Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Nettle Powder Preparation
2.2. Physicochemical and Techno-Functional Characteristics
2.3. Phytochemicals
2.4. Antioxidant Capacity
2.5. Mineral Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Techno-Functional Characteristics
3.1.1. Moisture Content and Water Activity
3.1.2. Flow Properties
3.1.3. Hygroscopicity, Water-Holding Capacity and Water Solubility Index
3.1.4. Colorimetric Parameters
3.2. Bio-Active and Nutritional Characteristics of Differently Processed NPs
3.2.1. Phytochemicals
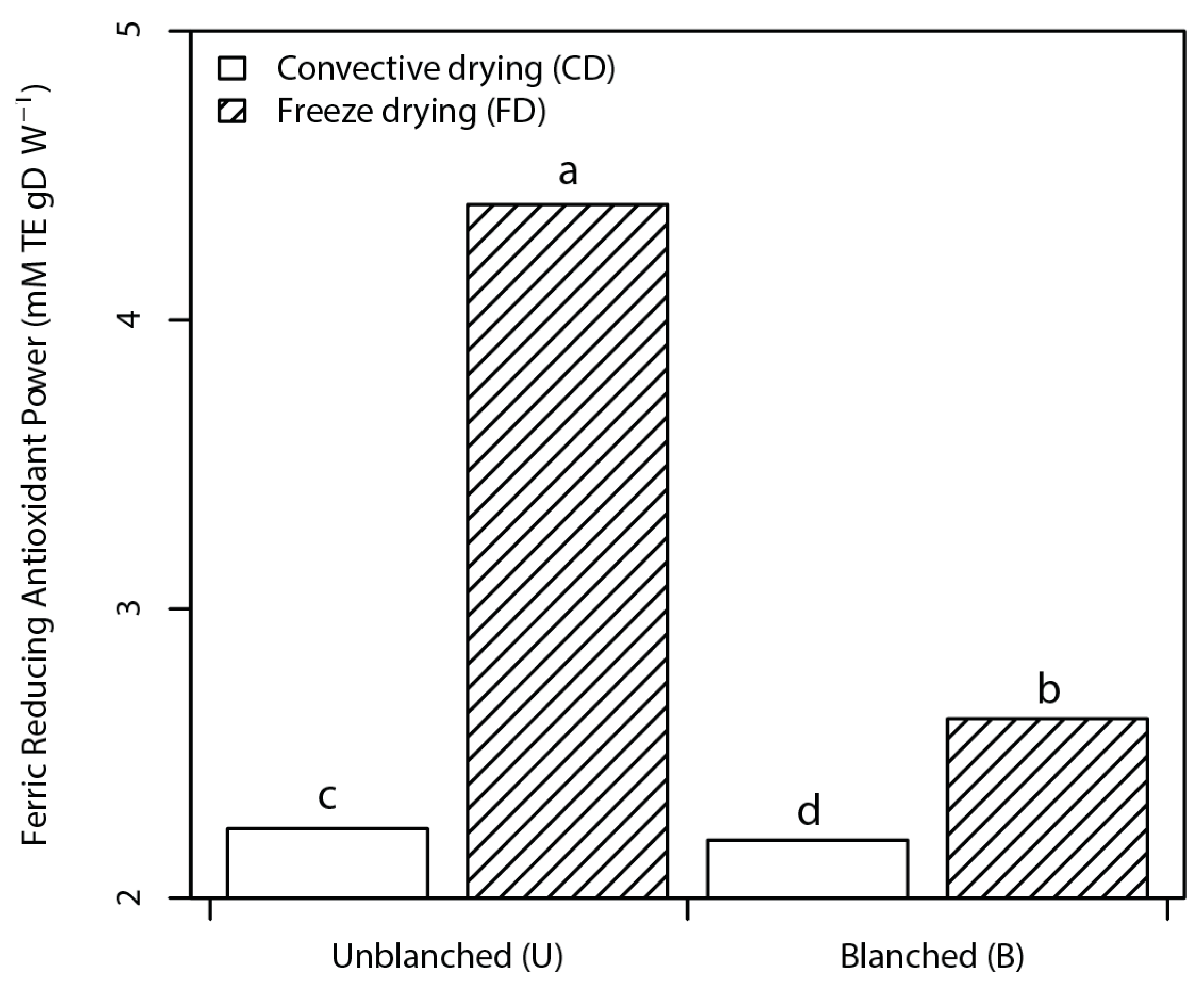
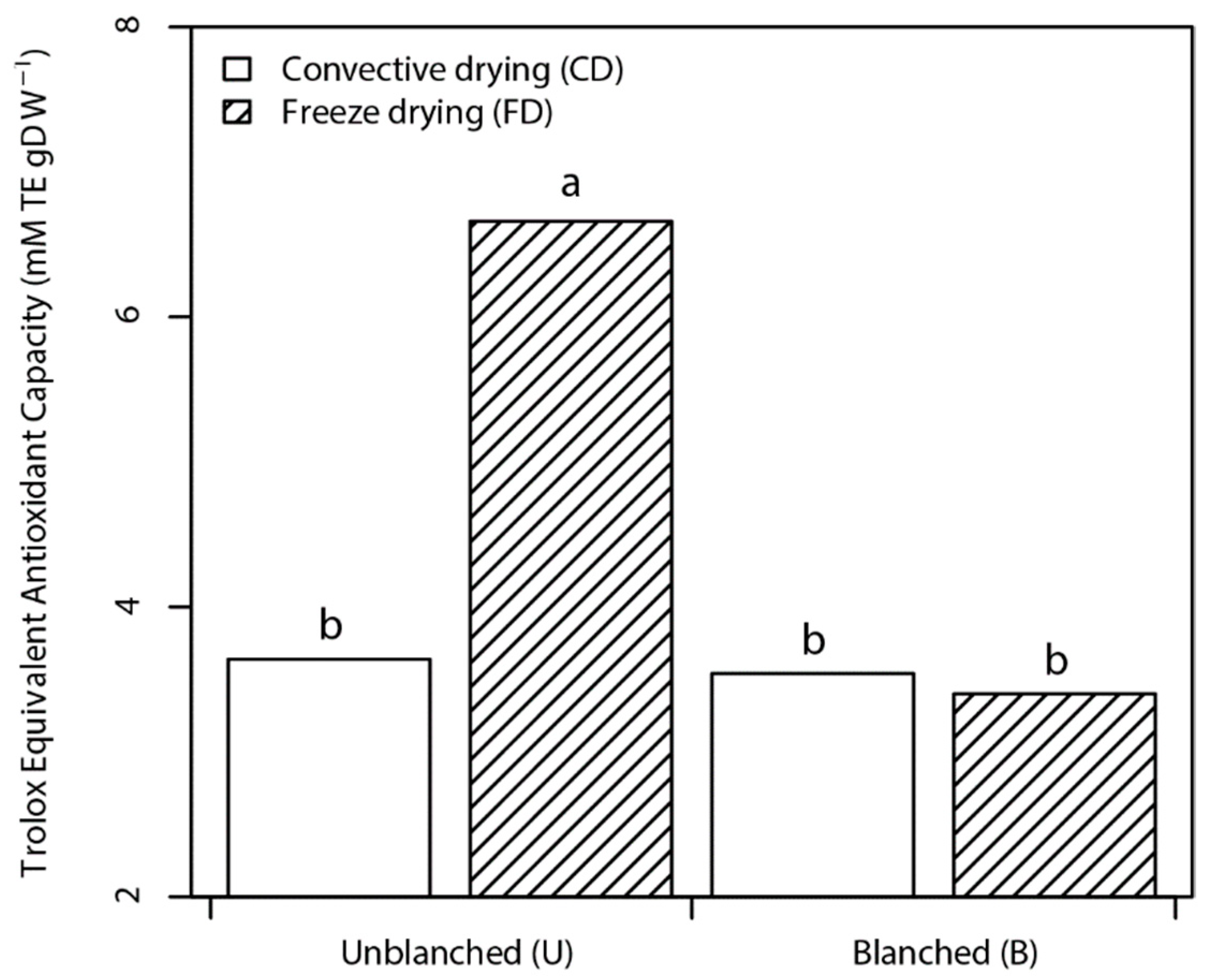
3.2.2. Antioxidant Activity
3.3. Mineral Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The Potential of stinging nettle (Urtica Dioica L.) as a crop with multiple uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica Dioica L. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef]
- Upton, R. Stinging nettles leaf (Urtica Dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nettle leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef]
- Rutakhli, A.; Sabahi, H.; Riazi, G.H. Nanocomposite of montmorillonite/nettle extract: A potential ingredient for functional foods development. J. Fun. Foods 2019, 57, 166–172. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of stinging Nettle (Urtica Dioica) flour with wheat and barley flours. Food Sci. Nutr. 2016, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Đurović, S.; Pavlić, B.; Šorgić, S.; Popov, S.; Savić, S.; Pertonijević, M.; Radojković, M.; Cvetanović, A.; Zeković, Z. Chemical composition of stinging nettle leaves obtained by different analytical approaches. J. Funct. Foods 2017, 32, 18–26. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rebolloso-Fuentes, M.M.; Torija Isasa, M.E. Fatty acids and carotenoids from stinging Nettle (Urtica Dioica L.). J. Food Compos. Anal. 2003, 16, 111–119. