Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham
Abstract
1. Introduction
2. Materials and Methods
2.1. Dry-Cured Ham Samples
2.2. Physicochemical Analysis
2.3. Colour
2.4. Texture Profile Analysis
2.5. Analysis of Volatile Compounds
2.6. Statistical Data Analyses
3. Results and Discussion
3.1. Changes in Physicochemical Parameters during the Production of Smoked Dry-Cured Ham
3.2. Instrumental Measurement of Color and Texture of Smoked Dry-Cured Ham
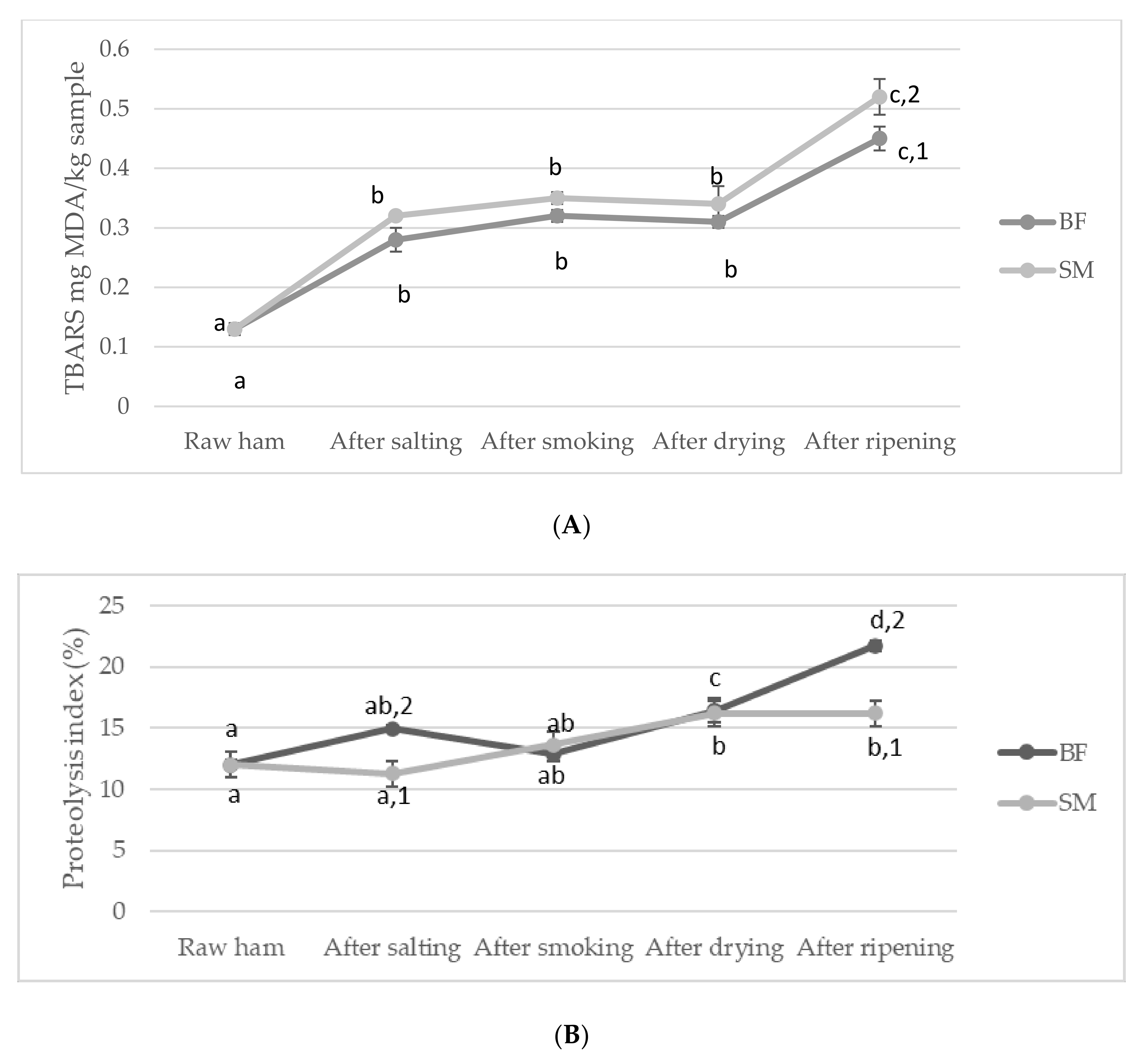
3.3. Effects of Processing Time and Muscle Type on Lipolysis and Proteolysis in Smoked Dry-Cured Ham
3.4. Evaluation of Volatile Compounds during the Processing of Smoked Dry-Cured Ham
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marušić Radovčić, N.; Vidaček, S.; Janči, T.; Medić, H. Characterization of volatile compounds, physico-chemical and sensory characteristics of smoked dry-cured ham. J. Food Sci. Technol. 2016, 53, 4093–4105. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.; Aasen, I.M.; Rustad, T.; Eikevik, T.M. Manufacture of dry-cured ham: A review. Part 1. Biochemical changes during the technological process. Eur. Food Res. Technol. 2015, 241, 587–599. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Škrlep, M. Factors in pig production that impact the quality of dry-cured ham: A review. Animal 2012, 6, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.I.; Ventanas, S.; Ventanas, J.; Cava, R.; Ruiz, J. Physicochemical changes throughout the ripening of dry cured hams with different salt content and processing conditions. Eur. Food Res. Technol. 2005, 221, 30–35. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Chemical changes in volatile aldehydes and ketones from subcutaneous fat during ripening of Iberian dry-cured ham. Prediction of the curing time. Food Res. Int. 2014, 55, 381–390. [Google Scholar] [CrossRef]
- Cava, R.; Ruiz, J.; Ventanas, J.; Antequera, T. Oxidative and lipolytic changes during ripening of Iberian hams as affected by feeding regime: Extensive feeding and alpha-tocopheryl acetate supplementation. Meat Sci. 1999, 52, 165–172. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Lipolysis, lipid peroxidation and texture of Serrano ham processed under different ripening temperature conditions. Int. J. Food Sci. Technol. 2016, 51, 1793–1800. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Physicochemical changes during manufacture and final sensory characteristics of dry-cured Celta ham. Effect of muscle type. Food Control 2014, 43, 263–269. [Google Scholar] [CrossRef]
- Harkouss, R.; Astruc, T.; Lebert, A.; Gatellier, P.; Loison, O.; Safa, H.; Portanguen, S.; Parafita, E.; Mirade, P.S. Quantitative study of the relationships among proteolysis, lipid oxidation, structure and texture throughout the dry-cured ham process. Food Chem. 2015, 166, 522–530. [Google Scholar] [CrossRef]
- Sirtori, F.; Dimauro, C.; Bozzi, R.; Aquilani, C.; Franci, O.; Calamai, L.; Pezzati, A.; Pugliese, C. Evolution of volatile compounds and physical, chemical and sensory characteristics of Toscano PDO ham from fresh to dry-cured product. Eur. Food Res. Technol. 2020, 246, 409–424. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Calamai, L.; Franci, O. The evolution of volatile compounds profile of “Toscano” dry-cured ham during ripening as revealed by SPME-GC-MS approach. J. Mass Spectrom. 2010, 45, 1056–1064. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, G.; Zhao, G.; Xu, X.; Peng, Z. Changes in flavor compounds of dry-cured Chinese Jinhua ham during processing. Meat Sci. 2005, 71, 291–299. [Google Scholar] [CrossRef]
- Karpiński, P.; Kruszewski, B.; Stachelska, M.A.; Szabłowska, E. Development of volatile profile of Kumpiak podlaski dry-cured ham during traditional ripening. Int. J. Food Sci. Technol. 2020, 55, 3630–3638. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Škrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Čandek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Bicep femoris and Semimembranosus muscles of the Slovenian dry-cured ham Kraški pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of type of muscle on volatile compounds throughout the manufacture of Celta dry-cured ham. Food Sci. Technol. Int. 2015, 21, 581–592. [Google Scholar] [CrossRef]
- Gilles, G. Dry cured ham quality as related to lipid quality of raw material and lipid changes during processing: A review. Grasas y Aceites 2009, 60, 297–307. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Sentandreu, M.Á.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists [AOAC]. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburgh, MD, USA, 2000. [Google Scholar]
- International Organization for Standardization [ISO] 937. Meat and Meat Products. Determination of Nitrogen Content (Reference Method); International Organization for Standardization: Geneva, Switzerland, 1978. [Google Scholar]
