Development of a Sodium Alginate-Based Active Package with Controlled Release of Cinnamaldehyde Loaded on Halloysite Nanotubes
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Etching of HNTs
2.3. Fabrication of T-HNTs-CIN Nanoparticles
2.4. Preparation of the SA Composite Film
2.5. Characterization of Nanoparticles
2.6. Film Thickness
2.7. Microscopic Images
2.8. Water Vapor Permeability (WVP)
2.9. Mechanical Properties
2.10. Light Transmittance and Opacity of Film
2.11. The Sustained-Release of CIN in Film in Food Simulants
2.12. In Vitro Antibacterial Activity of the Film in the Release Experiment
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.2. Surface Morphology of Composite Film
3.3. WVP
3.4. Mechanical Properties
3.5. Light Transmittance and Opacity of Film
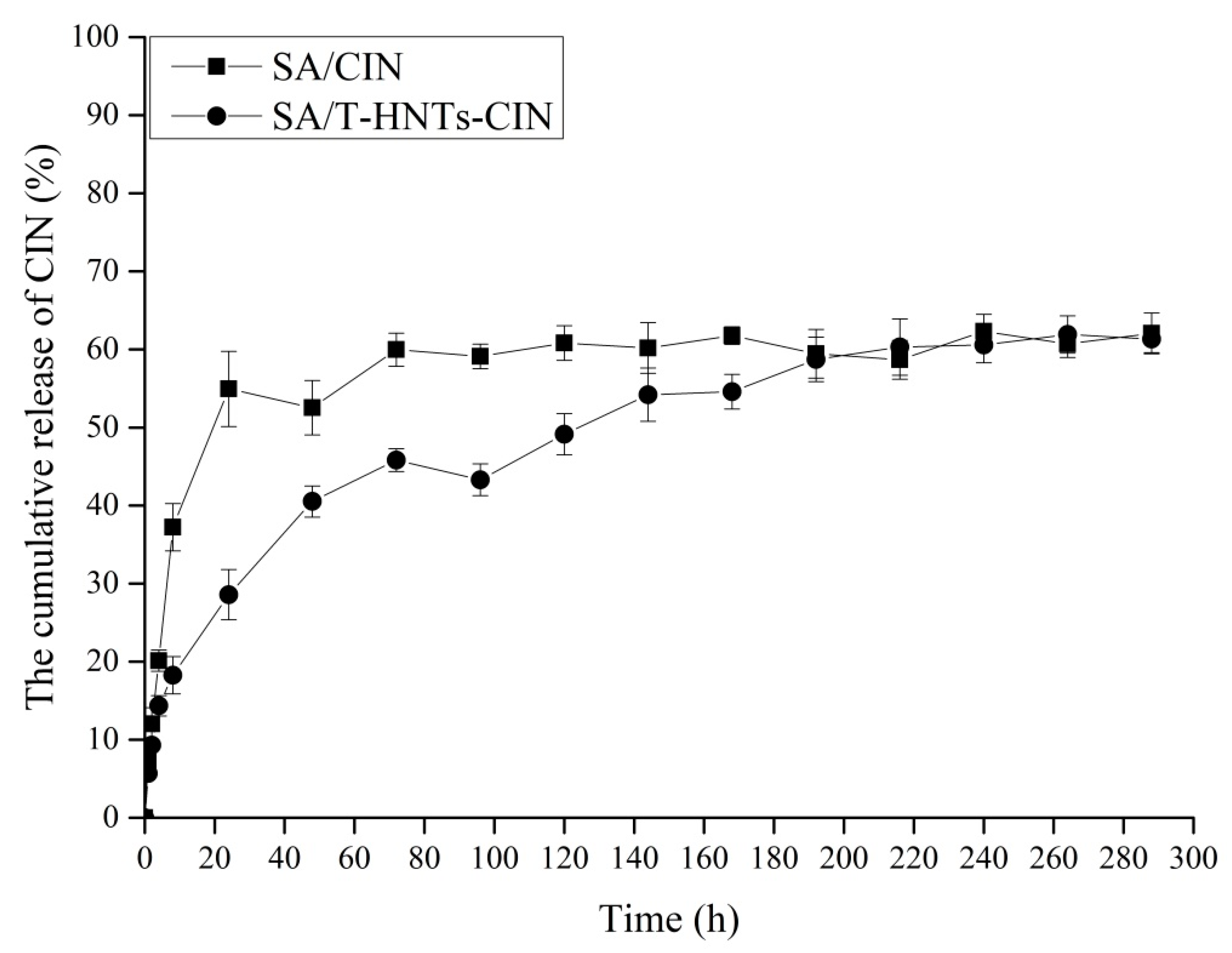
3.6. Slow-Release Behavior of the CIN in Food Simulants
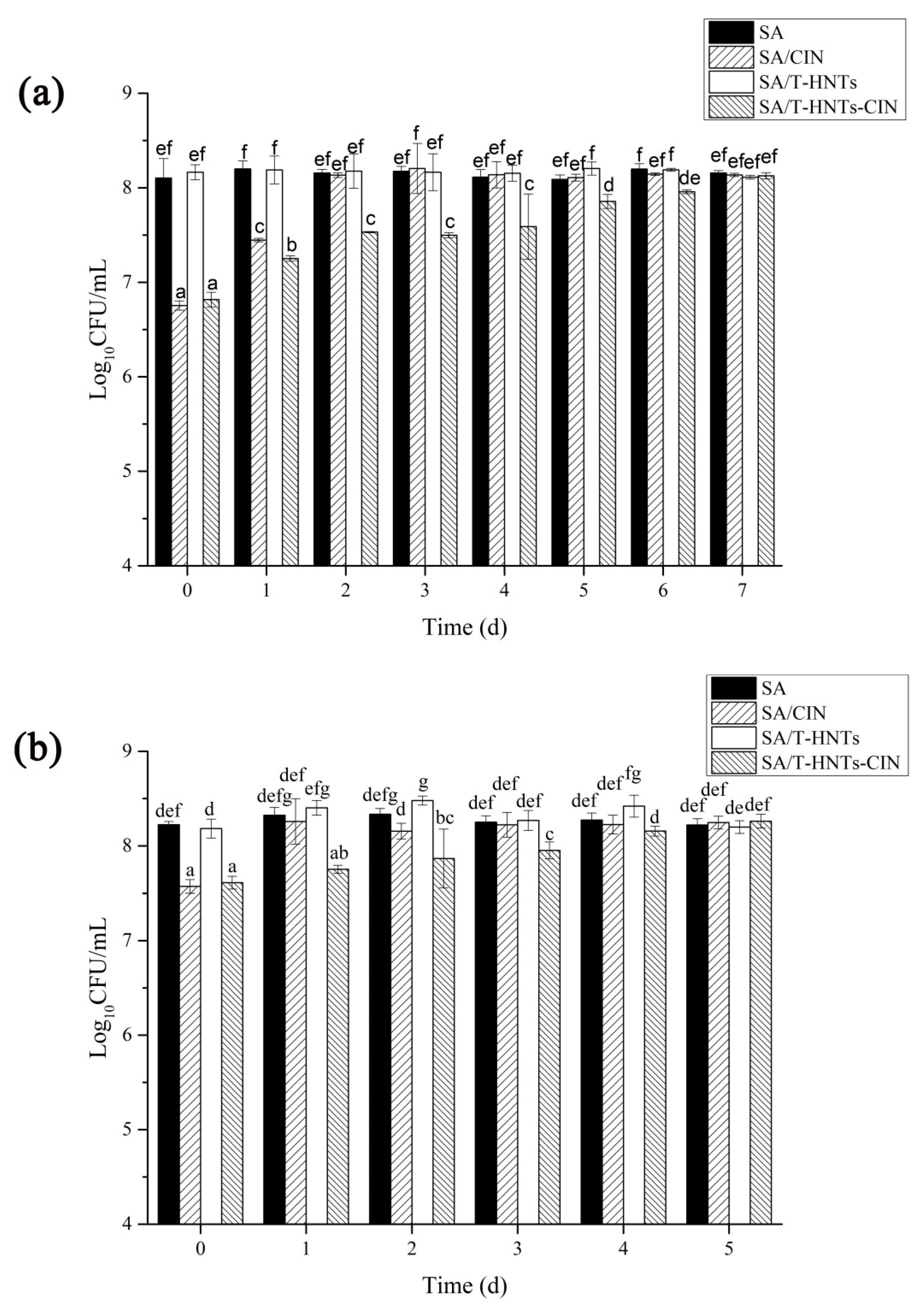
3.7. In Vitro Antibacterial Activity of the Film in the Release Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Progr. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- King, A.H. Brown seaweed extracts (alginates). Food Hydrocoll. 1983, 2, 115–188. [Google Scholar]
- Rhim, J.W. Physical and mechanical properties of water resistant sodium alginate films. Lebensm. Wiss. Technol. Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Shankar, S.; Kasapis, S.; Rhim, J.-W. Alginate-based nanocomposite films reinforced with halloysite nanotubes functionalized by alkali treatment and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1824–1832. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Krepker, M.; Shemesh, R.; Poleg, Y.D.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Lee, M.H.; Seo, H.-S.; Park, H.J. Thyme Oil Encapsulated in Halloysite Nanotubes for Antimicrobial Packaging System. J. Food Sci. 2017, 82, 922–932. [Google Scholar] [CrossRef]
- Loke, X.-J.; Shemesh, R.; Poleg, Y.D.; Kashi, Y.; Vaxman, A.; Segal, E. Plasma-treated polyethylene coated with polysaccharide and protein containing cinnamaldehyde for active packaging films and applications on tilapia (Orechromis niloticus) fillet preservation. Food Control. 2021, 125, 117–126. [Google Scholar] [CrossRef]
