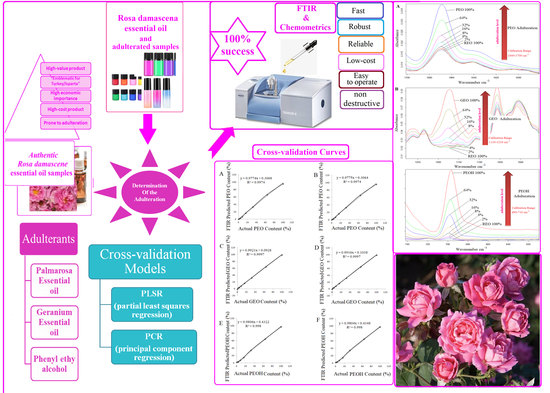
Quantification of the Geranium Essential Oil, Palmarosa Essential Oil and Phenylethyl Alcohol in Rosa damascena Essential Oil Using ATR-FTIR Spectroscopy Combined with Chemometrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Essential Oils and Spiked Samples
2.3. ATR-FTIR Measurements
2.4. Chemometrics
2.4.1. Classification by Hierarchical Cluster Analysis (HCA) and Principal Component Analysis (PCA)
2.4.2. Construction of PLSR and PCR Calibration Models
3. Results
3.1. ATR-FTIR Spectra of Rosa Damascena Essential Oil and Adulterants
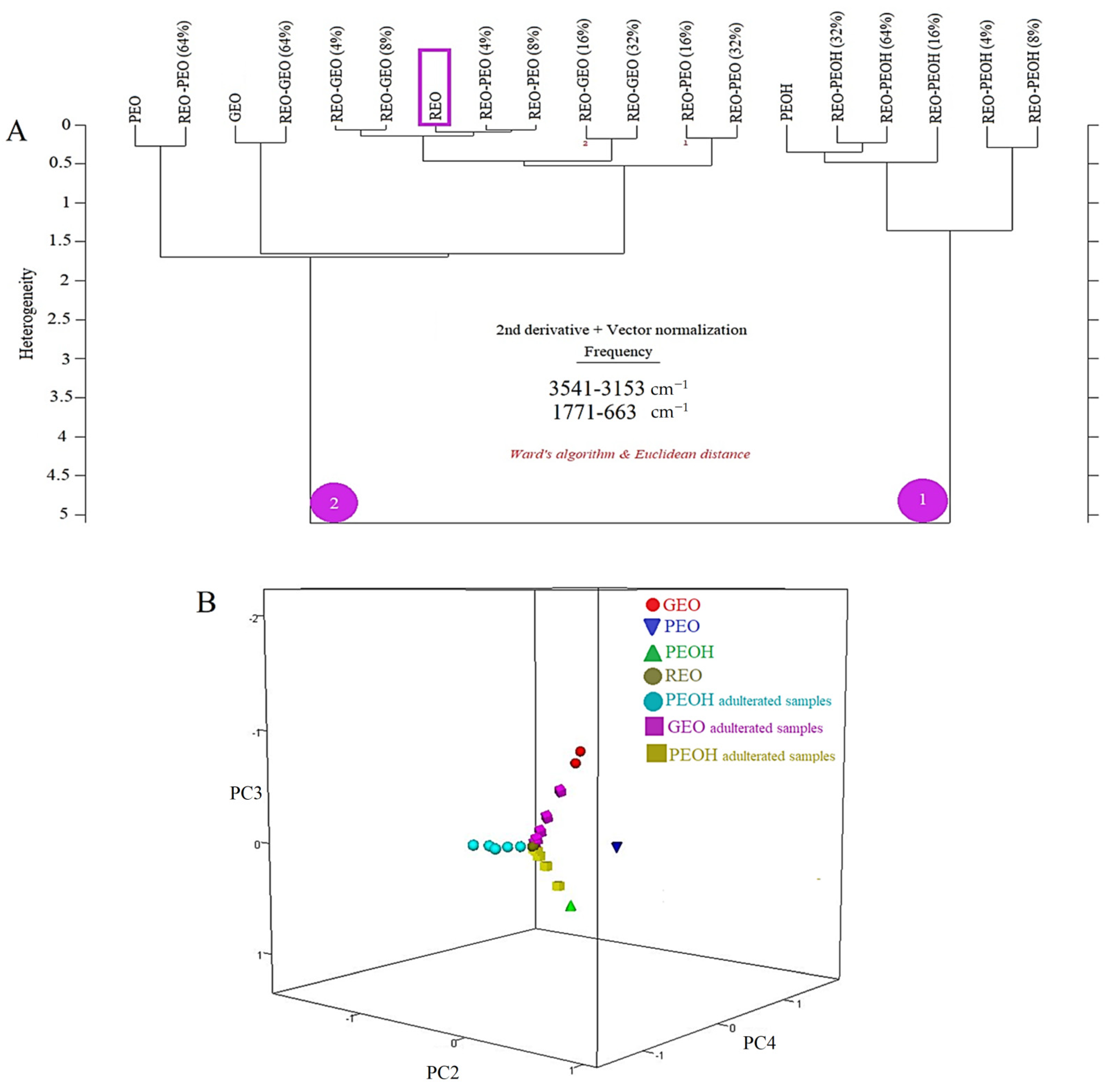
3.2. Interpretation of the Hierarchical Cluster Analysis (HCA) and Principal Component Analysis (PCA) Results
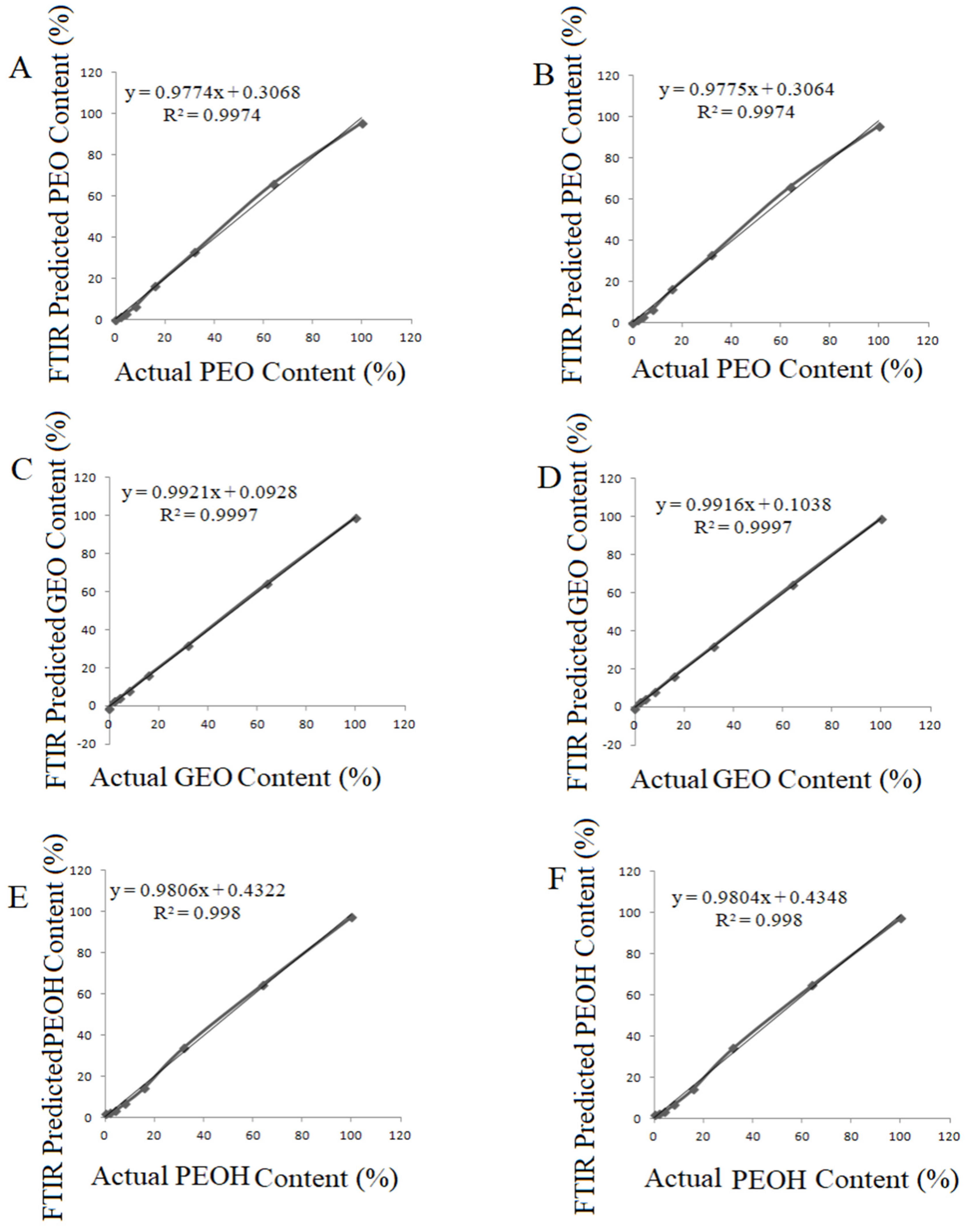
3.3. Prediction of GEO, PEO and PEOH Contents of Rosa Damascena Essential Oil Using PLSR and PCR Calibration Models
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cebi, N.; Arici, M.; Sagdic, O. The famous Turkish rose essential oil: Characterization and authenticity monitoring by FTIR, Raman and GC–MS techniques combined with chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena mill.) cultivars from north indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- ISO. International standard ISO 9842:2003(E) Oil of rose (Rosa × damascena Miller); ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Baydar, H. Yağ gülü Tarımı ve Endüstrisi. In Tıbbi ve Aromatik Bitkiler Bilimi ve Teknolojisi (Genişletilmiş 5. Baskı); Süleyman Demirel University: Isparta, Turkey, 2016; pp. 1–11. [Google Scholar]
- Toluei, Z.; Hosseini Tafreshi, S.; Arefi Torkabadi, M. Comparative Chemical Composition Analysis of Essential Oils in Different Populations of Damask Rose from Iran. J. Agric. Sci. Technol. 2019, 21, 423–437. [Google Scholar]