- Monin, G.; Marinova, P.; Talmant, A.; Martin, J.F.; Cornet, M.; Lanore, D.; Grasso, F. Chemical and structural changes in dry-cured hams (bayonne hams) during processing and effects of the dehairing technique. Meat Sci. 1997, 47, 29–47. [Google Scholar] [CrossRef]
- Bruna, J.M.; Ordóñez, J.A.; Fernández, M.; Herranz, B.; De La Hoz, L. Microbial and physico-chemical changes during the ripening of dry fermented sausages superficially inoculated with or having added an intracellular cell-free extract of Penicillium aurantiogriseum. Meat Sci. 2001, 59, 87–96. [Google Scholar] [CrossRef]
- Marušić, N.; Petroví, M.; Vidaček, S.; Petrak, T.; Medić, H. Characterization of traditional Istrian dry-cured ham by means of physical and chemical analyses and volatile compounds. Meat Sci. 2011, 88, 786–790. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by GCMS, 3rd ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2001. [Google Scholar]
- Toldrá, F.; Flores, M. The role of muscle proteases and lipases in flavor development during the processing of dry-cured ham. Crit. Rev. Food Sci. Nutr. 1998, 38, 331–352. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, D.; Zhou, G.; Wang, Y.; Dang, Y.; He, J.; Li, G.; Cao, J. The Changes of the Volatile Compounds Derived from Lipid Oxidation of Boneless Dry-Cured Hams During Processing. Eur. J. Lipid Sci. Technol. 2019, 121, 1–9. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Ventanas, J.; Toldrá, F. Nutritional composition of dry-cured ham and its role in a healthy diet. Meat Sci. 2010, 84, 585–593. [Google Scholar] [CrossRef]
- Schivazappa, C.; Degni, M.; Nanni Costa, L.; Russo, V.; Buttazzoni, L.; Virgili, R. Analysis of raw meat to predict proteolysis in Parma ham. Meat Sci. 2002, 60, 77–83. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Purriños, L. Changes on Physico-chemical, Textural, Proteolysis, Lipolysis and Volatile Compounds During the Manufacture of Dry-cured “Lacón” from Celta Pig Breed. J. Biol. Sci. 2013, 13, 168–182. [Google Scholar] [CrossRef][Green Version]
- Martuscelli, M.; Pittia, P.; Casamassima, L.M.; Manetta, A.C.; Lupieri, L.; Neri, L. Effect of intensity of smoking treatment on the free amino acids and biogenic amines occurrence in dry cured ham. Food Chem. 2009, 116, 955–962. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, J.; Arnau, J.; Serra, X.; Gou, P. Effect of pH24, NaCl content and proteolysis index on the relationship between water content and texture parameters in biceps femoris and semimembranosus muscles in dry-cured ham. Meat Sci. 2006, 72, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Sensory properties and physico-chemical changes in the biceps femoris muscle during processing of dry-cured ham from celta pigs. Effects of cross-breeding with duroc and landrace pigs. Ital. J. Food Sci. 2017, 29, 123–137. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Ruiz, J.; Martín, D.; Barat, J.M.; Antequera, T. Pre-cure freezing effect on physicochemical, texture and sensory characteristics of Iberian ham. Food Sci. Technol. Int. 2011, 17, 127–133. [Google Scholar] [CrossRef]
- Cilla, I.; Martínez, L.; Beltrán, J.A.; Roncalés, P. Factors affecting acceptability of dry-cured ham throughout extended maturation under “bodega” conditions. Meat Sci. 2005, 69, 789–795. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Pérez-Santaescolástica, C.; Franco, D.; Fulladosa, E.; Carballo, J.; Zapata, C.; Lorenzo, J.M. Comparative proteomic profiling of myofibrillar proteins in dry-cured ham with different proteolysis indices and adhesiveness. Food Chem. 2018, 244, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Serra, X.; Ruiz-Ramírez, J.; Arnau, J.; Gou, P. Texture parameters of dry-cured ham m. biceps femoris samples dried at different levels as a function of water activity and water content. Meat Sci. 2005, 69, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Andronikov, D.; Gašperlin, L.; Polak, T.; Žlender, B. Texture and quality parameters of slovenian dry-cured ham kraški pršut according to mass and salt levels. Food Technol. Biotechnol. 2013, 51, 112–122. [Google Scholar]
- Lorenzo, J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “cecina”. Meat Sci. 2014, 96, 256–263. [Google Scholar] [CrossRef]
- Bozkurt, H.; Bayram, M. Colour and textural attributes of sucuk during ripening. Meat Sci. 2006, 73, 344–350. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of instrumental texture properties from textural profile analysis (TPA) with eating behaviours and macronutrient composition for a wide range of solid foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef]
