Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animal Rearing and Batches
2.3. Carcass Sampling
2.3.1. Subcutaneous Back-Fat Sampling
2.3.2. Meat Sampling
2.4. Fat and Meat Characteristics Analysis
2.4.1. pH and Instrumental Color
2.4.2. Proximate Analysis
2.4.3. Determination of the Fatty Acid Profile
2.4.4. Cooking Losses and Texture Analysis
2.4.5. Sensory Analysis
2.5. Shelf Life of Meat
2.5.1. Sample Preparation
2.5.2. Gas Composition and Microbiological Analysis
2.5.3. Sensory Evaluation
2.6. Statistical Analysis
3. Results
3.1. Effect of Diet on the Carcass Yield
3.2. Effect of Diet on Fatty Acid Profile
3.2.1. Fatty Acid Profile of Subcutaneous Fat
3.2.2. Fatty Acid Profile of Intramuscular Fat
3.3. Effect of Diet on Meat Quality Traits
Sensory Analysis
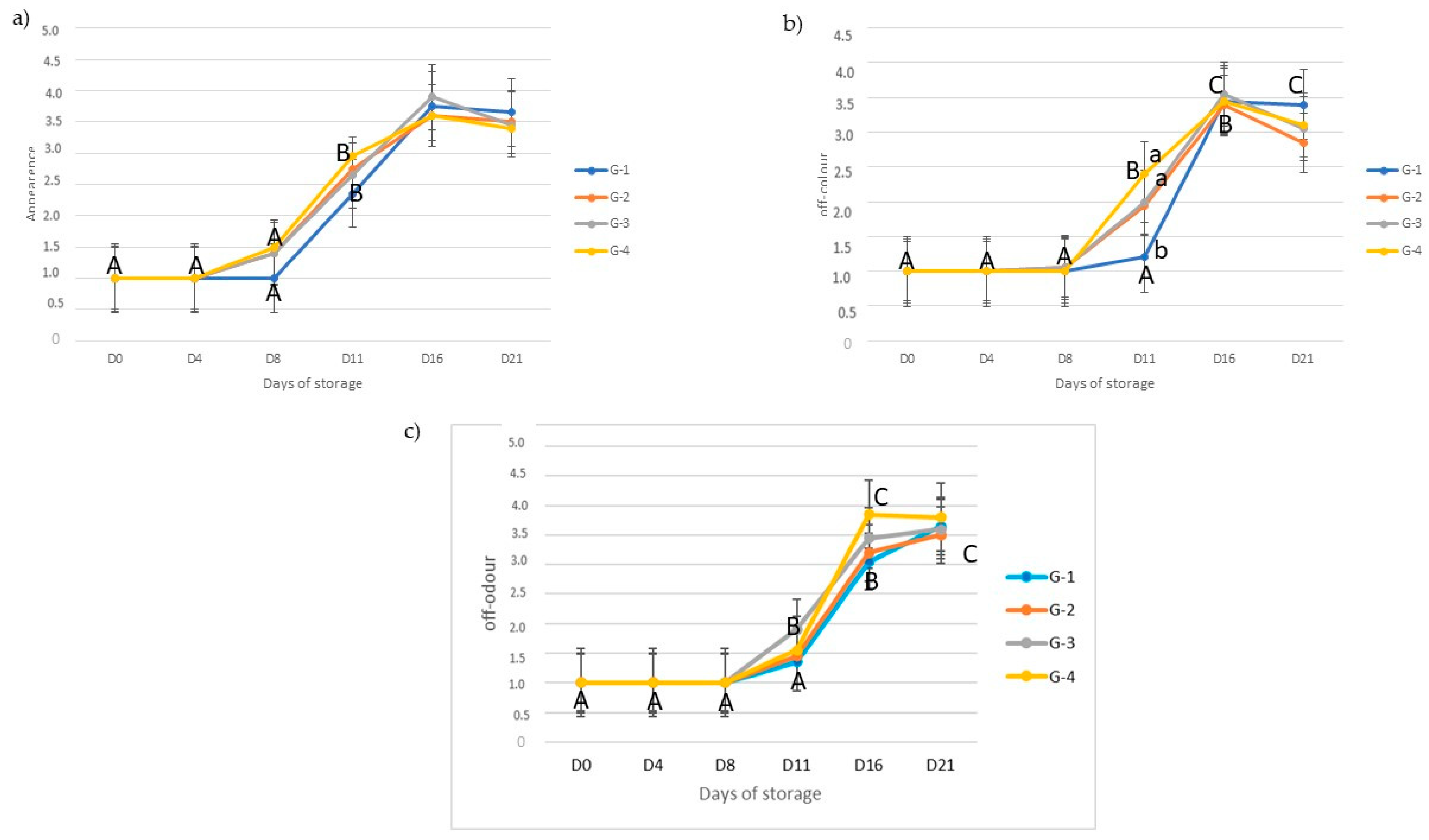
3.4. Effect of Diet on Shelf Life of Meat Packaged in Modified Atmosphere during Storage
Microbiological Analyses
4. Discussion
4.1. Carcass Yield and Meat and Fat Composition
4.2. Meat Quality Traits
4.3. Shelf Life of Meat in Retail Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauw, W.M.; Casco, J.M.; Carballar, F.G.; Fito, E.D.; Granados, P.P.; Barroso, M.Á.; Raya, L.G. Feed efficiency and loin meat quality in Iberian pigs. Rev. Bras. Zootec. 2020, 49, 49. [Google Scholar] [CrossRef]
- Bonneau, M.; Lebret, B. Production systems and influence on eating quality of pork. Meat Sci. 2010, 84, 293–300. [Google Scholar] [CrossRef]
- Pascual, J.; Rafecas, M.; Canela, M.; Boatella, J.; Bou, R.; Barroeta, A.C.; Codony, R. Effect of increasing amounts of a linoleic-rich dietary fat on the fat composition of four pig breeds. Part II: Fatty acid composition in muscle and fat tissues. Food Chem. 2007, 100, 1639–1648. [Google Scholar] [CrossRef]
- Registro Informativo de Organismos Independientes de Control Del Ibérico (RIBER); Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2019. (In Spanish)
- Tejerina, D.; García-Torres, S.; Cava, R. Water-holding capacity and instrumental texture properties of m. Longissimus dorsi and m. Serratus ventralis from Iberian pigs as affected by the production system. Livest. Sci. 2012, 148, 46–51. [Google Scholar] [CrossRef]
- Rincker, P.J.; Killefer, J.; Ellis, M.; Brewer, M.S.; McKeith, F.K. Intramuscular fat content has little influence on the eating quality of fresh pork loin chops. J. Anim. Sci. 2008, 86, 730–737. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Hernández-Matamoros, A.; Tejeda, J.F. Two by-products of the olive oil extraction industry as oleic acid supplement source for Iberian pigs: Effect on the meat’s chemical composition and induced lipoperoxidation. J. Sci. Food Agric. 2012, 92, 2543–2551. [Google Scholar] [CrossRef]