- Gorji-Chakespari, A.; Nikbakht, A.M.; Sefidkon, F.; Ghasemi-Varnamkhasti, M.; Valero, E.L. Classification of essential oil composition in Rosa damascena Mill. genotypes using an electronic nose. J. Appl. Res. Med. Aromat. Plants 2017, 4, 27–34. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Kaundal, M.; Sood, S.; Agnihotri, V.K. Variation in Essential Oil Content and Composition of Damask Rose (Rosa damascena Mill) Flowers by Salt Application Under Mid Hills of the Western Himalayas. J. Essent. Oil Bear. Plants 2016, 19, 297–306. [Google Scholar] [CrossRef]
- Nunes, H.S.; Miguel, M.G. Rosa damascena essential oils: A brief review about chemical composition and biological properties. Trends Phytochem. Res. Trends Phytochem. Res. 2017, 1, 111–128. [Google Scholar]
- Schmidt, E.; Wanner, J. Adulteration of Essential Oils. Handbook of Essential Oils, 2nd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Pres: Boca Raton, FL, USA, 2009. [Google Scholar]
- Boukhatem, M.N.; Kameli, A.; Saidi, F. Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control. 2013, 34, 208–213. [Google Scholar] [CrossRef]
- Rao, B.R.R.; Kaul, P.N.; Syamasundar, K.V.; Ramesh, S. Chemical profiles of primary and secondary essential oils of palmarosa (Cymbopogon martinii (Roxb.) Wats var. motia Burk). Ind. Crops Prod. 2005, 21, 121–127. [Google Scholar] [CrossRef]
- Cebi, N.; Taylan, O.; Abusurrah, M.; Sagdic, O. Detection of orange essential oil, isopropyl myristate, and benzyl alcohol in lemon essential oil by ftir spectroscopy combined with chemometrics. Foods 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Tankeu, S.Y.; Vermaak, I.; Kamatou, G.P.P.; Viljoen, A.M. Vibrational spectroscopy and chemometric modeling: An economical and robust quality control method for lavender oil. Ind. Crops Prod. 2014, 59, 234–240. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H.; Walter, A.; Rösch, P.; Quilitzsch, R.; Lösing, G.; Popp, J. Investigation of eucalyptus essential oil by using vibrational spectroscopy methods. Vib. Spectrosc. 2006, 42, 341–345. [Google Scholar] [CrossRef]