- Toldrá, F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Koutina, G.; Jongberg, S.; Skibsted, L.H. Protein and lipid oxidation in Parma ham during production. J. Agric. Food Chem. 2012, 60, 9737–9745. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Huang, T.; Li, F.; Sun, J. Lipolysis and lipid oxidation during processing of Chinese traditional smoke-cured bacon. Food Chem. 2014, 149, 31–39. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Zhang, J.; Yu, X.; Wang, J.; Huang, F. Effects of temperature and NaCl percentage on lipid oxidation in pork muscle and exploration of the controlling method using response surface methodology (RSM). Food Chem. 2012, 131, 817–825. [Google Scholar] [CrossRef]
- Zhou, G.H.; Zhao, G.M. Biochemical changes during processing of traditional Jinhua ham. Meat Sci. 2007, 77, 114–120. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, C.; Pan, D.; Wang, Y.; Cao, J. The effect of sodium chloride levels on the taste and texture of dry-cured ham. J. Food Meas. Charact. 2020, 14, 2646–2655. [Google Scholar] [CrossRef]
- Harkouss, R.; Mirade, P.S.; Gatellier, P. Development of a rapid, specific and efficient procedure for the determination of proteolytic activity in dry-cured ham: Definition of a new proteolysis index. Meat Sci. 2012, 92, 84–88. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J.; Franco, D. Effect of the inclusion of chestnut in the finishing diet on volatile compounds of dry-cured ham from celta pig breed. J. Integr. Agric. 2013, 12, 2002–2012. [Google Scholar] [CrossRef]
- Pastorelli, G.; Magni, S.; Rossi, R.; Pagliarini, E.; Baldini, P.; Dirinck, P.; Van Opstaele, F.; Corino, C. Influence of dietary fat, on fatty acid composition and sensory properties of dry-cured Parma ham. Meat Sci. 2003, 65, 571–580. [Google Scholar] [CrossRef]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Generation of volatile flavour compounds as affected by the chemical composition of different dry-cured ham sections. Eur. Food Res. Technol. 2006, 222, 658–666. [Google Scholar] [CrossRef]
- Petričević, S.; Marušić Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R.; Aparicio-Ruiz, R. Volatile and amino acid profiling of dry cured hams from different swine breeds and processing methods. Molecules 2013, 18, 3927–3947. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids in muscles and adipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Purriños, L.; Bermúdez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Development of Volatile Compounds during the Manufacture of Dry-Cured “Lacón,” a Spanish Traditional Meat Product. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef]
| Phase of Production | |||||
|---|---|---|---|---|---|
| Raw Ham | After Salting | After Smoking | After Drying | After Ripening | |
| Water (%) | |||||
| BF | 74.82 ± 0.12 e | 72.33 ± 0.37 d,2 | 68.93 ± 0.85 c,2 | 63.41 ± 0.55 b,2 | 56.78 ± 0.30 a,2 |
| SM | 74.42 ± 0.22 d | 64.12 ± 0.36 c,1 | 63.79 ± 0.29 c,1 | 59.21 ± 0.53 b,1 | 42.54 ± 0.75 a,1 |
| Intramuscular fat (% of dry matter) | |||||
| BF | 5.54 ± 0.54 b | 5.05 ± 0.19 b,1 | 2.57 ± 0.18 a,1 | 5.38 ± 0.44 b,1 | 12.45 ± 0.45 c,1 |
| SM | 6.00 ± 0.44 a | 5.72 ± 0.23 a,2 | 5.55 ± 0.25 a,2 | 11.02 ± 0.71 b,2 | 14.86 ± 0.35 c,2 |
| Protein (% of dry matter) | |||||
| BF | 88.19 ± 0.91 c | 87.11 ± 1.60 c,1 | 78.20 ± 0.82 b,1 | 70.35 ± 1.23 a,1 | 69.16 ± 1.22 a,1 |
| SM | 89.54 ± 1.12 c | 75.96 ± 0.89 ab,2 | 79.82 ± 1.76 b,2 | 78.26 ± 0.92 ab,2 | 74.72 ± 0.72 a,2 |
| Ash (% of dry matter) | |||||
| BF | 4.37 ± 0.11 a | 8.24 ± 0.35 b,1 | 13.44 ± 0.32 c,1 | 17.68 ± 0.35 d,1 | 19.33 ± 0.48 e,2 |
| SM | 4.21 ± 0.08 a | 18.97 ± 0.52 c,2 | 18.21 ± 0.35 c,2 | 18.36 ± 0.23 c,2 | 12.91 ± 0.36 b,1 |
| NaCl (% of dry matter) | |||||
| BF | 0.20 ± 0.00 a | 4.87 ± 0.44 b,1 | 12.47 ± 0.20 c,1 | 15.06 ± 0.32 d | 17.55 ± 0.50 e,2 |
| SM | 0.18 ± 0.00 a | 16.78 ± 0.61 c,2 | 16.39 ± 0.27 c,2 | 16.29 ± 0.52 c | 11.20 ± 0.35 b,1 |
| aw | |||||
| BF | 0.97 ± 0.00 c | 0.97 ± 0.00 c,2 | 0.96 ± 0.00 c,2 | 0.93 ± 0.00 b,2 | 0.88 ± 0.00 a,2 |
| SM | 0.96 ± 0.00 d | 0.90 ± 0.01 b,1 | 0.93 ± 0.00 c,1 | 0.91 ± 0.00 bc,1 | 0.86 ± 0.00 a,1 |
| pH | |||||
| BF | 5.83 ± 0.05 | 5.74 ± 0.06 | 6.02 ± 0.31 | 5.65 ± 0.02 | 5.93 ± 0.03 |
| SM | 5.78 ± 0.10 bc | 5.74 ± 0.04 abc | 5.64 ± 0.04 ab | 5.51 ± 0.07 a | 5.99 ± 0.02 c |
| Phase of Production | |||||
|---|---|---|---|---|---|
| Raw Ham | After Salting | After Smoking | After Drying | After Ripening | |
| L* | |||||
| BF | 54.02 ± 0.48 c | 51.55 ± 0.18 a,2 | 53.53 ± 0.27 bc,2 | 54.31 ± 0.47 c,2 | 52.06 ± 0.40 ab,2 |
| SM | 54.41 ± 0.75 c | 48.21 ± 0.21 ab,1 | 49.98 ± 0.57 b,1 | 49.88 ± 0.41 b,1 | 47.83 ± 0.34 a,1 |
| a* | |||||
| BF | 2.96 ± 0.18 b,2 | 1.95 ± 0.11 a | 1.88 ± 0.14 a | 1.86 ± 0.16 a,2 | 2.98 ± 0.18 b,2 |
| SM | 1.77 ± 0.09 ab,1 | 1.84 ± 0.13 ab | 1.76 ± 0.14 ab | 1.38 ± 0.09 a,1 | 2.25 ± 0.28 b,1 |
| b* | |||||
| BF | 8.30 ± 0.37 c | 8.32 ± 0.19 c | 3.74 ± 0.20 a,2 | 4.20 ± 0.09 a,2 | 5.28 ± 0.19 b,2 |
| SM | 8.20 ± 0.39 c | 8.21 ± 0.14 c | 3.26 ± 0.12 a,1 | 3.41 ± 0.08 a,1 | 4.47 ± 0.16 b,1 |
| Phase of Production | |||||
|---|---|---|---|---|---|