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Vilela, D.D.; Peixoto, L.G.; Martins, M.M.; Teixeira, R.R.; Espindol, F.S. Peel of araticum fruit (Annona crassiflora Mart.) as a source of antioxidant compounds with a-amylase, a-glucosidase and glycation inhibitory activities. Bioorg. Chem. 2016, 69, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Durgo, K.; Vojvodić, A.; Bušić, A. Nettle (Urtica Dioica L.) Extracts as functional ingredients for production of chocolates with improved bioactive composition and sensory properties. J. Food Sci. Technol. 2015, 52, 7723–7734. [Google Scholar] [CrossRef]
- Marchetti, N.; Bonetti, G.; Brandolini, V.; Cavazzini, A.; Maietti, A.; Meca, G.; Mañes, J. Stinging Nettle (Urtica Dioica L.) as a functional food additive in egg pasta: Enrichment and bioaccessibility of lutein and β-carotene. J. Funct. Foods 2018, 47, 547–553. [Google Scholar] [CrossRef]
- Shonte, T.T.; de Kock, H.L. Descriptive sensory evaluation of cooked stinging Nettle (Urtica Dioica L.) leaves and leaf infusions: Effect of using fresh or oven-dried leaves. S. Afr. J. Bot. 2017, 110, 167–176. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Branisa, J.; Jomova, K.; Porubska, M.; Kollar, V.; Simunkova, M.; Valko, M. Effect of drying methods on the content of natural pigments and antioxidant capacity in extracts from medicinal plants: A spectroscopic study. Chem. Pap. 2017, 71, 1993–2002. [Google Scholar] [CrossRef]
- Alibas, I. Energy consumption and colour characteristics of nettle leaves during microwave, vacuum and convective drying. Biosyst. Eng. 2007, 96, 495–502. [Google Scholar] [CrossRef]
- Movagharnejad, K.; Vahdatkhoram, F.; Nanvakenari, S. Optimization of microwave and infrared drying process of nettle leaves using design of experiments. J. Therm. Anal. Calorim. 2019, 135, 1677–1685. [Google Scholar] [CrossRef]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral properties and dietary value of raw and processed stinging Nettle (Urtica Dioica L.). Int. J. Food Sci. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Korus, A. Effect of preliminary processing, method of drying and storage temperature on the level of antioxidants in Kale (Brassica Oleracea L. var. acephala) leaves. LWT Food Sci. Technol. 2011, 44, 1711–1716. [Google Scholar] [CrossRef]