- Sandasi, M.; Kamatou, G.P.P.; Gavaghan, C.; Baranska, M.; Viljoen, A.M. A quality control method for geranium oil based on vibrational spectroscopy and chemometric data analysis. Vib. Spectrosc. 2011, 57, 242–247. [Google Scholar] [CrossRef]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid screening of mentha spicata essential oil and l-menthol in mentha piperita essential oil by atr-ftir spectroscopy coupled with multivariate analyses. Foods 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Mihai, C.T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational spectroscopy fingerprinting in medicine: From molecular to clinical practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Irudayaraj, J.; Paradkar, M.M. Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem. 2005, 93, 25–32. [Google Scholar] [CrossRef]
- Li, Y.Q.; Kong, D.X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC-MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Berechet, M.D.; Calinescu, I.; Stelescu, M.D.; Manaila, E.; Craciun, G.; Purcareanu, B.; Mihaiescu, D.E.; Rosca, S.; Fudulu, A.; Niculescu-Aron, I.G.; et al. Composition of the essential oil of Rosa damascena Mill. cultivated in Romania. Rev. Chim. 2015, 66, 1986–1991. [Google Scholar]
- Bardakçı, B.; Seçilmiş, H. Isparta bölgesindeki gül yağinin kimyasal içeriğinin GC-MS ve FTIR spektroskopisi tekniği ile incelenmesi. SDÜ Fen Edeb. Fakültesi Fen Derg. 2006, 1, 64–69. [Google Scholar]
- Schulz, H.; Özkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Nedeltcheva-Antonova, D.; Stoicheva, P.; Antonov, L. Chemical profiling of Bulgarian rose absolute (Rosa damascena Mill.) using gas chromatography–mass spectrometry and trimethylsilyl derivatives. Ind. Crops Prod. 2017, 108, 36–43. [Google Scholar] [CrossRef]