| Raw Ham | After Salting | After Smoking | After Drying | After Ripening | |
| Hardness (N) | |||||
| BF | 89.78 ± 3.50 b | 93.81 ± 1.71 bc | 105.30 ± 5.68 c | 92.98 ± 1.74 bc,1 | 58.00 ± 3.09 a,1 |
| SM | 82.21 ± 2.25 a | 98.24 ± 1.31 b | 114.72 ± 5.15 c | 121.04 ± 2.08 c,2 | 82.62 ± 2.05 a,2 |
| Gumminess (N) | |||||
| BF | 45.50 ± 1.43 b | 47.48 ± 1.18 b | 51.89 ± 0.49 c,1 | 43.85 ± 0.52 b,1 | 23.37 ± 0.99 a,1 |
| SM | 42.35 ± 1.24 ab | 50.41 ± 3.09 bc | 56.51 ± 2.02 cd,2 | 63.53 ± 0.76 d,2 | 40.19 ± 2.45 a,2 |
| Chewiness (N x mm) | |||||
| BF | 54.75 ± 1.73 b | 61.43 ± 1.37 cd,1 | 69.49 ± 1.18 d,1 | 59.61 ± 1.29 bc,1 | 29.87 ± 1.23 a,1 |
| SM | 58.21 ± 1.45 b | 64.82 ± 0.31 c,2 | 72.82 ± 0.85 d,2 | 79.98 ± 1.00 e,2 | 52.69 ± 0.59 a,2 |
| Adhesiveness (N x mm) | |||||
| BF | 0.94 ± 0.07 c | 0.83 ± 0.12 bc,2 | 0.77 ± 0.04 bc,2 | 0.59 ± 0.06 ab,2 | 0.45 ± 0.04 a,2 |
| SM | 0.89 ± 0.02 d | 0.62 ± 0.04 c,1 | 0.58 ± 0.02 c,1 | 0.43 ± 0.01 b,1 | 0.34 ± 0.02 a,1 |
| Springiness (mm) | |||||
| BF | 0.80 ± 0.08 b | 0.85 ± 0.01 b,2 | 0.91 ± 0.02 b,2 | 0.56 ± 0.01 a,2 | 0.45 ± 0.01 a,2 |
| SM | 0.75 ± 0.01 b | 0.81 ± 0.01 c,1 | 0.83 ± 0.01 c,1 | 0.41 ± 0.01 a,1 | 0.40 ± 0.01 a,1 |
| Cohesiveness | |||||
| BF | 0.50 ± 0.02 b,1 | 0.49 ± 0.02 b | 0.46 ± 0.01 b,2 | 0.43 ± 0.01 ab | 0.38 ± 0.01 a,2 |
| SM | 0.59 ± 0.02 d,2 | 0.53 ± 0.01 c | 0.42 ± 0.01 b,1 | 0.40 ± 0.01 b | 0.33 ± 0.01 a,1 |
| Resilience | |||||
| BF | 0.40 ± 0.02 a,1 | 0.42 ± 0.02 a | 0.36 ± 0.02 a,1 | 0.54 ± 0.03 b,2 | 0.43 ± 0.01 a |
| SM | 0.47 ± 0.02 ab,2 | 0.47 ± 0.02 ab | 0.50 ± 0.02 b,2 | 0.47 ± 0.01 ab,1 | 0.40 ± 0.02 a |
| Muscle (M) | Phase of Production (P) | M × P | |
|---|---|---|---|
| Physicochemical parameter | |||
| Water | 0.000 | 0.000 | 0.000 |
| Intramuscular fat | 0.000 | 0.000 | 0.000 |
| Protein | 0.156 | 0.000 | 0.000 |
| Ash | 0.000 | 0.000 | 0.000 |
| NaCl | 0.000 | 0.000 | 0.000 |
| aw | 0.000 | 0.000 | 0.000 |
| pH | 0.166 | 0.020 | 0.329 |
| mg MDA/kg sample | 0.003 | 0.000 | 0.708 |
| Proteolysis index | 0.004 | 0.000 | 0.003 |
| Color parameters | |||
| L* | 0.000 | 0.000 | 0.000 |
| a* | 0.000 | 0.000 | 0.005 |
| b* | 0.001 | 0.000 | 0.283 |
| Texture parameters | |||
| Hardness (N) | 0.000 | 0.000 | 0.000 |
| Gumminess (N) | 0.000 | 0.000 | 0.000 |
| Chewiness (N × mm) | 0.000 | 0.000 | 0.000 |
| Adhesiveness (N × mm) | 0.000 | 0.000 | 0.000 |
| Springiness (mm) | 0.000 | 0.000 | 0.301 |
| Cohesiveness | 0.965 | 0.000 | 0.040 |
| Resilience | 0.011 | 0.000 | 0.000 |
| Volatiles | |||
| Aldehydes | 0.000 | 0.000 | 0.000 |
| Alcohols | 0.000 | 0.000 | 0.000 |
| Ketones | 0.000 | 0.000 | 0.011 |
| Aromatic hydrocarbons | 0.047 | 0.000 | 0.000 |
| Esters | 0.010 | 0.000 | 0.000 |
| Aliphatic hydrocarbons | 0.539 | 0.000 | 0.231 |
| Phenols | 0.000 | 0.000 | 0.000 |
| Terpenes | 0.000 | 0.000 | 0.000 |
| Volatile Compounds | RI | Muscle | Raw Ham | After Salting | After Smoking | After Drying | After Ripening | Identification |
|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||
| 3-Methylbutanal | 645 | BF | 0.30 ± 0.06 b,2 | 0.00 ± 0.00 a | 0.03 ± 0.00 a | 0.07 ± 0.01 a | 0.42 ± 0.10 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a | 0.04 ± 0.02 ab | 0.08 ± 0.02 bc | 0.13 ± 0.01 c,1 | MS, RI | ||
| 2-Methylbutanal | 654 | BF | 0.96 ± 0.20 b,2 | 0.00 ± 0.00 a | 0.07 ± 0.01 a | 0.14 ± 0.02 a | 0.30 ± 0.08 a,2 | MS, RI |
| SM | 0.20 ± 0.121 | 0.04 ± 0.03 | 0.05 ± 0.00 | 0.09 ± 0.05 | 0.10 ± 0.011 | MS, RI | ||
| Butanal | 657 | BF | 0.52 ± 0.16 b | 0.33 ± 0.08 ab | 0.19 ± 0.02 ab | 0.30 ± 0.05 ab | 0.00 ± 0.00 a | MS, RI |
| SM | 0.62 ± 0.13 c | 0.29 ± 0.05 b | 0.16 ± 0.02 ab | 0.24 ± 0.02 ab | 0.00 ± 0.00 a | MS, RI | ||
| Pentanal | 713 | BF | 1.88 ± 0.35 ab | 2.57 ± 0.48 b | 1.55 ± 0.14 ab | 1.49 ± 0.10 ab | 0.89 ± 0.16 a,2 | MS, RI |
| SM | 1.23 ± 0.59 ab | 1.98 ± 0.22 b | 1.08 ± 0.21 ab | 2.07 ± 0.47 b | 0.17 ± 0.03 a,1 | MS, RI | ||
| Hexanal | 799 | BF | 44.27 ± 3.18 bc | 54.44 ± 2.49 c | 44.30 ± 3.06 bc | 39.22 ± 2.53 b,2 | 10.90 ± 0.45 a,2 | MS, RI |
| SM | 48.58 ± 3.16 c | 55.53 ± 1.18 c | 41.37 ± 1.40 b | 7.26 ± 0.67 a,1 | 1.62 ± 0.07 a,1 | MS, RI | ||
| Heptanal | 903 | BF | 0.30 ± 0.13 a,2 | 1.08 ± 0.14 ab | 1.87 ± 0.22 c | 0.64 ± 0.04 a,1 | 3.23 ± 0.08 d,2 | MS, RI |
| SM | 0.00 ± 0.00 a,1 | 1.11 ± 0.14 bc | 1.31 ± 0.19 c | 1.52 ± 0.27 c,2 | 0.49 ± 0.06 ab,2 | MS, RI | ||
| Benzenaldehyde | 965 | BF | 1.70 ± 0.16 a | 1.40 ± 0.08 a | 3.20 ± 0.84 a | 1.08 ± 0.12 a,1 | 6.50 ± 1.42 b,2 | MS, RI |
| SM | 1.62 ± 0.08 a | 1.44 ± 0.11 a | 1.51 ± 0.12 a | 1.59 ± 0.21 a,2 | 2.24 ± 0.10 b,1 | MS, RI | ||
| 2,4-Heptadienal | 998 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.26 ± 0.03 b,1 | 0.17 ± 0.02 b,1 | 0.51 ± 0.05 c,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.11 ± 0.03 ab,2 | 0.68 ± 0.13 c,2 | 0.37 ± 0.06 b,2 | 0.00 ± 0.00 a,1 | MS, RI | ||