- Rocha, T.; Marty-Audouin, C.; Lebert, A. Effect of drying temperature and blanching on the degradation of chlorophyll a and b in Mint (Mentha Spicata Huds.) and Basil (Ocimum Basilicum): Analysis by high performance liquid chromatography with photodiode array detection. Chromatographia 1993, 36, 152–156. [Google Scholar] [CrossRef]
- Moscetti, R.; Haff, R.P.; Ferri, S.; Raponi, F.; Monarca, D.; Liang, P.; Massantini, R. Real-time monitoring of organic carrot (Var. Romance) during hot-air drying using near-infrared spectroscopy. Food Bioprocess Technol. 2017, 10, 2046–2059. [Google Scholar] [CrossRef]
- Association of Analytical Chemists. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of drying methods on the physical properties and microstructures of mango (Philippine “Carabao” var.) powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Koç, G.Ç.; Dirim, S.N. Spray dried spinach juice: Powder properties. J. Food Meas. Charact. 2018, 12, 1654–1668. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Foudari, M.; Al-Salman, F.; Almusallam, A.S. Effect of particle size and temperature on rheological, thermal, and structural properties of pumpkin flour dispersion. J. Food Eng. 2014, 124, 43–53. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Cataldi, T.R.I.; Margiotta, G.; Del Fiore, A.; Bufo, S.A. Ionic content in plant extracts determined by ion chromatography with conductivity detection. Phytochem. Anal. 2003, 14, 176–183. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic, T.; Ratti, C. Freeze-drying of maple syrup: Efficient protocol formulation and evaluation of powder physicochemical properties. Dry. Technol. 2019, 38, 1138–1150. [Google Scholar] [CrossRef]
- Bian, Q.; Sittipod, S.; Garg, A.; Ambrose, R.P.K. Bulk flow properties of hard and soft wheat flours. J. Cereal Sci. 2015, 63, 88–94. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Khashawi, R. Influence of hot-air drying and freeze-drying on functional, rheological, structural and dielectric properties of green banana flour and dispersions. Food Hydrocoll. 2020, 99, 105331. [Google Scholar] [CrossRef]