- Rey, A.; Daza, A.; López-Carrasco, C.; López-Bote, C. Feeding Iberian pigs with acorns and grass in either free-range or confinement affects the carcass characteristics and fatty acids and tocopherols accumulation in Longissimus dorsi muscle and backfat. Meat Sci. 2006, 73, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, D.; González, A.; Peña, F.; Hernández-García, F.; Izquierdo, M. Effect of fattening period length on intramuscular and subcutaneous fatty acid profiles in Iberian pigs finished in the Montanera sustainable system. Sustainability 2020, 12, 7937. [Google Scholar] [CrossRef]
- Pena, R.N.; Noguera, J.L.; García-Santana, M.J.; González, E.; Tejeda, J.F.; Ros-Freixedes, R.; Ibáñez-Escriche, N. Five genomic regions have a major impact on fat composition in Iberian pigs. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Cava, R.; Ventanas, J.; Tejeda, J.F.; Ruiz, J.; Antequera, T. Effect of free-range rearing and α-tocopherol and copper supplementation on fatty acid profiles and susceptibility to lipid oxidation of fresh meat from Iberian pigs. Food Chem. 2000, 68, 51–59. [Google Scholar] [CrossRef]
- Ovilo, C.; Fernández, A.; Fernández, A.I.; Martín Palomino, P.; Rodrigáñez, J.; Rodríguez, C.; Silió, L.; López-Bote, C.J. Effect of dietary oleic acid content: Different genetic regulation of fatty acid metabolism on muscle and fat of Iberian pigs. In Proceedings of the 7th International Symposium on the Mediterranean Pig, Cordoba, Spain, 14–16 October 2010; CIHEAM: Zaragoza, Spain, 2012; Volume 101, pp. 39–46. [Google Scholar]
- Ventanas Canillas, S.; Ruiz Carrascal, J. Influencia de La Raza y de La Alimentación Sobre El Contenido y Características de La Grasa Intramuscular del Lomo de Cerdo Ibérico: Efecto Sobre Parámetros Determinantes de La Calidad. Ph.D. Thesis, Universidad de Extremadura, Badajoz, Spain, 2006. (In Spanish). [Google Scholar]
- Theron, M.M.; Lues, J.F. Organic acids and meat preservation: A review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Ricke, S. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of acetic and citric acids to inhibit Escherichia coli O157:H7, Salmonella Typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.S.; Dora, K.C.; Chowdhury, S.; Sarkar, S.; Mishra, R. Effect of chitosan and acetic acid on the shelf life of sea bass fillets stored at refrigerated temperature. J. Appl. Nat. Sci. 2017, 9, 2175–2181. [Google Scholar] [CrossRef]
- Berry, E.D.; Cutter, C.N. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microbiol. 2000, 66, 1493–1498. [Google Scholar] [CrossRef]