| Octanal | 1003 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.98 ± 0.31 b | 5.24 ± 0.72 c,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.59 ± 0.14 b | 1.53 ± 0.11 b,1 | MS, RI | ||
| Benzeneacetaldehyde | 1049 | BF | 1.04 ± 0.42 ab | 0.28 ± 0.07 a | 0.28 ± 0.02 a | 0.33 ± 0.11 a | 1.44 ± 0.15 b,1 | MS, RI |
| SM | 0.84 ± 0.19 a | 0.17 ± 0.05 a | 0.20 ± 0.04 a | 0.35 ± 0.06 a | 4.87 ± 1.66 b,2 | MS, RI | ||
| 2-Nonenal | 1063 | BF | 0.21 ± 0.10 a | 0.61 ± 0.06 c | 0.94 ± 0.07 d,1 | 0.49 ± 0.06 bc,1 | 0.33 ± 0.03 ab | MS, RI |
| SM | 0.16 ± 0.07 a | 0.69 ± 0.06 ab | 1.63 ± 0.30 c,2 | 0.88 ± 0.08 b,2 | 0.31 ± 0.02 ab | MS, RI | ||
| Nonanal | 1105 | BF | 3.27 ± 0.40 a | 2.71 ± 0.23 a | 3.88 ± 0.35 ab | 3.40 ± 0.43 a.1 | 5.31 ± 0.35 c,2 | MS, RI |
| SM | 3.79 ± 0.41 a | 2.90 ± 0.29 a | 4.01 ± 0.37 ab | 5.54 ± 0.67 b,2 | 3.23 ± 0.14 a,1 | MS, RI | ||
| Decanal | 1207 | BF | 0.00 ± 0.00 a | 0.32 ± 0.05 bc,2 | 0.27 ± 0.03 bc,1 | 0.42 ± 0.07 c,1 | 0.21 ± 0.01 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.18 ± 0.02 a,1 | 0.61 ± 0.12 b,2 | 0.65 ± 0.04 b,2 | 0.22 ± 0.01 a | MS, RI | ||
| 2,4-Nonadienal | 1213 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.30 ± 0.03 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.12 ± 0.02 b,1 | MS, RI | ||
| 2E-Decenal | 1284 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.91 ± 0.12 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.82 ± 0.04 b | MS, RI | ||
| 2,4-Decadienal | 1318 | BF | 0.00 ± 0.00 a | 0.34 ± 0.06 c | 0.19 ± 0.02 b | 0.00 ± 0.00 a | 0.34 ± 0.04 c,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.24 ± 0.04 a | 0.21 ± 0.03 a | 0.00 ± 0.00 a | 1.94 ± 0.18 b,2 | MS, RI | ||
| Tetradecanal | 1613 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.26 ± 0.02 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.13 ± 0.03 b,1 | MS, RI | ||
| Pentadecanal | 1716 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.23 ± 0.02 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.17 ± 0.03 b | MS, RI | ||
| Hexadecanal | 1818 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.73 ± 0.07 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.49 ± 0.08 b,1 | MS, RI | ||
| 9-Octadecanal | 1998 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.24 ± 0.03 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.17 ± 0.02 b | MS, RI | ||
| Total | BF | 54.44 ± 3.32 bc | 64.09 ± 2.53 c | 57.11 ± 2.45 bc | 49.72 ± 2.66 b,2 | 38.29 ± 2.24 a,2 | MS, RI | |
| SM | 58.04 ± 3.35 bc | 64.68 ± 1.43 c | 52.90 ± 1.32 b | 22.22 ± 0.89 a,1 | 18.74 ± 0.58 a,1 | MS, RI | ||
| Alcohols | ||||||||
| 2-Propen-1-ol | 625 | BF | 0.89 ± 0.21 b,2 | 0.27 ± 0.05 a | 0.13 ± 0.02 a,1 | 1.08 ± 0.23 b,2 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.41 ± 0.05 bc,1 | 0.28 ± 0.06 b | 0.24 ± 0.03 b,2 | 0.48 ± 0.05 c,1 | 0.00 ± 0.00 a | MS, RI | ||
| 1-Penten-3-ol | 706 | BF | 0.00 ± 0.00 a | 0.24 ± 0.04 bc | 0.13 ± 0.03 b | 0.25 ± 0.04 c,1 | 0.16 ± 0.02 bc,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.20 ± 0.05 b | 0.12 ± 0.02 b | 0.74 ± 0.00 c,2 | 0.00 ± 0.00 a,1 | MS, RI | ||
| 3-Buten-1-ol | 739 | BF | 1.65 ± 0.24 b,2 | 0.10 ± 0.05 a,1 | 0.10 ± 0.02 a | 0.22 ± 0.04 a | 0.00 ± 0.00 a | MS, RI |
| SM | 0.74 ± 0.14 c,1 | 0.30 ± 0.05 b,2 | 0.08 ± 0.03 ab | 0.16 ± 0.03 ab | 0.00 ± 0.00 a | MS, RI | ||
| 3-Metyl-1-butanol | 742 | BF | 0.32 ± 0.14 b | 0.02 ± 0.01 a | 0.13 ± 0.01 ab,1 | 0.35 ± 0.05 b | 0.00 ± 0.00 a | MS, RI |
| SM | 0.09 ± 0.08 ab | 0.00 ± 0.00 a | 0.19 ± 0.03 b,2 | 0.40 ± 0.07 c | 0.00 ± 0.00 a | MS, RI | ||
| 1-Pentanol | 770 | BF | 0.00 ± 0.00 a,1 | 3.40 ± 0.34 d,1 | 2.76 ± 0.12 cd,2 | 2.11 ± 0.38 bc,2 | 1.48 ± 0.34 b,2 | MS, RI |
| SM | 5.34 ± 0.72 b,2 | 5.44 ± 0.65 b,2 | 1.71 ± 0.24 a,1 | 1.17 ± 0.21 a,1 | 0.54 ± 0.03 a,1 | MS, RI | ||
| 3-Methyl-2-buten-1-ol | 778 | BF | 2.30 ± 0.43 b | 0.18 ± 0.06 a | 0.24 ± 0.03 a | 0.26 ± 0.04 a | 0.83 ± 0.15 b,2 | MS, RI |
| SM | 2.15 ± 0.29 b | 0.27 ± 0.10 a | 0.32 ± 0.05 a | 0.20 ± 0.04 a | 0.14 ± 0.01 a,1 | MS, RI | ||
| 2-Furanmethanol | 859 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.24 ± 0.03 b,1 | 0.50 ± 0.00 a,1 | 1.21 ± 0.30 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.54 ± 0.14 ab,2 | 1.46 ± 0.12 bc,2 | 2.30 ± 0.63 c,2 | MS, RI | ||
| 1-Hexanol | 872 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.98 ± 0.13 b,2 | 0.00 ± 0.00 a,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.44 ± 0.15 b.1 | 0.20 ± 0.01 ab,1 | MS, RI | ||
| 1-Heptanol | 979 | BF | 0.91 ± 0.10 bc,2 | 0.45 ± 0.03 a | 0.67 ± 0.04 ab,2 | 0.49 ± 0.08 ab | 1.33 ± 0.21 c,2 | MS, RI |
| SM | 0.40 ± 0.131 | 0.44 ± 0.04 | 0.42 ± 0.091 | 0.44 ± 0.06 | 0.21 ± 0.031 | MS, RI | ||
| 1-Octen-3-ol | 986 | BF | 4.19 ± 0.36 a,1 | 8.88 ± 0.81 b | 8.73 ± 0.43 b,2 | 5.19 ± 0.39 a,2 | 5.31 ± 0.59 a,2 | MS, RI |
| SM | 5.40 ± 0.33 b,2 | 7.18 ± 0.37 c | 6.86 ± 0.35 c,1 | 2.51 ± 0.21 a,1 | 1.71 ± 0.15 a,1 | MS, RI | ||
| 2-Ethylhexanol | 1035 | BF | 1.15 ± 0.28 b | 0.31 ± 0.03 a.2 | 0.00 ± 0.00 a,1 | 1.17 ± 0.06 b,2 | 0.30 ± 0.07 a,2 | MS, RI |