- Chuesiang, P.; Sanguandeekul, R.; Siripatrawan, U. Enhancing effect of nanoemulsion on antimicrobial activity of cinnamon essential oil against foodborne pathogens in refrigerated Asian seabass (Lutes calcarifer) fillets. Food Control 2021, 122, 107782. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de F F Soares, N.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, M.; Wang, X.; Wang, J. Bioactive mesoporous nano-silica/potato starch films against molds commonly found in post-harvest white mushrooms. Food Hydrocoll. 2019, 95, 517–525. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Liu, Y.; Liu, Z.; Fan, L.; Dong, T.; Jin, Y.; Saldana, M.D.A.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by beta-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]
- Massaro, M.; Barone, G.; Biddeci, G.; Cavallaro, G.; di Blasi, F.; Lazzara, G.; Nicotra, G.; Spinella, C.; Spinelli, G.; Riela, S. Halloysite nanotubes-carbon dots hybrids multifunctional nanocarrier with positive cell target ability as a potential non-viral vector for oral gene therapy. J. Colloid Interface Sci. 2019, 552, 236–246. [Google Scholar] [CrossRef]
- Lee, M.H.; Park, H.J. Preparation of halloysite nanotubes coated with Eudragit for a controlled release of thyme essential oil. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Abdullayev, E.; Price, R.; Shchukin, D.; Lvov, Y. Halloysite Tubes as Nanocontainers for Anticorrosion Coating with Benzotriazole. ACS Appl. Mater. Interface 2009, 1, 1437–1443. [Google Scholar] [CrossRef]
- Zhong, B.; Wang, S.; Dong, H.; Luo, Y.; Jia, Z.; Zhou, X.; Chen, M.; Xie, D.; Jia, D. Halloysite Tubes as Nanocontainers for Herbicide and Its Controlled Release in Biodegradable Poly(vinyl alcohol)/Starch Film. J. Agric. Food Chem. 2017, 65, 10445–10451. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of Halloysite Clay Nanotube Lumen by Selective Etching of Aluminum Oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, C.; Wang, P.; Zhang, Y.; Zhang, H. Electrospun chitosan/polycaprolactone nanofibers containing chlorogenic acid-loaded halloysite nanotube for active food packaging. Carbohydr. Polym. 2020, 247, 116711. [Google Scholar] [CrossRef]