- Bacon, R.T.; Belk, K.E.; Sofos, J.N.; Clayton, R.P.; Reagan, J.O.; Smith, G.C. Microbial populations on animal hides and beef carcasses at different stages of slaughter in plants employing multiple-sequential interventions for decontamination. J. Food Prot. 2000, 63, 1080–1086. [Google Scholar] [CrossRef]
- European Parliament. Directive 2010/63/EU of the European Parliament and of the Council; European Parliament: Brussels, Belgium, 2010; pp. 33–79.
- Comisión Europea. Reglamento (UE) 2017/893 de la Comisión. Que modifica los Anexos I y IV del Reglamento (CE) No. 999/2001 del Parlamento Europeo y del Consejo y los anexos X, XIV y XV del Reglamento (UE) No. 142/2011 de la Comisión Por lo que se Refiere a las Disposiciones Sobre Proteína Animal Transformada; Comisión Europea: Brussels, Belgium, 2017; pp. 1–25. (In Spanish) [Google Scholar]
- Lurueña-Martínez, M.; Palacios, C.; Vivar-Quintana, A.; Revilla, I. Effect of the addition of calcium soap to ewes’ diet on fatty acid composition of ewe milk and subcutaneous fat of suckling lambs reared on ewe milk. Meat Sci. 2010, 84, 677–683. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Greer, G.G.; Murray, A.C. Effects of pork muscle quality on bacterial growth and retail case life. Meat Sci. 1988, 24, 61–71. [Google Scholar] [CrossRef]
- López-Bote, C. Sustained utilization of the Iberian pig breed. Meat Sci. 1998, 49, S17–S27. [Google Scholar] [CrossRef]
- Ventanas, S.; Ventanas, J.; Tovar, J.; Garcia, C.; Estévez, M. Extensive feeding versus oleic acid and tocopherol enriched mixed diets for the production of Iberian dry-cured hams: Effect on chemical composition, oxidative status and sensory traits. Meat Sci. 2007, 77, 246–256. [Google Scholar] [CrossRef]
- Daza, A.; Rey, A.; Ruiz, J.; Lopez-Bote, C. Effects of feeding in free-range conditions or in confinement with different dietary MUFA/PUFA ratios and α-tocopheryl acetate, on antioxidants accumulation and oxidative stability in Iberian pigs. Meat Sci. 2005, 69, 151–163. [Google Scholar] [CrossRef]
- Huxley, J.S. Constant differential growth-ratios and their significance. Nat. Cell Biol. 1924, 114, 895–896. [Google Scholar] [CrossRef]
- Nuernberg, K.; Fischer, K.; Nuernberg, G.; Kuechenmeister, U.; Klosowska, D.; Eliminowska-Wenda, G.; Fiedler, I.; Ender, K. Effects of dietary olive and linseed oil on lipid composition, meat quality, sensory characteristics and muscle structure in pigs. Meat Sci. 2005, 70, 63–74. [Google Scholar] [CrossRef]