| SM | 0.72 ± 0.30 c | 0.00 ± 0.00 a,1 | 0.16 ± 0.01 ab,2 | 0.65 ± 0.07 bc,1 | 0.14 ± 0.01 ab,1 | MS, RI | ||
| Benzylalcohol | 1037 | BF | 0.95 ± 0.27 b | 0.30 ± 0.07 a | 0.30 ± 0.03 a | 0.35 ± 0.05 a | 0.55 ± 0.03 ab,2 | MS, RI |
| SM | 1.05 ± 0.47 b | 0.17 ± 0.02 a | 0.46 ± 0.07 ab | 0.33 ± 0.03 ab | 0.24 ± 0.01 ab,1 | MS, RI | ||
| 3-Octen-1-ol | 1073 | BF | 0.00 ± 0.00 a | 0.84 ± 0.10 c | 0.92 ± 0.05 c,2 | 0.45 ± 0.03 b,1 | 0.74 ± 0.14 bc,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.71 ± 0.03 bc | 0.66 ± 0.09 bc,1 | 1.03 ± 0.23 c,2 | 0.41 ± 0.04 ab,1 | MS, RI | ||
| 1-Octanol | 1076 | BF | 7.62 ± 0.82 b,2 | 1.26 ± 0.10 a | 1.56 ± 0.19 a,1 | 1.35 ± 0.26 a | 2.88 ± 0.42 a,2 | MS, RI |
| SM | 4.94 ± 0.26 c,1 | 1.42 ± 0.11 a | 2.03 ± 0.11 b,2 | 1.42 ± 0.09 a | 1.01 ± 0.07 a,1 | MS, RI | ||
| Phenylethyl alcohol | 1113 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.96 ± 0.12 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.14 ± 0.02 b | 0.11 ± 0.01 ab | 0.38 ± 0.06 c,1 | MS, RI | ||
| 1-Undecanol | 1174 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.09 ± 0.02 b,1 | 0.07 ± 0.00 b,1 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.18 ± 0.02 b,2 | 0.19 ± 0.03 b,2 | 0.00 ± 0.00 a | MS, RI | ||
| 2-Cyclohexen-1-ol | 1366 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.51 ± 0.14 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.34 ± 0.05 b | MS, RI | ||
| Total | BF | 19.97 ± 1.62 b | 16.25 ± 0.98 ab | 16.13 ± 0.60 ab,2 | 14.91 ± 0.53 a,2 | 15.95 ± 0.90 ab,2 | MS, RI | |
| SM | 21.23 ± 1.13 d | 16.42 ± 0.71 c | 14.12 ± 0.64 bc,1 | 12.56 ± 0.72,.1 | 6.46 ± 0.10 a,1 | MS, RI | ||
| Phenols | ||||||||
| 2-Methylphenol | 1061 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 2.07 ± 0.36 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 4.58 ± 0.26 b,2 | 6.05 ± 0.23 c,2 | MS, RI | ||
| 4-Methylphenol | 1080 | BF | 0.00 ± 0.00 a | 0.64 ± 0.11 a,2 | 0.87 ± 0.17 a | 0.83 ± 0.09 a,1 | 5.13 ± 0.54 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.30 ± 0.04 ab,1 | 1.25 ± 0.15 b | 5.87 ± 0.57 c,2 | 7.22 ± 0.25 d,2 | MS, RI | ||
| 2-Methoxyphenol | 1090 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.98 ± 0.12 ab,1 | 2.08 ± 0.09 b,1 | 9.32 ± 0.85 c | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 8.28 ± 0.62 b,2 | 8.26 ± 0.11 b,2 | 11.08 ± 0.12 c | MS, RI | ||
| 4-Methoxyphenol | 1098 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a | 0.38 ± 0.03 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.54 ± 0.09 b,2 | 0.70 ± 0.13 b | 0.42 ± 0.02 b | MS, RI | ||
| 2-Ethylphenol | 1140 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.18 ± 0.02 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.33 ± 0.07 b,2 | 0.68 ± 0.04 c,2 | MS, RI | ||
| 2.5-Dimethylphenol (2.5-Xylenol) | 1153 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.33 ± 0.06 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 2.85 ± 0.20 b,2 | MS, RI | ||
| 2,6-Dimethylphenol | 1151 | BF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | 0.00 ± 0.001 | 0.00 ± 0.00 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.26 ± 0.05 b,2 | 1.17 ± 0.10 c,2 | 0.00 ± 0.00 a | MS, RI | ||
| 3-Ethylphenol | 1169 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.43 ± 0.07 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.29 ± 0.05 b,2 | 0.72 ± 0.04 c,2 | MS, RI | ||
| 3,5-Dimethylphenol | 1172 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.12 ± 0.01 b,1 | 0.48 ± 0.04 c,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.21 ± 0.03 a,2 | 0.54 ± 0.06 b,2 | 1.12 ± 0.03 c,2 | MS, RI | ||
| 3,4-Dimethylphenol | 1152 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 2.00 ± 0.22 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 9.72 ± 0.47 b,2 | MS, RI | ||
| 2-Methoxy-4-methylphenol | 1193 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a,1 | 0.88 ± 0.25 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.43 ± 0.49 b,2 | 0.25 ± 0.03 a,2 | 2.25 ± 0.16 b,2 | MS, RI | ||
| 3,4-Dimethylphenol | 1191 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.07 ± 0.01 b,1 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.18 ± 0.05 b,2 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | MS, RI | ||
| 2,3,5-Trimethylphenol | 1201 | BF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.35 ± 0.04 b,2 | MS, RI | ||
| 2,4,5-Trimethylphenol | 1268 | BF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.40 ± 0.04 b,2 | MS, RI | ||
| 2,4,6-Trimethylphenol | 1273 | BF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.50 ± 0.08 b,2 | MS, RI | ||
| 3,4-Dimethoxyphenol | 1277 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.11 ± 0.02 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.26 ± 0.02 b | MS, RI | ||
| 4-Ethyl-2-methoxy-phenol | 1281 | BF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | 0.00 ± 0.001 | 0.00 ± 0.001 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.66 ± 0.14 b,2 | 2.65 ± 0.27 c,2 | 6.57 ± 0.04 d,2 | MS, RI | ||
