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Chapter 45: Antioxidant Capacity of Hops. In Beer in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Elsevier Incorporated Academic Publishing: Amsterdam, The Netherlands, 2009; pp. 467–474. [Google Scholar]
- Aron, P.M.; Shellhammer, T.H. A discussion of polyphenols in beer physical and flavor stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Dvorakova, M.; Hulin, P.; Karabin, M.; Dostalek, P. Determination of polyphenols in beer by an effective method based on solid-phase extraction and high performance liquid chromatography with diode-array detection. Czech J. Food Sci. 2007, 25, 182–188. [Google Scholar] [CrossRef]
- Krofta, K.; Hervert, J.; Mikyska, A.; Dusek, M. Hop beta acids- from cones to beer. Acta Hortic. 2019, 1236, 15–22. [Google Scholar] [CrossRef]
- Bamforth, C.W. Scientific Principle of Malting and Brewing, 3rd ed.; American Society of Brewing Chemists: St. Paul, MN, USA, 2018; pp. 83–95. [Google Scholar]
- Ferreira, C.S.; Collins, S. Fate of bitter compounds through dry-hopped beer aging. Why cis-humulinones should be as feared as trans-isohumulones? J. Am. Soc. Brew. Chem. 2020, 78, 103–113. [Google Scholar] [CrossRef]
- Araki, S.; Takashio, M.; Shinotsuka, K. A new parameter for determination of the extent of staling in beer. J. Am. Soc. Brew. Chem. 2000, 60, 26–30. [Google Scholar] [CrossRef]
- Malfliet, S.; Van Opstaele, F.; De Clippeleer, J.; Syryn, E.; Goiris, K.; De Cooman, L.; Aerts, G. Flavour Instability of pale lager beers: Determination of analytical markers in relation to sensory ageing. J. Inst. Brew. 2008, 114, 180–192. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Donley, J.R. Solid-Phase Extraction of Hop Acids from Beer or Wort for Subsequent Analysis. J. Am. Soc. Brew. Chem. 1992, 50, 89–93. [Google Scholar] [CrossRef]
- Jaskula, B.; Goiris, K.; De Rouck, G.; Aerts, G.; De Cooman, L. Enhanced Quantitative Extraction and HPLC Determination of Hop and Beer Bitter Acids. J. Inst. Brew. 2012, 113, 381–390. [Google Scholar] [CrossRef]
- Van Opstaele, F.; De Rouck, G.; De Clippeleer, J.; Aerts, G.; De Cooman, L.; Brew, J.I. Analytical and Sensory Assessment of Hoppy Aroma and Bitterness of Conventionally Hopped and Advanced Hopped Pilsner Beers. J. Inst. Brew. Distill. 2010, 116, 445–458. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Prediction of flavor differences between beers from their chemical composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Jarauta, I.; Ferreira, V.; Cacho, J.F. Synergic, additive and antagonistic effects between odorants with similar odour properties. Dev. Food Sci. 2006, 43, 205–208. [Google Scholar]
- Siebert, T.E.; Barker, A.; Pearson, W.; Barter, S.R.; de Barros Lopes, M.A.; Darriet, P.; Herderich, M.J.; Francis, I.L. Volatile Compounds Related to ‘Stone Fruit’ Aroma Attributes in Viognier and Chardonnay Wines. J. Agric. Food Chem. 2018, 66, 2838–2850. [Google Scholar] [CrossRef]
- Parker, D.K. Beer: Production, sensory characteristics and sensory analysis. In Alcoholic Beverages: Sensory Evaluation and Consumer Research; Woodhead Publishing: Cambridge, UK, 2012; pp. 133–158. [Google Scholar]