- Nieto, R.; García-Casco, J.; Lara, L.; Palma-Granados, P.; Izquierdo, M.; Hernandez, F.; Dieguez, E.; Luis Duarte, J.; Bato-rek-Lukač, N. Ibérico (Iberian) Pig. In European Local Pig Breeds—Diversity and Performance. A Study of Project TREASURE; IntechOpen: London, UK, 2019. [Google Scholar]
- Daza, A.; Menoyo, D.; Olivares, A.; Cordero, G.; López-Bote, C. Effect of Iberian pig feeding system on tissue fatty-acid composition and backfat rheological properties. J. Anim. Feed. Sci. 2007, 16, 408–419. [Google Scholar] [CrossRef]
- Ruiz, J.; Cava, R.; Antequera, T.; Martín, L.; Ventanas, J.; López-Bote, C.J. Prediction of the feeding background of Iberian pigs using the fatty acid profile of subcutaneous, muscle and hepatic fat. Meat Sci. 1998, 49, 155–163. [Google Scholar] [CrossRef]
- Benítez, R.; Núñez, Y.; Fernández, A.; Isabel, B.; Rodríguez, C.; Daza, A.; López-Bote, C.; Silió, L.; Óvilo, C. Adipose tissue transcriptional response of lipid metabolism genes in growing Iberian pigs fed oleic acid v. carbohydrate enriched diets. Animal 2016, 10, 939–946. [Google Scholar] [CrossRef]
- Allee, G.L.; Baker, D.H.; Leveille, G.A. Influence of level of dietary fat on adipose tissue lipogenesis and enzymatic activity in the pig. J. Anim. Sci. 1971, 33, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, G. Digestión y metabolismo de las grasas en cerdos. Av. Tecnol. Porc. 2017, 14, 32–35. (In Spanish) [Google Scholar]
- Serrano, M.; Valencia, D.; Nieto, M.; Lázaro, R.; Mateos, G. Influence of sex and terminal sire line on performance and carcass and meat quality of Iberian pigs reared under intensive production systems. Meat Sci. 2008, 78, 420–428. [Google Scholar] [CrossRef]
- Asghar, A.; Lin, C.; Gray, J.; Buckley, D.; Booren, A.; Flegal, C. Effects of dietary oils and tocopherol supplementation on membranal lipid oxidation in broiler meat. J. Food Sci. 1990, 55, 46–50. [Google Scholar] [CrossRef]
- Hoz, L.; Lopez-Bote, C.; Cambero, M.; D’Arrigo, M.; Pin, C.; Santos, C.; Ordóñez, J. Effect of dietary linseed oil and α-tocopherol on pork tenderloin (Psoas major) muscle. Meat Sci. 2003, 65, 1039–1044. [Google Scholar] [CrossRef]
- Rezar, V.; Salobir, J.; Levart, A.; Tomažin, U.; Škrlep, M.; Lukač, N.B.; Čandek-Potokar, M. Supplementing entire male pig diet with hydrolysable tannins: Effect on carcass traits, meat quality and oxidative stability. Meat Sci. 2017, 133, 95–102. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; López, R.C. Physico-chemical characteristics of M. Longissimus dorsi from three lines of free-range reared Iberian pigs slaughtered at 90 kg live-weight and commercial pigs: A comparative study. Meat Sci. 2003, 64, 499–506. [Google Scholar] [CrossRef]