- Corrêa, S.C.; Clerici, M.T.P.S.; Garcia, J.S.; Ferreira, E.B.; Eberlin, M.N.; Azevedo, L. Evaluation of dehydrated marolo (Annona Crassiflora) flour and carpels by freeze-drying and convective hot-air drying. Food Res. Int. 2011, 44, 2385–2390. [Google Scholar] [CrossRef]
- Fombang, E.N.; Mbofung, C.M.F.M.F. The effect of steam blanching and drying method on nutrients, phytochemicals and antioxidant activity of moringa (Moringa Oleifera L.) leaves. Am. J. Food Sci. Technol. 2017, 5, 53–60. [Google Scholar]
- Braga, M.C.; Vieira, E.C.S.; de Oliveira, T.F. Curcuma Longa L. leaves: Characterization (bioactive and antinutritional compounds) for use in human food in Brazil. Food Chem. 2018, 265, 308–315. [Google Scholar] [CrossRef]
- Joshi, P. Physical aspects of color in foods. ACS Symp. Ser. 2001, 775, 43–53. [Google Scholar]
- Hunter, R.S.; Harold, R.W. The Measurement of Appearance; John Wiley & Sons: Hoboken, NJ, USA, 1987. [Google Scholar]
- Krokida, M.K.; Maroulis, Z.B.; Saravacos, G.D. the effect of the method of drying on the colour of dehydrated products. Int. J. Food Sci. Technol. 2001, 36, 53–59. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Isolation of chlorophylls from stinging Nettle (Urtica Dioica L.). Sep. Purif. Technol. 2007, 57, 37–46. [Google Scholar] [CrossRef]
- Rocha, T.; Lebert, A.; Marty-Audouin, C. Effect of pretreatments and drying conditions on drying rate and colour retention of Basil (Ocimum Basilicum). LWT Food Sci. Technol. 1993, 26, 456–463. [Google Scholar] [CrossRef]
- Korus, A. Effect of preliminary and technological treatments on the content of chlorophylls and carotenoids in Kale (Brassica Oleracea L. var. acephala). J. Food Process. Preserv. 2013, 37, 335–344. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching impact on pigments, glucosinolates, and phenolics of dehydrated broccoli by-products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Parma, Italy, 2006. [Google Scholar]

| Factor | Yield (%) | Moisture Content * (g 100 g−1) | Water Activity (aw) | Bulk Density (g cm−3) | Tapped Density (g cm−3) |
|---|---|---|---|---|---|
| Pretreatment (PR) | |||||
| Unblanched (U) | 13.99 ± 1.93a | 8.06 ± 2.28 | 0.42 ± 0.12 | 0.30 ± 0.13 | 0.44 ± 0.10 |