- de la Mata, P.; Dominguez-Vidal, A.; Bosque-Sendra, J.M.; Ruiz-Medina, A.; Cuadros-Rodríguez, L.; Ayora-Cañada, M.J. Olive oil assessment in edible oil blends by means of ATR-FTIR and chemometrics. Food Control 2012, 23, 449–455. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for Rapid Authentication and Detection of Adulteration of Food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Xin, Z.; Ren, D.; Yi, L. GC-MS fingerprinting combined with chemometric methods reveals key bioactive components in Acori tatarinowii rhizoma. Int. J. Mol. Sci. 2017, 18, 1342. [Google Scholar] [CrossRef]
- Temizkan, R.; Can, A.; Dogan, M.A.; Mortas, M.; Ayvaz, H. Rapid detection of milk fat adulteration in yoghurts using near and mid-infrared spectroscopy. Int. Dairy J. 2020, 110, 104795. [Google Scholar] [CrossRef]
- Paradkar, M.M.; Sivakesava, S.; Irudayaraj, J. Discrimination and classification of adulterants in maple syrup with the use of infrared spectroscopic techniques. J. Sci. Food Agric. 2003, 83, 714–721. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Application of Fourier Transform Infrared Spectroscopy for Authentication of Functional Food Oils. Appl. Spectrosc. Rev. 2012, 47, 1–13. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J. A rapid spectroscopic technique for determining honey adulteration with corn syrup. J. Food Sci. 2001, 66, 787–792. [Google Scholar] [CrossRef]
- Fagan, C.C.; Everard, C.; O’Donnell, C.P.; Downey, G.; Sheehan, E.M.; Delahunty, C.M.; O’Callaghan, D.J.; Howard, V. Prediction of processed cheese instrumental texture and meltability by mid-infrared spectroscopy coupled with chemometric tools. J. Food Eng. 2007, 80, 1068–1077. [Google Scholar] [CrossRef]
- Vongsvivut, J.; Heraud, P.; Zhang, W.; Kralovec, J.A.; McNaughton, D.; Barrow, C.J. Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem. 2012, 135, 603–609. [Google Scholar] [CrossRef]