- Franco, D.; Vazquez, J.A.; Lorenzo, J.M. Growth performance, carcass and meat quality of the Celta pig crossbred with Duroc and Landrance genotypes. Meat Sci. 2014, 96, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Auqui, S.; Egea, M.; Peñaranda, I.; Garrido, M.; Linares, M.; Auqui, M. Rustic Chato Murciano pig breed: Effect of the weight on carcass and meat quality. Meat Sci. 2019, 156, 105–110. [Google Scholar] [CrossRef]
- Ruiz, J.; Ventanas, J.; Cava, R.; Andrés, A.; García, C. Volatile compounds of dry-cured Iberian ham as affected by the length of the curing process. Meat Sci. 1999, 52, 19–27. [Google Scholar] [CrossRef]
- Savell, J.W.; Cross, H.R. The role of fat in the palatability of beef, pork, and lamb. Designing foods: Animal product options in the marketplace. In Committee on Technological Options to Improve the Nutritional Attributes of Animal Products; National Academy Press: Washington, DC, USA, 1988; pp. 345–355. [Google Scholar]
- Ruiz, J.; De La Hoz, L.; Isabel, B.; Rey, A.I.; Daza, A.; López-Bote, C.J.; Ruiz-Carrascal, J. Improvement of dry-cured Iberian ham quality characteristics through modifications of dietary fat composition and supplementation with vitamin E. Food Sci. Technol. Int. 2005, 11, 327–335. [Google Scholar] [CrossRef]
- Luong, N.-D.M.; Coroller, L.; Zagorec, M.; Membré, J.-M.; Guillou, S. Spoilage of chilled fresh meat products during storage: A quantitative analysis of literature data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef]
- Benson, A.K.; David, J.R.D.; Gilbreth, S.E.; Smith, G.; Nietfeldt, J.; Legge, R.; Kim, J.; Sinha, R.; Duncan, C.E.; Ma, J.; et al. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf life study. Appl. Environ. Microbiol. 2014, 80, 5178–5194. [Google Scholar] [CrossRef]
- Reglamento (CE) No. 2073/2005 de La Comisión, de 15 de Noviembre de 2005, Relativo a Los Criterios Microbiológicos Aplicables a Los Productos Alimenticios; Comisión Europea: Brussels, Belgium, 2005; pp. 141–166. (In Spanish)
- Insausti, K.; Beriain, M.J.; Purroy, A.; Alberti, P.; Gorraiz, C.; Alzueta, M.J. Shelf life of beef from local Spanish cattle breeds stored under modified atmosphere. Meat Sci. 2001, 57, 273–281. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.-J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R. The International Commission on Microbiological Specifications for Foods (ICMSF): Update. Food Control 1996, 7, 99–101. [Google Scholar] [CrossRef]