| Blanched (B) | 11.67 ± 1.14b | 6.79 ± 3.13 | 0.34 ± 0.17 | 0.30 ± 0.14 | 0.45 ± 0.13 |
| p value | ≤0.05 | ≤0.05 | ≤0.05 | ns | ns |
| HSD | 0.035 | ||||
| Drying (DR) | |||||
| Convective drying (CD) | 13.05 ± 2.24 | 9.89 ± 0.29 | 0.51 ± 0.02 | 0.41 ± 0.02a | 0.54 ± 0.02 |
| Freeze drying (FD) | 12.61 ± 1.77 | 4.96 ± 1.12 | 0.24 ± 0.07 | 0.18 ± 0.01b | 0.35 ± 0.02 |
| p value | ns | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 |
| HSD | 0.001 | ||||
| PR × DR | |||||
| U × CD | 14.81 ± 1.27 | 10.13 ± 0.14a | 0.53 ± 0.01a | 0.41 ± 0.02 | 0.53 ± 0.02b |
| U × FD | 13.18 ± 2.38 | 5.98 ± 0.02c | 0.30 ± 0.01c | 0.19 ± 0.01 | 0.36 ± 0.01c |
| B × CD | 11.29 ± 1.30 | 9.64 ± 0.10b | 0.49 ± 0.01b | 0.42 ± 0.01 | 0.56 ± 0.01a |
| B × FD | 12.04 ± 1.07 | 3.93 ± 0.07d | 0.18 ± 0.02d | 0.18 ± 0.01 | 0.34 ± 0.01c |
| p value | ns | ≤0.05 | ≤0.05 | ns | ≤0.05 |
| HSD | 0.001 | 0.001 | 0.02 |
| Factor | Carr Index (%) | Hausner Ratio | Hygroscopicity (g H2O 100 g DW−1) | WHC (g H2O g DW−1) | WSI (%) |
|---|---|---|---|---|---|
| Pretreatment (PR) | |||||
| Unblanched (U) | 36.00 ± 15.03 | 1.63 ± 0.38 | 7.49 ± 2.90 | 6.18 ± 0.32 | 11.90 ± 1.47 |
| Blanched (B) | 36.00 ± 12.73 | 1.60 ± 0.35 | 8.59 ± 4.27 | 6.14 ± 1.19 | 5.97 ± 0.57 |
| p value | ns | ns | ns | ns | ≤0.05 |
| HSD | |||||
| Drying (DR) | |||||
| Convective drying (CD) | 24.00 ± 1.15 | 1.30 ± 0.01b | 4.85 ± 0.42b | 5.49 ± 0.49 | 8.14 ± 2.73 |
| Freeze drying (FD) | 48.00 ± 1.63 | 1.93 ± 0.05a | 11.23 ± 1.66a | 6.83 ± 0.43 | 9.72 ± 3.82 |
| p-value | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 |
| HSD | 0.001 | 0.001 | |||
| PR × DR | |||||
| U × CD | 23.00 ± 0.01b | 1.30 ± 0.01 | 4.86 ± 0.61 | 5.92 ± 0.12c | 10.62 ± 0.58b |
| U × FD | 49.00 ± 1.41a | 1.95 ± 0.07 | 10.12 ± 0.14 | 6.45 ± 0.17b | 13.17 ± 0.39a |
| B × CD | 25.00 ± 0.01b | 1.30 ± 0.01 | 4.84 ± 0.24 | 5.06 ± 0.20d | 5.67 ± 0.06c |
| B × FD | 47.00 ± 1.41a | 1.90 ± 0.01 | 12.34 ± 1.78 | 7.22 ± 0.01a | 6.27 ± 0.73c |
| p value | ≤0.05 | ns | ns | ≤0.05 | ≤0.05 |
| HSD | 0.05 | 0.001 | 0.01 |
| Factor | Luminance (L*) | Redness (a*) | Yellowness (b*) | Chroma (C*) | Hue Angle (h) | ΔE* |
|---|---|---|---|---|---|---|