- Achachlouei, B.F.; Zahedi, Y. Fabrication and characterization of CMC-based nanocomposites reinforced with sodium montmorillonite and TiO2 nanomaterials. Carbohydr. Polym. 2018, 199, 415–425. [Google Scholar] [CrossRef]
- Muller, J.; Quesada, A.C.; Gonzalez-Martinez, C.; Chiralt, A. Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur. Polym. J. 2017, 96, 316–325. [Google Scholar] [CrossRef]
- Cui, R.; Jiang, K.; Yuan, M.; Cao, J.; Li, L.; Tang, Z.; Qin, Y. Antimicrobial film based on polylactic acid and carbon nanotube for controlled cinnamaldehyde release. J. Mater. Res. Technol. Jmr T 2020, 9, 10130–10138. [Google Scholar] [CrossRef]
- Zhang, A.-B.; Pan, L.; Zhang, H.; Liu, S.; Ye, Y.; Xia, M.; Chen, X. Effects of acid treatment on the physico-chemical and pore characteristics of halloysite. Colloids Surf. Physicochem. Eng. Asp. 2012, 396, 182–188. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Ripoll, L.; Hidalgo, M.; Lopez-Martinez, J.; Balart, R. Characterization of selectively etched halloysite nanotubes by acid treatment. Appl. Surf. Sci. 2017, 422, 616–625. [Google Scholar] [CrossRef]
- Echeverría, I.; Lopez-Caballero, M.E.; Gomez-Guillen, M.C.; Mauri, A.N.; Montero, M.P. Structure, Functionality, and Active Release of Nanoclay–Soy Protein Films Affected by Clove Essential Oil. Food Bioprocess. Technol. 2016, 9, 1937–1950. [Google Scholar] [CrossRef]
- Yousefi, P.; Hamedi, S.; Garmaroody, E.R.; Koosha, M. Antibacterial nanobiocomposite based on halloysite nanotubes and extracted xylan from bagasse pith. Int. J. Biol. Macromol. 2020, 160, 276–287. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Ghorbani, M.; Mahmoodzadeh, F.; Nikzad, B. Development of reinforced aldehyde-modified kappa-carrageenan/gelatin film by incorporation of halloysite nanotubes for biomedical applications. Int. J. Biol. Macromol. 2020, 160, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Casariego, A.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Cruz, L.; Diaz, R.; Vicente, A.A. Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles concentrations. Food Hydrocoll. 2009, 23, 1895–1902. [Google Scholar] [CrossRef]