| 4-Ethyl-2-methoxyphenol | 1291 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.84 ± 0.12 b,2 | MS, RI |
| SM | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | MS, RI | ||
| 4-Methoxy-2,3,6-trimethylphenol | 1326 | BF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.001 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.37 ± 0.08 b,2 | MS, RI | ||
| 2,6-Dimethoxyphenol | 1353 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.09 ± 0.24 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.71 ± 0.11 b,2 | MS, RI | ||
| Total | BF | 0.00 ± 0.00 a | 0.64 ± 0.11 a,2 | 1.92 ± 0.24 a,1 | 3.03 ± 0.14 a,1 | 23.22 ± 1.97 b,1 | MS, RI | |
| SM | 0.00 ± 0.00 a | 0.30 ± 0.04 a,1 | 12.82 ± 1.43 b,2 | 24.65 ± 0.91 c,2 | 52.27 ± 0.37 d,2 | MS, RI | ||
| Ketones | ||||||||
| 2-Butanone | 673 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.26 ± 0.06 b | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.87 ± 0.15 b,2 | 0.28 ± 0.07 a | 0.00 ± 0.00 a | MS, RI | ||
| 2-Heptanone | 893 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.31 ± 0.04 a,2 | 0.94 ± 0.21 b,2 | 0.89 ± 0.17 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.14 ± 0.04 ab,2 | 0.18 ± 0.05 b,1 | 0.18 ± 0.06 b,1 | 0.20 ± 0.02 b,2 | MS, RI | ||
| 2-Methyl-2-cyclopenten-1-one | 905 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.31 ± 0.04 b,1 | 0.00 ± 0.00 a,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.01 ± 0.34 b,2 | 1.64 ± 0.15 b,2 | 0.29 ± 0.03 a,2 | MS, RI | ||
| Octen-3-one | 984 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.29 ± 0.02 b,1 | 0.41 ± 0.07 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.32 ± 0.04 a,2 | 1.29 ± 0.39 b,2 | 0.21 ± 0.02 a,1 | MS, RI | ||
| 2,3-Octanedione | 990 | BF | 4.56 ± 0.48 b | 6.67 ± 0.62 cd | 8.04 ± 0.34 d,2 | 5.36 ± 0.53 bc | 1.27 ± 0.13 a,1 | MS, RI |
| SM | 5.58 ± 0.53 abc | 6.89 ± 0.46 c | 6.32 ± 0.41 bc,1 | 4.38 ± 0.86 ab | 3.88 ± 0.25 a,2 | MS, RI | ||
| 3,5-Dimethyl-2(5H)-furanone | 1001 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.69 ± 0.09 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.46 ± 0.02 b,1 | MS, RI | ||
| 3,4-Dimethyl-2-cyclopenten-1-one | 1022 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.32 ± 0.04 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.27 ± 0.02 b | MS, RI | ||
| 2,3-Dimethyl-2-cyclopenten-1-one | 1040 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.65 ± 0.07 b,1 | 1.96 ± 0.17 c,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 2.64 ± 0.44 c,2 | 2.43 ± 0.38 c,2 | 1.14 ± 0.07 b,1 | MS, RI | ||
| 3-Octen-2-one | 1044 | BF | 0.00 ± 0.00 a | 0.13 ± 0.04 b | 0.15 ± 0.01 b,1 | 0.24 ± 0.04 bc,2 | 0.32 ± 0.04 c | MS, RI |
| SM | 0.00 ± 0.00 a | 0.09 ± 0.02 b | 0.23 ± 0.03 cd,2 | 0.15 ± 0.02 bc,1 | 0.27 ± 0.01 d | MS, RI | ||
| 2-Nonanone | 1094 | BF | 0.22 ± 0.07 b | 0.18 ± 0.02 ab | 0.09 ± 0.01 ab,1 | 0.20 ± 0.03 ab,1 | 1.17 ± 0.08 c,2 | MS, RI |
| SM | 0.24 ± 0.10 ab | 0.14 ± 0.03 a | 0.27 ± 0.08 ab,2 | 0.74 ± 0.11 bc,2 | 0.86 ± 0.04 c,1 | MS, RI | ||
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 1111 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.32 ± 0.03 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.35 ± 0.02 b | MS, RI | ||
| 1-Cyclohexyl-1-propanone | 1125 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.14 ± 0.01 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.22 ± 0.01 b,2 | MS, RI | ||
| Total | BF | 4.78 ± 0.47 a | 6.98 ± 0.64 b | 8.59 ± 0.37 b,1 | 8.05 ± 0.55 b,1 | 7.42 ± 0.42 b | MS, RI | |
| SM | 5.82 ± 0.51 a | 7.27 ± 0.46 a | 11.85 ± 1.22 b,2 | 11.09 ± 1.04 b,2 | 8.08 ± 0.13 ab | MS, RI | ||
| Esters | ||||||||
| Methyl butanoate | 747 | BF | 1.49 ± 0.21 c | 0.32 ± 0.07 a,1 | 0.38 ± 0.06 a,1 | 0.94 ± 0.11 b,2 | 0.00 ± 0.00 a | MS, RI |
| SM | 1.20 ± 0.18 b | 1.02 ± 0.32 b,2 | 1.01 ± 0.19 b,2 | 0.24 ± 0.03 a,1 | 0.00 ± 0.00 a | MS, RI | ||
| Ethylhexanoate | 1004 | BF | 3.15 ± 0.81 c,2 | 1.20 ± 0.07 ab | 2.29 ± 0.27 bc,2 | 0.00 ± 0.00 a,1 | 0.35 ± 0.05 a,2 | MS, RI |
| SM | 1.16 ± 0.21 b,1 | 1.38 ± 0.12 b | 1.62 ± 0.16 b,1 | 0.43 ± 0.13 a,2 | 0.12 ± 0.04 a,1 | MS, RI | ||
| Isohexyl hexanoate | 1345 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.32 ± 0.12 b | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.23 ± 0.02 b | MS, RI | ||
| Buthyl benzoate | 1346 | BF | 0.24 ± 0.09 ab,2 | 0.45 ± 0.06 b | 0.39 ± 0.05 b,2 | 0.00 ± 0.00 a | 0.32 ± 0.06 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a,1 | 0.46 ± 0.08 c | 0.22 ± 0.03 b,1 | 0.00 ± 0.00 a | 0.13 ± 0.02 ab,1 | MS, RI | ||
| Total | BF | 4.88 ± 0.80 c,2 | 1.98 ± 0.09 ab,1 | 3.06 ± 0.32 b | 0.94 ± 0.11 a | 0.99 ± 0.18 a,2 | MS, RI | |
| SM | 2.35 ± 0.33 b,1 | 2.87 ± 0.25 b,2 | 2.86 ± 0.10 b | 0.66 ± 0.13 a | 0.47 ± 0.01 a,1 | MS, RI | ||
| Aromatic hydrocarbons | ||||||||