| Pretreatment (PR) | ||||||
| Unblanched (U) | 43.59 ± 2.87 | −3.76 ± 0.70 | 18.77 ± 1.12 | 19.15 ± 1.23 | 101.27 ± 1.42 | 9.14 ± 1.46 |
| Blanched (B) | 41.14 ± 0.90 | −7.21 ± 1.82 | 18.50 ± 2.59 | 19.87 ± 3.07 | 111.01 ± 2.24 | 5.61 ± 0.88 |
| p value | ≤0.05 | ≤0.05 | ns | ≤0.05 | ≤0.05 | ≤0.05 |
| HSD | ||||||
| Drying (DR) | ||||||
| Convective drying (CD) | 40.89 ± 1.13 | −4.34 ± 1.33 | 16.96 ± 0.91 | 17.56 ± 0.58 | 104.48 ± 4.92 | 7.14 ± 0.97 |
| Freeze-drying (FD) | 43.85 ± 2.46 | −6.64 ± 2.45 | 20.32 ± 0.64 | 21.46 ± 1.34 | 107.80 ± 5.76 | 7.62 ± 3.05 |
| p value | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 | ns |
| HSD | ||||||
| PR × DR | ||||||
| U × CD | 41.15 ± 1.48b | −3.13 ± 0.02a | 17.76 ± 0.06c | 18.03 ± 0.05c | 99.99 ± 0.10d | 7.93 ± 0.70b |
| U × FD | 46.02 ± 0.75a | −4.40 ± 0.10b | 19.78 ± 0.22b | 20.27 ± 0.21b | 102.55 ± 0.34c | 10.36 ± 0.66a |
| B × CD | 40.62 ± 0.90b | −5.55 ± 0.17c | 16.16 ± 0.40d | 17.09 ± 0.43d | 108.97 ± 0.22b | 6.35 ± 0.16c |
| B × FD | 41.67 ± 0.61b | −8.87 ± 0.11d | 20.85 ± 0.38a | 22.66 ± 0.39a | 113.05 ± 0.14a | 4.87 ± 0.53d |
| p value | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 |
| HSD | 0.01 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Factor | Chlorophyll a (mg 100 g DW−1 NP) | Chlorophyll b (mg 100 g DW−1 NP) | Total Carotenoids (mg 100 g DW−1 NP) |
|---|---|---|---|
| Pretreatment (PR) | |||
| Unblanched (U) | 512.68 ± 105.51b | 215.35 ± 38.53 | 128.13 ± 29.58 |
| Blanched (B) | 585.31 ± 155.52a | 229.08 ± 55.07 | 162.49 ± 42.31 |
| p value | ≤0.05 | ns | ≤0.05 |
| HSD | 0.005 | ||
| Drying (DR) | |||
| Convective drying (CD) | 432.61 ± 21.35b | 180.73 ± 3.98b | 112.88 ± 13.19 |
| Freeze-drying (FD) | 665.38 ± 74.29a | 263.70 ± 22.02a | 177.75 ± 25.99 |
| p value | ≤0.05 | ≤0.05 | ≤0.05 |
| HSD | 0.001 | 0.001 | |
| PR × DR | |||
| U × CD | 417.54 ± 19.63 | 180.53 ± 5.67 | 101.39 ± 4.97d |
| U × FD | 607.82 ± 17.09 | 250.17 ± 6.50 | 154.87 ± 4.30b |
| B × CD | 447.68 ± 8.53 | 180.94 ± 2.72 | 124.36 ± 3.77c |
| B × FD | 722.94 ± 59.70 | 277.23 ± 24.92 | 200.63 ± 10.00a |
| p value | ns | ns | ≤0.05 |
| HSD | 0.014 |