- Dainty, R.H.; Mackey, B.M. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Bacteriol. 1992, 73, 103s–114s. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, R.-Q.; Li, Y.-F. Correlations between growth parameters of spoilage micro-organisms and shelf-life of pork stored under air and modified atmosphere at −2, 4 and 10 °C. Food Microbiol. 2006, 23, 578–583. [Google Scholar] [CrossRef]
- Vieira, C.; Rubio, B.; Martínez, B.; Mantecón, A.; Manso, T. Suckling lamb meat quality from ewes fed with different sources of fat, during storage under display conditions. Small Rumin. Res. 2019, 176, 47–54. [Google Scholar] [CrossRef]
- Del Río, E.; Panizo-Morán, M.; Prieto, M.; Alonso-Calleja, C.; Capita, R. Effect of various chemical decontamination treatments on natural microflora and sensory characteristics of poultry. Int. J. Food Microbiol. 2007, 115, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, K.; Sharma, R.; Demirci, A.; Cutter, C. Comparison of electrolyzed oxidizing water with various antimicrobial interventions to reduce Salmonella species on poultry. Poult. Sci. 2002, 81, 1598–1605. [Google Scholar] [CrossRef]
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of varying oxygen concentrations on the shelf-life of fresh pork sausages packaged in modified atmosphere. Food Chem. 2006, 94, 219–225. [Google Scholar] [CrossRef]
- Kapetanakou, A.; Agathaggelou, E.; Skandamis, P. Storage of pork meat under modified atmospheres containing vapors from commercial alcoholic beverages. Int. J. Food Microbiol. 2014, 178, 65–75. [Google Scholar] [CrossRef] [PubMed]
| Pig Fat | Solid Oleic Acid | Oleic High Sunflower Oil | |
|---|---|---|---|
| Metabolizable Energy (kcal/kg) | 8288.00 | 6640.00 | 8100.00 |
| Crude fat (%) | 99.00 | 83.00 | 95.10 |
| Fatty acid profile (g/100 g of fat) | |||
| C14:0 | 1.30 | 0.25 | nd |
| C16:0 | 23.80 | 10.50 | 6.40 |
| C18:0 | 13.50 | 3.50 | 5.00 |
| C18:1 n-9 | 41.20 | 68.50 | 22.60 |
| C18:2 n-6 | 10.20 | 8.00 | 63.00 |
| C18:3 n-3 | 1.00 | 0.70 | 0.50 |
| Growing 2 | Finishing | |||||||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| Ingredients (g/100 g) | ||||||||
| Barley | 50.00 | 50.00 | 50.00 | 50.00 | 48.21 | 45.60 | 46.94 | 46.94 |
| Wheat | 30.65 | 30.54 | 30.65 | 30.61 | 35.00 | 36.06 | 35.00 | 35.08 |
| Soybean meal | 12.49 | 12.48 | 12.50 | 12.48 | 8.23 | 8.59 | 8.54 | 8.54 |
| Wheat bran | 2.50 | 2.50 | 2.50 | 2.50 | 3.00 | 3.00 | 3.00 | 3.00 |
| Calcium carbonate | 1.66 | 1.55 | 1.55 | 1.55 | 1.48 | 1.44 | 1.82 | 1.70 |
| Monocalcium Phosphate | 0.58 | 0.58 | 0.58 | 0.58 | 0.45 | 0.45 | 0.45 | 0.45 |
| Common Salt | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Premix 1 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Pig Fat | 1.20 | 0.00 | 0.00 | 0.00 | 2.61 | |||