| Benzene | 691 | BF | 0.45 ± 0.28 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a | 2.33 ± 0.12 b | 0.28 ± 0.04 a,2 | MS, RI |
| SM | 0.10 ± 0.07 a | 0.21 ± 0.04 a | 0.10 ± 0.02 a | 2.55 ± 0.63 b | 0.10 ± 0.00 a,1 | MS, RI | ||
| 1-Methyl-2-propyl-cyclohexane | 789 | BF | 0.00 ± 0.00 a | 0.36 ± 0.05 b | 0.10 ± 0.02 a,1 | 0.34 ± 0.08 b | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 0.33 ± 0.06 b | 0.38 ± 0.08 b,2 | 0.48 ± 0.10 b | 0.00 ± 0.00 a | MS, RI | ||
| 1,4-Dimethyl-benzene (p-xylene) | 874 | BF | 0.00 ± 0.00 a | 0.63 ± 0.03 b,1 | 1.25 ± 0.16 c,2 | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 1.27 ± 0.26 b,2 | 0.57 ± 0.07 ab,1 | 0.68 ± 0.31 ab,2 | 0.00 ± 0.00 a | MS, RI | ||
| Methoxy-phenil-oxime | 918 | BF | 6.24 ± 0.81 c,2 | 0.37 ± 0.05 b,2 | 0.23 ± 0.09 a,1 | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.67 ± 0.321 | 0.00 ± 0.001 | 0.71 ± 0.182 | 0.45 ± 0.192 | 0.00 ± 0.00 | MS, RI | ||
| 2,5-Dimethylfuran | 971 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.39 ± 0.04 b,1 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.10 ± 0.12 b,2 | 0.00 ± 0.00 a | MS, RI | ||
| 1,3,5-Trimethyl-benzene | 973 | BF | 1.07 ± 0.19 b | 0.16 ± 0.02 a | 0.19 ± 0.02 a | 0.82 ± 0.01 b,2 | 0.36 ± 0.04 a,1 | MS, RI |
| SM | 0.72 ± 0.15 b | 0.21 ± 0.04 a | 0.32 ± 0.06 a | 0.31 ± 0.04 a,1 | 0.47 ± 0.01 ab,2 | MS, RI | ||
| 1,4-Dimethoxy-benzene | 1179 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.34 ± 0.03 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.60 ± 0.01 b,2 | 0.00 ± 0.00 a,1 | MS, RI | ||
| 2,3-Dimetoxytoluene | 1275 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.15 ± 0.03 b,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.48 ± 0.10 b,2 | MS, RI | ||
| Total | BF | 7.76 ± 0.77 c,2 | 1.67 ± 0.10 a | 1.90 ± 0.15 a | 3.89 ± 0.20 b,1 | 1.13 ± 0.07 a,2 | MS, RI | |
| SM | 1.49 ± 0.24 a,1 | 2.02 ± 0.25 a | 2.08 ± 0.19 a | 6.16 ± 0.87 b,2 | 2.04 ± 0.03 a,1 | MS, RI | ||
| Aliphatic hydrocarbons | ||||||||
| 3,3,4-Trimethyl heptane | 753 | BF | 0.53 ± 0.14 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.56 ± 0.26 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.31 ± 0.06 ab,2 | 0.00 ± 0.00 a | MS, RI | ||
| Hexane | 757 | BF | 0.91 ± 0.26 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | MS, RI |
| SM | 0.83 ± 0.34 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | MS, RI | ||
| 1-Tridecene | 952 | BF | 0.00 ± 0.00 a | 0.12 ± 0.02 b,2 | 0.11 ± 0.02 b | 0.12 ± 0.02 b | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a,1 | 0.13 ± 0.02 b | 0.14 ± 0.01 b | 0.00 ± 0.00 a | MS, RI | ||
| 3-Ethyl-2-methyl-1,3-hexadiene | 1034 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.64 ± 0.07 b,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.09 ± 0.01 b,1 | MS, RI | ||
| Total | BF | 1.44 ± 0.37 b | 0.12 ± 0.02 a,2 | 0.11 ± 0.02 a | 0.12 ± 0.03 a,1 | 0.64 ± 0.07 a,2 | MS, RI | |
| SM | 1.39 ± 0.45 b | 0.00 ± 0.00 a,1 | 0.13 ± 0.02 a | 0.45 ± 0.07 a,2 | 0.09 ± 0.01 a,1 | MS, RI | ||
| Terpenes | ||||||||
| Myrcene | 994 | BF | 0.00 ± 0.00 a | 1.13 ± 0.09 b | 0.97 ± 0.08 b | 0.00 ± 0.00 a,1 | 0.00 ± 0.00 a | MS, RI |
| SM | 0.00 ± 0.00 a | 1.10 ± 0.08 b | 1.50 ± 0.28 b | 1.10 ± 0.20 b,2 | 0.00 ± 0.00 a | MS, RI | ||
| Limonene | 1033 | BF | 0.00 ± 0.00 a | 0.40 ± 0.07 b | 0.45 ± 0.05 b | 0.28 ± 0.04 b,1 | 0.71 ± 0.06 c,2 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.30 ± 0.07 ab | 0.46 ± 0.08 b | 0.90 ± 0.16 c,2 | 0.53 ± 0.05 b,1 | MS, RI | ||
| Linalool | 1101 | BF | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.12 ± 0.02 b,1 | 0.12 ± 0.03 b,1 | 0.00 ± 0.00 a,1 | MS, RI |
| SM | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 1.00 ± 0.16 c,2 | 0.54 ± 0.16 b,2 | 0.47 ± 0.02 b,2 | MS, RI | ||
| Total | BF | 0.00 ± 0.00 a | 1.54 ± 0.13 c | 1.54 ± 0.15 c,1 | 0.40 ± 0.06 b,1 | 0.71 ± 0.06 b,1 | MS, RI | |
| SM | 0.00 ± 0.00 a | 1.40 ± 0.11 b | 2.95 ± 0.40 c,2 | 2.53 ± 0.29 c,2 | 1.00 ± 0.06 b,2 | MS, RI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marušić Radovčić, N.; Poljanec, I.; Petričević, S.; Mora, L.; Medić, H. Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham. Foods 2021, 10, 1228. https://doi.org/10.3390/foods10061228
Marušić Radovčić N, Poljanec I, Petričević S, Mora L, Medić H. Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham. Foods. 2021; 10(6):1228. https://doi.org/10.3390/foods10061228
Chicago/Turabian StyleMarušić Radovčić, Nives, Ivna Poljanec, Sandra Petričević, Leticia Mora, and Helga Medić. 2021. "Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham" Foods 10, no. 6: 1228. https://doi.org/10.3390/foods10061228
APA StyleMarušić Radovčić, N., Poljanec, I., Petričević, S., Mora, L., & Medić, H. (2021). Influence of Muscle Type on Physicochemical Parameters, Lipolysis, Proteolysis, and Volatile Compounds throughout the Processing of Smoked Dry-Cured Ham. Foods, 10(6), 1228. https://doi.org/10.3390/foods10061228