Optimization of an Untargeted DART-HRMS Method Envisaging Identification of Potential Markers for Saffron Authenticity Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. DART-HRMS Analysis
2.4. Data Processing and Statistical Analysis
3. Results
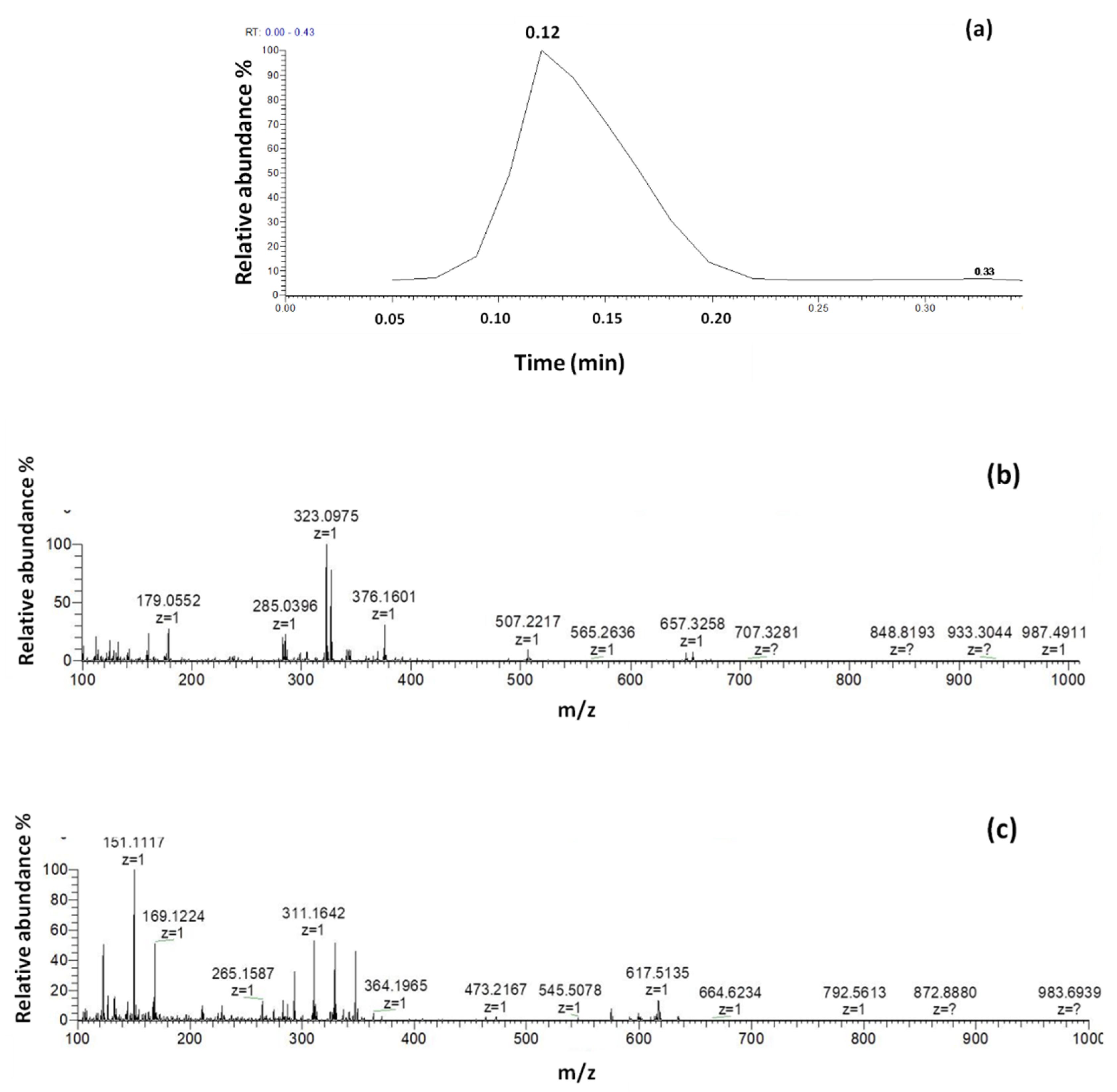
3.1. DART-HRMS Conditions Optimization
3.2. Data Processing and Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kianbakht, S.; Mozaffari, K. Effects of saffron and its active constituents, crocin and safranal, on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J. Med. Plants 2009, 8, 30–38. [Google Scholar]
- Tseng, T.H.; Chu, C.Y.; Huang, J.M.; Shiow, S.J.; Wang, C.J. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 1995, 97, 61–67. [Google Scholar] [CrossRef]
- Boskabady, M.H.; SeyedhosseiniTamijani, S.M.; Rafatpanah, H.; Rezaei, A.; Alavinejad, A. The effect of Crocus sativus extract on human lymphocytes’ cytokines and T helper 2/T helper 1 balance. J. Med. Food 2011, 14, 1538–1545. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H. Antinociceptive and anti-inflammatory effects of Crocus sativus L: Stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 1–8. [Google Scholar]
- Yousefi, E.; Eskandari, A.; Gharavi, M.J.; Khademvatan, S. In vitro activity and cytotoxicity of Crocus sativus extract against leihmania major (MRHO/IR/75/ER). Infect Disord. Drug Targets 2014, 14, 56–60. [Google Scholar] [CrossRef]
- Xu, G.L.; Yu, S.Q.; Gong, Z.N.; Zhang, S.Q. Study of the effect of crocin on rat experimental hyperlipemia and the underlying mechanisms. China J. Chin. Meteria Med. 2005, 30, 369–372. [Google Scholar]
- Zhang, R.; Qian, Z.Y.; Han, X.Y.; Chen, Z.; Yan, J.L.; Hamid, A. Comparison of the effects of crocetin and crocin on myocardial injury in rats. Chin. J. Nat. Med. 2009, 7, 223–227. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A.; Hassan, M.H.; Aly, H.A.A.; Doghish, A.S.; Alghaithy, A.A.A. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol. Environ. Saf. 2012, 83, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Bahashawan, S.A.; Abdelghany, T.M.; Abd-Allah, G.M.; Ghobara, M.M. Crocin abrogates carbon tetrachloride-induced renal toxicity in rats via modulation of metabolizing enzymes and diminution of oxidative stress, apoptosis, and inflammatory cytokines. J. Biochem. Mol. Toxicol. 2015, 29, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Reed, M. Saffron marketing: Challenges and opportunities. In Saffron: Science, Technology and Health; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Amsterdam, The Netherland, 2020; pp. 357–365. [Google Scholar]
- Winterhalter, P.; Straubinger, M. Saffron—Renewed interest in an ancient spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghorbani, M.; Jafari, S.M.; Mahoonak, A.S.; Rajabzadeh, G. Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum arabic and gelatin as wall materials. Food Hydrocolloids 2015, 51, 327–337. [Google Scholar] [CrossRef]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Valle García-Rodríguez, M.; Serrano-Díaz, J.; Tarantilis, P.A.; López-Córcoles, H.; Carmona, M.; Alonso, G.L. Determination of saffron quality by high-performance liquid chromatography. J. Agric. Food Chem. 2014, 62, 8068–8074. [Google Scholar] [CrossRef]