- Bombarda, I.; Dupuy, N.; Le Van Da, J.P.; Gaydou, E.M. Comparative chemometric analyses of geographic origins and compositions of lavandin var. Grosso essential oils by mid infrared spectroscopy and gas chromatography. Anal. Chim. Acta 2008, 613, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hirri, A.; Bassbasi, M.; Kzaiber, F.; Oussama, A. Prediction of oil content in olive fruit using Fourier transformed infrared spectroscopy FT-IR coupled with partial least squares regression. Int. Food Res. J. 2014, 21, 723–727. [Google Scholar]
- Aureli, R.; Ueberschlag, Q.; Klein, F.; Noël, C.; Guggenbuhl, P. Use of near infrared reflectance spectroscopy to predict phytate phosphorus, total phosphorus, and crude protein of common poultry feed ingredients. Poult. Sci. 2017, 96, 160–168. [Google Scholar] [CrossRef]
- Boren, K.E.; Young, D.G.; Woolley, C.L.; Smith, B.L.; Carlson, R.E. Detecting Essential Oil Adulteration. J. Environ. Anal. Chem. 2015, 2, 1–4. [Google Scholar]
- Pellati, F.; Orlandini, G.; Van Leeuwen, K.A.; Anesin, G.; Bertelli, D.; Paolini, M.; Benvenuti, S.; Camin, F. Gas chromatography combined with mass spectrometry, flame ionization detection and elemental analyzer/isotope ratio mass spectrometry for characterizing and detecting the authenticity of commercial essential oils of Rosa damascena Mill. Rapid Commun. Mass Spectrom. 2013, 27, 591–602. [Google Scholar] [CrossRef]
- Krupčík, J.; Gorovenko, R.; Špánik, I.; Sandra, P.; Armstrong, D.W. Enantioselective comprehensive two-dimensional gas chromatography. A route to elucidate the authenticity and origin of Rosa damascena Miller essential oils. J. Sep. Sci. 2015, 38, 3397–3403. [Google Scholar] [CrossRef] [PubMed]
- Mahboubifar, M.; Hemmateenejad, B.; Jassbi, A.R. Evaluation of adulteration in distillate samples of Rosa damascena Mill using colorimetric sensor arrays, chemometric tools and dispersive liquid–liquid microextraction-GC-MS. Phytochem. Anal. 2021, 1–12. [Google Scholar]