| Factor | Ca (mg g DW−1 NP) | K (mg g DW−1 NP) | Mg (mg g DW−1 NP) | Na (mg g DW−1 NP) |
|---|---|---|---|---|
| Pretreatment (PR) | ||||
| Unblanched (U) | 16.83 ± 0.97 | 23.1 ± 1.26a | 2.59 ± 0.35 | 0.28 ± 0.02b |
| Blanched (B) | 15.99 ± 1.37 | 10.85 ± 0.88b | 2.26 ± 0.57 | 0.38 ± 0.03a |
| p value | ns | <0.05 | <0.05 | <0.05 |
| HSD | 0.001 | 0.032 | 0.001 | |
| Drying (DR) | ||||
| Convective drying (DR) | 16.00 ± 0.78 | 17.17 ± 6.45 | 2.04 ± 0.30b | 0.33 ± 0.07 |
| Freeze drying (FD) | 16.82 ± 1.50 | 16.78 ± 7.11 | 2.81 ± 0.27 | 0.33 ± 0.04 |
| p value | ns | ns | <0.05 | ns |
| HSD | 0.001 | |||
| PR × DR | ||||
| U × CD | 16.25 ± 0.94 | 22.98 ± 1.59 | 2.29 ± 0.16 | 0.26 ± 0.02 |
| U × FD | 17.41 ± 0.67 | 23.22 ± 1.19 | 2.89 ± 0.07 | 0.29 ± 0.01 |
| B × CD | 15.75 ± 0.67 | 11.36 ± 0.56 | 1.79 ± 0.12 | 0.39 ± 0.03 |
| B × FD | 16.23 ± 2.02 | 10.34 ± 0.91 | 2.73 ± 0.39 | 0.36 ± 0.02 |
| p value | ns | ns | ns | ns |
| HSD | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nallan Chakravartula, S.S.; Moscetti, R.; Farinon, B.; Vinciguerra, V.; Merendino, N.; Bedini, G.; Neri, L.; Pittia, P.; Massantini, R. Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders. Foods 2021, 10, 1152. https://doi.org/10.3390/foods10061152
Nallan Chakravartula SS, Moscetti R, Farinon B, Vinciguerra V, Merendino N, Bedini G, Neri L, Pittia P, Massantini R. Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders. Foods. 2021; 10(6):1152. https://doi.org/10.3390/foods10061152
Chicago/Turabian StyleNallan Chakravartula, Swathi Sirisha, Roberto Moscetti, Barbara Farinon, Vittorio Vinciguerra, Nicolò Merendino, Giacomo Bedini, Lilia Neri, Paola Pittia, and Riccardo Massantini. 2021. "Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders" Foods 10, no. 6: 1152. https://doi.org/10.3390/foods10061152
APA StyleNallan Chakravartula, S. S., Moscetti, R., Farinon, B., Vinciguerra, V., Merendino, N., Bedini, G., Neri, L., Pittia, P., & Massantini, R. (2021). Stinging Nettles as Potential Food Additive: Effect of Drying Processes on Quality Characteristics of Leaf Powders. Foods, 10(6), 1152. https://doi.org/10.3390/foods10061152