- Soffritti, G.; Busconi, M.; Sánchez, R.A.; Thiercelin, J.-M.; Polissiou, M.; Roldán, M.; Fernández, J.A. Genetic and epigenetic approaches for the possible detection of adulteration and autoadulteration in saffron (Crocus sativus L.) spice. Molecules 2016, 21, 343. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Hagh-Nazari, S.; Keifi, N. Saffron and various fraud manners in its production and trades. Acta Hortic. 2007, 739, 411–416. [Google Scholar] [CrossRef]
- Torelli, A.; Marieschi, M.; Bruni, R. Authentication of saffron (Crocus sativus L.) in different processed, retail products by means of SCAR markers. Food Control. 2014, 36, 126–131. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Maggi, L.; Carmona, M.; Alonso, G.L. Authentication of saffron spice (Crocus sativus L.). In Progress in Authentication of Food and Wine; Ebeler, S.E., Takeoka, G.R., Winterhalter, P., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1081, pp. 309–331. [Google Scholar]
- The International Organization for Standardization. International Standard ISO 3632-1: Saffron (Crocus sativus L.) Specification; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- The International Organization for Standardization. International Standard ISO3632-2:Saffron (Crocus sativus L.) Test Methods; International Organization for Standardization: Geneva, Switzerland, 2010. [Google Scholar]
- Sabatino, L.; Scordino, M.; Gargano, M.; Belligno, A.; Traulo, P.; Gagliano, G. HPLC/PDA/ESIMS evaluation of saffron (Crocus sativus L.) adulteration. Nat. Prod. Commun. 2011, 6, 1873–1876. [Google Scholar]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Instrumental approaches and innovative systems for saffron quality assessment. J. Food Eng. 2018, 216, 1–10. [Google Scholar] [CrossRef]
- Chenga, J.H.; Sun, D.W. Hyperspectral imaging as an effective tool for quality analysis and control of fish and other seafoods: Current research and potential applications. Trends Food Sci. Technol. 2014, 37, 78–91. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Liu, F.; He, Y.; Xiao, Y. Rapid and non-destructive measurement of spinach pigments content during storage using hyperspectral imaging with chemometrics. Measurement 2017, 97, 149–155. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Cagliani, L.R.; Polissiou, M.G.; Consonni, R. Evaluation of saffron (Crocus sativus L.) adulteration with plant adulterants by 1H NMR metabolite fingerprinting. Food Chem. 2015, 173, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Lacina, O.; Zachariasova, M.; Hajslova, J. Saffron authentication based on liquid chromatography high resolution tandem mass spectrometry and multivariate data analysis. Food Chem. 2016, 204, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Chevallier, O.P.; Elliott, C.T. The current and potential applications of ambient mass spectrometry in detecting food fraud. Trends Anal. Chem. 2016, 82, 268–278. [Google Scholar] [CrossRef]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Weston, D.J. Ambient ionization mass spectrometry: Current understanding of mechanistic theory; analytical performance and application areas. Analyst 2010, 135, 661–668. [Google Scholar] [CrossRef]