- Rehan, M.; El-Naggar, M.E.; Mashaly, H.M.; Wilken, R. Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr. Polym. 2018, 182, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Mulla, M.; Jacob, H.; Luciano, G.; Bini, T.B.; Almusallam, A. Polylactide/poly(ε-caprolactone)/zinc oxide/clove essential oil composite antimicrobial films for scrambled egg packaging. Food Packag. Shelf Life 2019, 21, 100355. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Utomo, D.P.; Raemas, a.A.; Kusworo, T.D.; Jos, B.; Djaeni, M. Alginate/κ-Carrageenan-Based Edible Films Incorporated with Clove Essential Oil: Physico-Chemical Characterization and Antioxidant-Antimicrobial Activity. Polymers 2021, 13, 354. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Huang, D.J.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocoll. 2020, 101, 105471. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Liu, Z.; Liu, Y.; Wei, H.; Li, J. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Int. J. Biol. Macromol. 2020, 164, 557–565. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, L.; Chafer, M.; Gonzalez-Martinez, C.; Chiralt, A.; Desobry, S. Study of the release of limonene present in chitosan films enriched with bergamot oil in food simulants. J. Food Eng. 2011, 105, 138–143. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Exp. Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Zhao, X.; Mu, Y.; Dong, H.; Zhang, H.; Zhang, H.; Chi, Y.; Song, G.; Li, H.; Wang, L. Effect of cinnamaldehyde incorporation on the structural and physical properties, functional activity of soy protein isolate-egg white composite edible films. J. Food Process. Preserv. 2021, 45, e15143. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fabra, M.J.; Castro-Mayorga, J.L.; Bourbon, A.I.; Pastrana, L.M.; Vicente, A.A.; Lagaron, J.M. Use of Electrospinning to Develop Antimicrobial Biodegradable Multilayer Systems: Encapsulation of Cinnamaldehyde and Their Physicochemical Characterization. Food Bioprocess Technol. 2016, 9, 1874–1884. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]



| Samples | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| HNTs | 25.50 | 0.30 | 1.88 |
| T-HNTs | 76.61 | 0.39 | 2.41 |