- Alvarez-Ordonez, A.; Prieto, M. Fourier Transform Infrared Spectroscopy in Food Microbiology; Hartel, R.W., Clark, J.P., Rodriguez-Lazaro, D., Topping, D., Eds.; Springer: New York, NY, USA, 2012; ISBN 9781461438120. [Google Scholar]
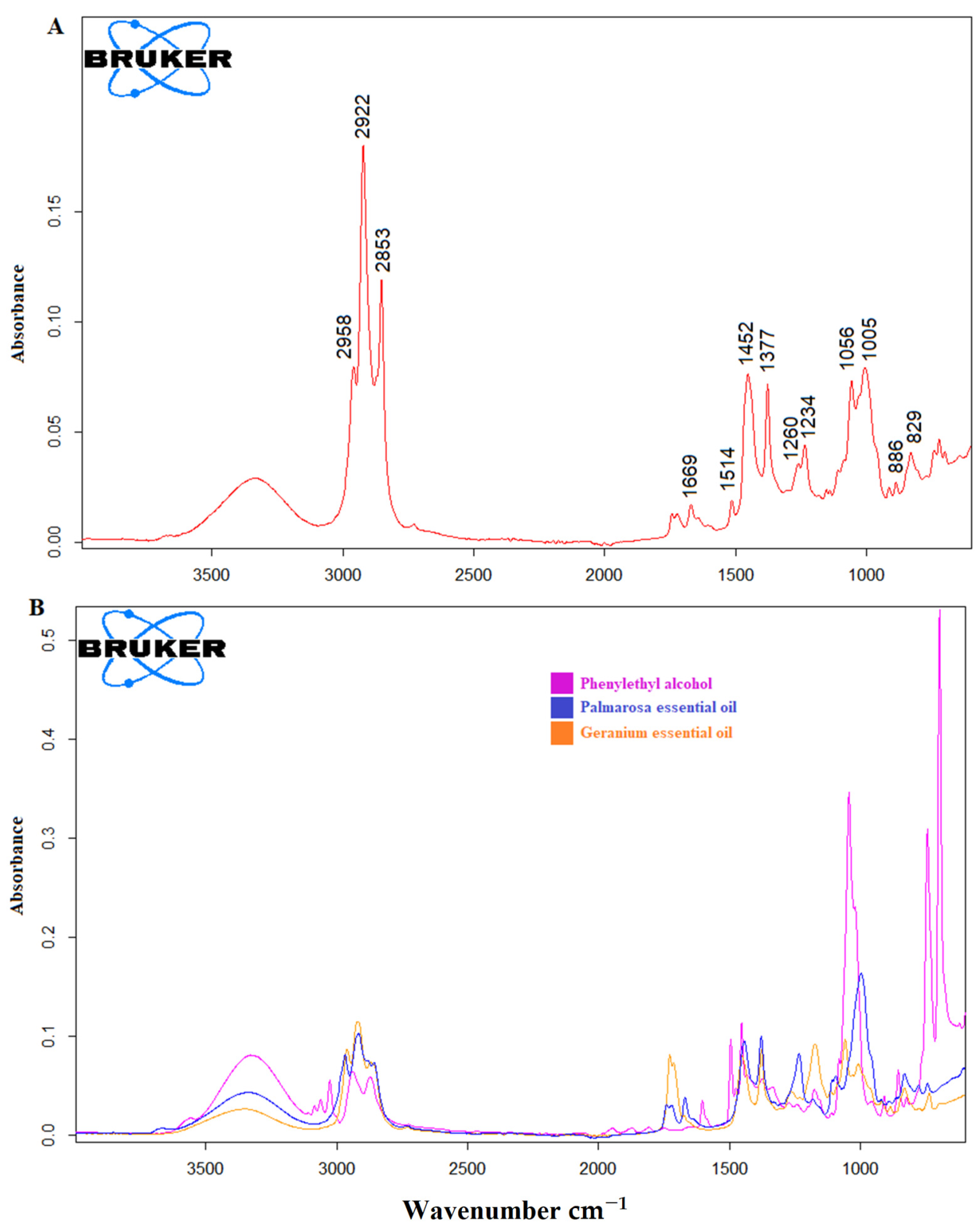

| Band (cm−1) | Band Assignment |
|---|---|
| 3345 | O–H stretching |
| 2958 | methyl C–H stretching |
| 2922 | C–H asymmetric stretching |
| 2853 | C–H symmetric stretching |
| 1669 | C=C stretching |
| 1514 | C=C stretching |
| 1452 | C–H bending |
| 1377 | C–H bending |
| 1260 | C–C–O stretching |
| 1234 | C–O stretching |
| 1056 | O–H stretching |
| 1005 | C–H bending |
| 829 | C–H stretching |
| Sample Codes | Model | Factors | Preprocessing | Calibration (Cross-Validation) | Press | SECV | Bias | |
|---|---|---|---|---|---|---|---|---|
| Equation | Regression Coefficient | |||||||
| PEO1 REO1 (PEO1 adulterated REO1) | PLSR | 3 | Raw | y = 0.9598x + 0.6122 | R² = 0.9969 | 34.57 | 2.08 | 1.96 |
| 3 | First derivative | y = 0.9549x + 0.6494 | R² = 0.9913 | 77.92 | 3.12 | 2.78 | ||
| 3 | Second derivative | y = 0.9528x + 0.7053 | R² =0.9922 | 107.36 | 3.66 | 2.64 | ||
| PCR | 3 | Raw | y = 0.9669x + 0.4623 | R² =0.9935 | 52.06 | 2.55 | 2.31 | |
| 3 | First derivative | y = 0.955x + 0.6493 | R² = 0.9913 | 86.83 | 3.29 | 2.78 | ||
| 3 | Second derivative | y = 0.9528x + 0.7065 | R² = 0.9923 | 101.95 | 3.57 | 2.64 | ||
| PEO2 REO2 (PEO2 adulterated REO2) | PLSR | 3 | Raw | y = 0.9774x + 0.3068 | R² = 0.9974 | 52.08 | 2.55 | 1.50 |
| 3 | First derivative | y = 0.9427x + 0.8437 | R² = 0.9937 | 80.15 | 3.16 | 2.75 | ||
| 3 | Second derivative | y = 0.9405x + 0.9284 | R² = 0.9921 | 61.41 | 2.77 | 2.91 | ||
| PCR | 3 | Raw | y = 0.9775x + 0.3064 | R² = 0.9974 | 36.78 | 2.14 | 1.50 | |
| 3 | First derivative | y = 0.9424x + 0.8368 | R² = 0.9850 | 110.19 | 3.71 | 3.57 | ||
| 3 | Second derivative | y = 0.9416x + 0.8863 | R² = 0.9863 | 137.90 | 4.15 | 3.41 | ||