- Vaclavik, L.; Hrbek, V.; Cajka, T.; Rohlik, B.-A.; Pipek, P.; Hajslova, J. Authentication of animal fats using direct analysis in real time (DART) ionization-mass spectrometry and chemometric tools. J. Agric. Food Chem. 2011, 59, 5919–5926. [Google Scholar] [CrossRef]
- Guo, T.; Yong, W.; Jin, Y.; Liu, J.; Wang, S.; Chen, Q.; Dong, Y.; Su, H.; Tan, T. Application of DART-MS for food quality and safety assurance in food supply chain. Mass Spectrom. Rev. 2017, 36, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Hrbek, V.; Cajka, T.; Hajslova, J. Ambient mass spectrometry employing direct analysis in real time ion source for olive oil quality and authenticity assessment. Anal. Chim. Acta 2009, 645, 56–63. [Google Scholar] [CrossRef]
- Cajka, T.; Riddellova, K.; Tomaniova, M.; Hajslova, J. Ambient mass spectrometry employing a DART ion source for metabolomic fingerprinting/profiling: A powerful tool for beer origin recognition. Metabolomics 2011, 7, 500–508. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Fauhl-Hassek, C.; Hajslova, J. Metabolic fingerprinting based on high-resolution tandem mass spectrometry: A reliable tool for wine authentication? Anal. Bioanal. Chem. 2014, 406, 6791–6803. [Google Scholar] [CrossRef]
- Hrbek, V.; Vaclavik, L.; Elich, O.; Hajslova, J. Authentication of milk and milk-based foods by direct analysis in real time ionization-high resolution mass spectrometry (DART-HRMS) technique: A critical assessment. Food Control 2014, 36, 138–145. [Google Scholar] [CrossRef]
- Fiorino, G.M.; Losito, I.; De Angelis, E.; Arlorio, M.; Logrieco, A.F.; Monaci, L. Assessing fish authenticity by direct analysis in real time-high resolution mass spectrometry and multivariate analysis: Discrimination between wildtype and farmed salmon. Food Res. Int. 2019, 116, 1258–1265. [Google Scholar] [CrossRef]
- Avula, B.; Smillie, T.J.; Wang, Y.H.; Zweigenbaum, J.; Khan, I.A. Authentication of true cinnamon (Cinnamon verum) utilising direct analysis in real time (DART)-QToF-MS. Food Addit. Contam. 2015, 32, 1–8. [Google Scholar] [CrossRef]
- Shen, Y.; Van Beek, T.A.; Claassen, F.W.; Zuilhof, H.; Chen, B.; Nielen, M.W. Rapid control of Chinese star anise fruits and teas for neurotoxic anisatin by direct analysis in real time high resolution mass spectrometry. J. Chromatogr. A. 2012, 1259, 179–186. [Google Scholar] [CrossRef]
- Sumner, L.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Schymanski, E.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Hajslova, J.; Cajka, T.; Vaclavik, L. Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. TrAC 2011, 30, 204–218. [Google Scholar] [CrossRef]