- Steinhaus, M.; Schieberle, P. Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. Variety Spalter Select) based on GC−olfactometry and odor dilution techniques. J. Agric. Food Chem. 2000, 48, 1776–1783. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. Fems Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef]
- Beaver, J.W.; Medina-Plaza, C.; Miller, K.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. Effects of the Temperature and Ethanol on the Kinetics of Proanthocyanidin Adsorption in Model Wine Systems. J. Agric. Food Chem. 2020, 68, 2891–2899. [Google Scholar] [CrossRef]
- Masel, R. Principles of Adsorption and Reaction on Solid Surfaces; Wiley Interscience: New York, NY, USA, 1996. [Google Scholar]
- Miller, K.V.; Noguera, R.; Beaver, J.; Medina-Plaza, C.; Oberholster, A.; Block, D.E. A Mechanistic Model for the Extraction of Phenolics from Grapes during Red Wine Fermentation. Molecules 2019, 24, 1275. [Google Scholar] [CrossRef]


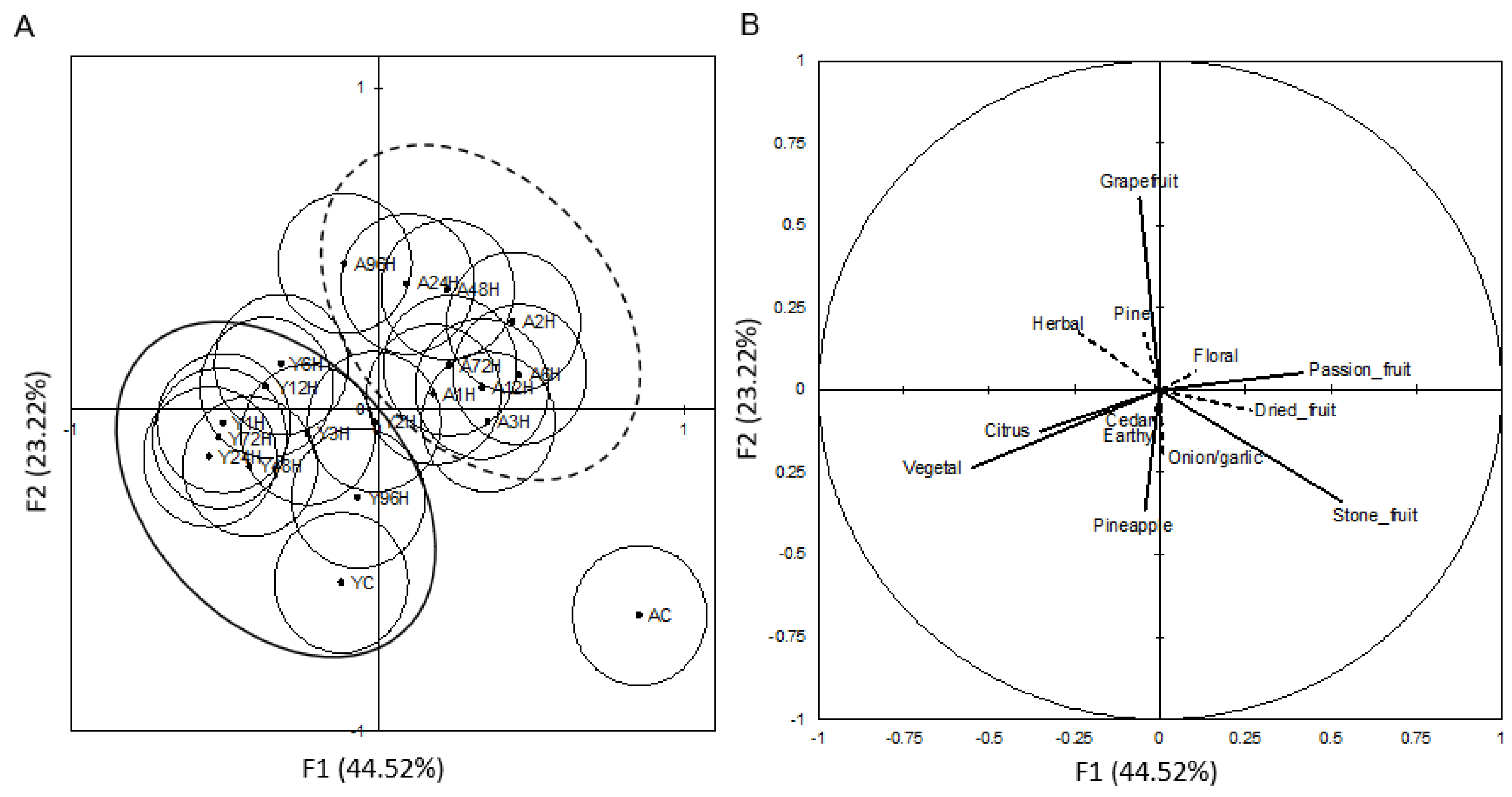


| Attribute | References Standard |
|---|---|
| Passion fruit | Fresh pulp of ¼ of a fresh passion fruit |
| Onion/garlic | Three 1 cm2 pieces of raw yellow onion and 20.0 g of raw, chopped garlic |
| Stone fruit | Four 0.5 cm3 pieces of white peach and four 0.5 cm3 pieces of nectarine |
| Pineapple | Six 0.5 cm3 pieces of fresh cut pineapple |
| Pine | One pinch of damp pine shaving with three drops of PineSol (Chlorox, Oakland, CA, USA) |
| Cedar | One 5 cm × 2 cm piece of cedar shaving soaked overnight in grain alcohol |
| Grapefruit | Six 0.5 cm3 pieces of fresh grapefruit rind |
| Herbal | Contents of ½ tea bag of green tea (Celestial Seasonings, Boulder, CA, USA) and ½ tea bag of chamomile tea (Stash Tea Co., Tigard, OR, USA) |
| Floral | Small sprig of fresh picked lavender |
| Citrus | Six 0.5 cm3 pieces of fresh naval orange rind and six 0.5 cm3 pieces of fresh lemon rind |
| Earthy | Two 0.25 cm2 pieces of dried Porcini mushrooms (Grapevine Trading Co., Santa Rosa, CA, USA) |
| Vegetal | One 1 cm3 pieces of fresh cut bell pepper, two 2.5 cm frozen green bean pieces, and two frozen oeas (Norpac Foods Inc., Brooks, OR, USA) |
| Dried fruit | Eight 1 cm3 pieces of dried apricot (Sun-Maid, Fresno, CA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titus, B.M.; Lerno, L.A.; Beaver, J.W.; Byrnes, N.K.; Heymann, H.; Oberholster, A. Impact of Dry Hopping on Beer Flavor Stability. Foods 2021, 10, 1264. https://doi.org/10.3390/foods10061264
Titus BM, Lerno LA, Beaver JW, Byrnes NK, Heymann H, Oberholster A. Impact of Dry Hopping on Beer Flavor Stability. Foods. 2021; 10(6):1264. https://doi.org/10.3390/foods10061264
Chicago/Turabian StyleTitus, Bradley M., Larry A. Lerno, Jordan W. Beaver, Nadia K. Byrnes, Hildegarde Heymann, and Anita Oberholster. 2021. "Impact of Dry Hopping on Beer Flavor Stability" Foods 10, no. 6: 1264. https://doi.org/10.3390/foods10061264
APA StyleTitus, B. M., Lerno, L. A., Beaver, J. W., Byrnes, N. K., Heymann, H., & Oberholster, A. (2021). Impact of Dry Hopping on Beer Flavor Stability. Foods, 10(6), 1264. https://doi.org/10.3390/foods10061264