| PEO3 REO3 (PEO3 adulterated REO3) | PLSR | 3 | Raw | y = 0.9779x + 0.309 | R² = 0.9971 | 45.63 | 2.39 | 1.50 |
| 3 | First derivative | y = 0.9516x + 0.6694 | R² = 0.9909 | 87.62 | 3.30 | 2.84 | ||
| 3 | Second derivative | y = 0.9593x + 0.5599 | R² = 0.9956 | 55.95 | 2.64 | 1.84 | ||
| PCR | 3 | Raw | y = 0.9779x + 0.3086 | R² = 0.9971 | 44.14 | 2.35 | 1.50 | |
| 3 | First derivative | y = 0.9516x + 0.6695 | R² = 0.9908 | 64.04 | 2.83 | 2.84 | ||
| 3 | Second derivative | y = 0.9518x + 0.6974 | R² = 0.9906 | 66.83 | 2.89 | 2.70 | ||
| GEO1 REO1 (GEO1 Adulterated REO1) | PLSR | 3 | Raw | y = 0.9921x + 0.0928 | R² = 0.9997 | 6.00 | 0.87 | 0.39 |
| 3 | First derivative | y = 0.9778x + 0.3265 | R² = 0.9987 | 15.83 | 1.40 | 1.20 | ||
| 3 | Second derivative | y = 0.9708x + 0.4261 | R² = 0.9961 | 47.13 | 2.43 | 1.89 | ||
| PCR | 3 | Raw | y = 0.9916x + 0.1038 | R² = 0.9997 | 2.62 | 0.57 | 0.41 | |
| 3 | First derivative | y = 0.9777x + 0.3203 | R² = 0.9987 | 18.51 | 1.52 | 1.22 | ||
| 3 | Second derivative | y = 0.9708x + 0.4262 | R² = 0.9961 | 43.05 | 2.32 | 1.89 | ||
| GEO2 REO2 (GEO2 Adulterated REO2) | PLSR | 3 | Raw | y = 1.0003x − 0.0249 | R² = 0.9998 | 1.49 | 0.43 | 0.28 |
| 3 | First derivative | y = 0.9799x + 0.2364 | R² = 0.9992 | 10.38 | 1.14 | 1.02 | ||
| 3 | Second derivative | y = 0.9783x + 0.1424 | R² = 0.9987 | 15.43 | 1.39 | 1.23 | ||
| PCR | 3 | Raw | y = 1.0014x − 0.0348 | R² = 0.9998 | 1.58 | 0.44 | 0.29 | |
| 3 | First derivative | y = 0.9716x + 0.3795 | R² = 0.9987 | 15.06 | 1.37 | 1.39 | ||
| 3 | Second derivative | y = 0.972x + 0.4117 | R² = 0.9971 | 27.31 | 1.85 | 1.67 | ||
| GEO3 REO3 (GEO3 Adulterated REO3) | PLSR | 3 | Raw | y = 1.0067x − 0.2283 | R² = 0.9998 | 2.17 | 0.52 | 0.34 |
| 3 | First derivative | y = 0.9856x + 0.1796 | R² = 0.9997 | 4.62 | 0.76 | 0.67 | ||
| 3 | Second derivative | y = 0.9844x + 0.1644 | R² = 0.9991 | 11.11 | 1.18 | 0.82 | ||