- Vaclavik, L.; Belkova, B.; Reblova, Z.; Riddellova, K.; Hajslova, J. Rapid monitoring of heat accelerated reactions in vegetable oils using direct analysis in real time ionization coupled with high resolution mass spectrometry. Food Chem. 2013, 138, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Vaclavik, L.; Capuano, E.; Gökmen, V.; Hajslova, J. Prediction of acrylamide formation in biscuits based on fingerprint data generated by ambient ionization mass spectrometry employing direct analysis in real time (DART) ion source. Food Chem. 2015, 173, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Senizza, B.; Rocchetti, G.; Ghisoni, S.; Busconi, M.; Pascual, M.D.L.M.; Fernandez, J.A.; Lucini, L.; Trevisan, M. Identification of phenolic markers for saffron authenticity and origin: An untargeted metabolomics approach. Food Res. Int. 2019, 126, 108584. [Google Scholar] [CrossRef] [PubMed]




| Analysis Mode (Polarity) | DART Parameters | MS Conditions | ||||
|---|---|---|---|---|---|---|
| Gas T °C | Grid V | Capillary T °C | Capillary V | Tube Lens | Skimmer V | |
| Positive | 450 | 250 | 250 | 55 | 130 | 26 |
| Negative | 350 | −350 | 200 | −50 | −120 | −25 |
| Adulterant | MS Characterization | Level of Inclusion (Signal Intensity) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Chemical Formula | Mass Accuracy (ppm) | m/z | Adduct | SFit% | 0% | 5% | 10% | 20% | |
| Safflower | €-2,4,6-trimethyltetracos-2-enoic acid | C27H52O2 | −2.56 | 409.4030 | [M+H]+ | 84 | ||||
| All-trans-4,4′-diapophytoene | C30H48 | −2.28 | 409.3817 | [M+H]+ | 75 | |||||
| Eicosapentenoic acid | C20H30O2 | −2.12 | 303.2311 | [M+H]+ | 95 | |||||
| Turmeric | Hymecromone | C10H8O3 | −1.41 | 177.0642 | [M+H]+ | 85 | ||||
| Adenylthiomethylpentose | C11H15N5O3S | −4.31 | 339.1218 | [M+AcN+H]+ | 60 | |||||
 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelis, E.D.; Pilolli, R.; Bejjani, A.; Guagnano, R.; Garino, C.; Arlorio, M.; Monaci, L. Optimization of an Untargeted DART-HRMS Method Envisaging Identification of Potential Markers for Saffron Authenticity Assessment. Foods 2021, 10, 1238. https://doi.org/10.3390/foods10061238
Angelis ED, Pilolli R, Bejjani A, Guagnano R, Garino C, Arlorio M, Monaci L. Optimization of an Untargeted DART-HRMS Method Envisaging Identification of Potential Markers for Saffron Authenticity Assessment. Foods. 2021; 10(6):1238. https://doi.org/10.3390/foods10061238
Chicago/Turabian StyleAngelis, Elisabetta De, Rosa Pilolli, Alice Bejjani, Rocco Guagnano, Cristiano Garino, Marco Arlorio, and Linda Monaci. 2021. "Optimization of an Untargeted DART-HRMS Method Envisaging Identification of Potential Markers for Saffron Authenticity Assessment" Foods 10, no. 6: 1238. https://doi.org/10.3390/foods10061238
APA StyleAngelis, E. D., Pilolli, R., Bejjani, A., Guagnano, R., Garino, C., Arlorio, M., & Monaci, L. (2021). Optimization of an Untargeted DART-HRMS Method Envisaging Identification of Potential Markers for Saffron Authenticity Assessment. Foods, 10(6), 1238. https://doi.org/10.3390/foods10061238