| T-HNTs-CIN | 51.39 | 0.37 | 2.13 |
| Films | Thickness (mm) | WVP (×10−2 g mm/24 h·KPa·m2) |
|---|---|---|
| SA | 0.030 ± 0.001 a | 1.59 ± 0.05 b |
| SA/CIN | 0.033 ± 0.004 a | 1.31 ± 0.15 a |
| SA/T-HNTs | 0.035 ± 0.001 a | 1.41 ± 0.02 a |
| SA/T-HNTs-CIN | 0.033 ± 0.003 a | 1.36 ± 0.11 a |
| Film | TS (MPa) | ε (%) |
|---|---|---|
| SA | 66.4 ± 4.28 a | 2.76 ± 0.30 a |
| SA/CIN | 70.4 ± 2.45 ab | 3.35 ± 0.07 b |
| SA/T-HNTs | 77.3 ± 7.50 bc | 2.66 ± 0.09 a |
| SA/T-HNTs-CIN | 80.2 ± 3.81 c | 2.97 ± 0.30 ab |
| Film Sample | UV-C (240 nm) T (%) | UV-B (300 nm) T (%) | UV-A (360 nm) T (%) | Visible (600 nm) T (%) | Opacity (AU. nm/mm) |
|---|---|---|---|---|---|
| SA | 69.5 ± 2.29 d | 81.8 ± 0.67 d | 85.0 ± 0.32 c | 87.4 ± 2.45 c | 2.50 ± 0.21 a |
| SA/CIN | 64.4 ± 1.13 c | 76.1 ± 1.13 c | 84.8 ± 0.57 c | 89.9 ± 0.85 c | 2.00 ± 0.10 a |
| SA/T-HNTs | 35.6 ± 1.62 b | 50.2 ± 1.07 b | 57.5 ± 0.79 b | 70.3 ± 0.50 b | 4.54 ± 0.55 b |
| SA/T-HNTs-CIN | 22.8 ± 2.40 a | 40.9 ± 4.62 a | 50.1 ± 3.27 a | 66.1 ± 0.57 a | 4.78 ± 1.15 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, R.; Zhu, B.; Yan, J.; Qin, Y.; Yuan, M.; Cheng, G.; Yuan, M. Development of a Sodium Alginate-Based Active Package with Controlled Release of Cinnamaldehyde Loaded on Halloysite Nanotubes. Foods 2021, 10, 1150. https://doi.org/10.3390/foods10061150
Cui R, Zhu B, Yan J, Qin Y, Yuan M, Cheng G, Yuan M. Development of a Sodium Alginate-Based Active Package with Controlled Release of Cinnamaldehyde Loaded on Halloysite Nanotubes. Foods. 2021; 10(6):1150. https://doi.org/10.3390/foods10061150
Chicago/Turabian StyleCui, Rui, Bifen Zhu, Jiatong Yan, Yuyue Qin, Mingwei Yuan, Guiguang Cheng, and Minglong Yuan. 2021. "Development of a Sodium Alginate-Based Active Package with Controlled Release of Cinnamaldehyde Loaded on Halloysite Nanotubes" Foods 10, no. 6: 1150. https://doi.org/10.3390/foods10061150
APA StyleCui, R., Zhu, B., Yan, J., Qin, Y., Yuan, M., Cheng, G., & Yuan, M. (2021). Development of a Sodium Alginate-Based Active Package with Controlled Release of Cinnamaldehyde Loaded on Halloysite Nanotubes. Foods, 10(6), 1150. https://doi.org/10.3390/foods10061150