| PCR | 3 | Raw | y = 0.9988x − 0.0033 | R² = 0.9998 | 2.33 | 0.54 | 0.34 | |
| 3 | First derivative | y = 0.9857x + 0.179 | R² = 0.9997 | 4.76 | 0.77 | 0.67 | ||
| 3 | Second derivative | y = 0.9847x + 0.2223 | R² = 0.9989 | 11.74 | 1.21 | 0.82 | ||
| PEOH REO1 (PEOH Adulterated REO1) | PLSR | 3 | Raw | y = 0.9806x + 0.4322 | R² = 0.9983 | 6.03 | 0.74 | 0.38 |
| 3 | First derivative | y = 0.9913x + 0.3094 | R² = 0.9996 | 4.37 | 0.87 | 0.39 | ||
| 3 | Second derivative | y = 0.9447x + 1.0213 | R² = 0.9881 | 98.56 | 3.51 | 2.87 | ||
| PEOH REO1 (PEOH Adulterated REO1) | PCR | 3 | Raw | y = 0.9804x + 0.4348 | R² = 0.9981 | 5.80 | 0.73 | 0.39 |
| 3 | First derivative | y = 0.9927x + 0.2686 | R² = 0.9996 | 4.26 | 0.85 | 0.42 | ||
| 3 | Second derivative | y = 0.9447x + 1.022 | R² = 0.9881 | 115.52 | 3.80 | 2.88 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cebi, N. Quantification of the Geranium Essential Oil, Palmarosa Essential Oil and Phenylethyl Alcohol in Rosa damascena Essential Oil Using ATR-FTIR Spectroscopy Combined with Chemometrics. Foods 2021, 10, 1848. https://doi.org/10.3390/foods10081848
Cebi N. Quantification of the Geranium Essential Oil, Palmarosa Essential Oil and Phenylethyl Alcohol in Rosa damascena Essential Oil Using ATR-FTIR Spectroscopy Combined with Chemometrics. Foods. 2021; 10(8):1848. https://doi.org/10.3390/foods10081848
Chicago/Turabian StyleCebi, Nur. 2021. "Quantification of the Geranium Essential Oil, Palmarosa Essential Oil and Phenylethyl Alcohol in Rosa damascena Essential Oil Using ATR-FTIR Spectroscopy Combined with Chemometrics" Foods 10, no. 8: 1848. https://doi.org/10.3390/foods10081848
APA StyleCebi, N. (2021). Quantification of the Geranium Essential Oil, Palmarosa Essential Oil and Phenylethyl Alcohol in Rosa damascena Essential Oil Using ATR-FTIR Spectroscopy Combined with Chemometrics. Foods, 10(8), 1848. https://doi.org/10.3390/foods10081848