| Lysine | 0.20 | 0.20 | 0.20 | 0.20 | 0.21 | 0.21 | 0.21 | 0.21 |
| Oleic high sunflower oil | 0.62 | 0.62 | 1.73 | 1.73 | ||||
| Oleic Solid Acid | 1.35 | 0.60 | 0.60 | 3.85 | 1.50 | 1.50 | ||
| Organic-acid mix | 0.05 | 0.05 | ||||||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Chemical composition (g/1000 g) | ||||||||
| Metabolizable Energy (kcal/kg) | 3153 | 3149 | 3153 | 3151 | 3250 | 3250 | 3250 | 3250 |
| Crude protein | 14.50 | 14.50 | 14.50 | 14.50 | 13.00 | 13.00 | 13.00 | 13.00 |
| Crude Fiber | 4.40 | 4.40 | 4.41 | 4.41 | 4.26 | 4.18 | 4.22 | 4.22 |
| Crude Fat | 3.00 | 3.00 | 3.00 | 3.00 | 4.50 | 5.05 | 4.84 | 4.84 |
| Ash (550 °C) | 5.52 | 5.44 | 5.49 | 5.48 | 5.03 | 5.43 | 5.54 | 5.42 |
| Starch | 45.43 | 45.45 | 45.43 | 45.37 | 47.18 | 46.43 | 46.51 | 46.51 |
| C16:0 | 0.58 | 0.43 | 0.39 | 0.39 | 0.94 | 0.64 | 0.52 | 0.52 |
| C18:0 | 0.18 | 0.07 | 0.06 | 0.06 | 0.37 | 0.16 | 0.12 | 0.12 |
| C18:1 n-9 | 0.70 | 1.02 | 1.08 | 1.08 | 1.34 | 2.50 | 2.50 | 2.50 |
| C18:2 n-6 | 0.87 | 0.89 | 0.89 | 0.89 | 0.99 | 1.09 | 1.06 | 1.06 |
| Calcium | 0.95 | 0.95 | 0.95 | 0.95 | 0.85 | 1.10 | 1.09 | 1.04 |
| Total Phosphorus | 0.55 | 0.55 | 0.55 | 0.55 | 0.50 | 0.50 | 0.50 | 0.50 |
| Methionine | 0.22 | 0.22 | 0.22 | 0.22 | 0.20 | 0.20 | 0.20 | 0.20 |
| M + C | 0.52 | 0.52 | 0.52 | 0.52 | 0.48 | 0.48 | 0.48 | 0.48 |
| Lysine | 0.80 | 0.80 | 0.80 | 0.80 | 0.70 | 0.70 | 0.70 | 0.70 |
| Tryptophan | 0.19 | 0.19 | 0.19 | 0.19 | 0.17 | 0.17 | 0.17 | 0.17 |
| Threonine | 0.50 | 0.50 | 0.50 | 0.50 | 0.44 | 0.44 | 0.44 | 0.44 |
| Sodium | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Slaughter Analysis | G1 | G2 | G3 | G4 | p |
|---|---|---|---|---|---|
| Live weight (kg) | 166.8 ± 11.56 | 165.04 ± 12.80 | 163.98 ± 17.80 | 162.84 ± 13.73 | 0.671 |
| Carcass weight (kg) | 75.25 ± 7.57 | 75.88 ± 5.30 | 76.14 ± 3.99 | 79.31 ± 6.52 | 0.365 |
| Thickness of backfat (6th rib) (cm) | 7.29 ± 1.14 | 7.25 ± 1.0 | 6.45 ± 1.11 | 6.5 ± 0.91 | 0.112 |
| Thickness of back fat (3rd lumbar vertebra) | 8.88 a ± 1.00 | 7.50 b ± 0.86 | 7.81 b ± 0.78 | 8.31 a,b ± 0.95 | 0.030 |
| G1 | G2 | G3 | G4 | p | |
|---|---|---|---|---|---|
| C12:0 | 0.08 ± 0.08 | 0.08 ± 0.01 | 0.08 ± 0.10 | 0.08 ± 0.01 | 0.413 |
| C14:0 | 1.44 ± 0.11 | 1.395 ± 0.07 | 1.43 ± 0.13 | 1.36 ± 0.12 | 0.615 |
| C16:0 | 24.81 ± 0.77 | 24.86 ± 0.19 | 25.44 ± 0.80 | 24.87 ± 0.49 | 0.266 |
| C16:1 n-7 | 2.43 ± 0.26 | 2.53 ± 0.21 | 2.20 ± 0.21 | 2.21 ± 0.39 | 0.142 |
| C17:0 | 0.39 ± 0.07 | 0.33 ± 0.10 | 0.36 ± 0.08 | 0.29 ± 0.08 | 0.202 |
| C17:1 n-6 | 0.40 ± 0.09 | 0.34 ± 0.10 | 0.33 ± 0.08 | 0.27 ± 0.07 | 0.113 |
| C18:0 | 12.99 a,b ± 0.75 | 12.37 b ± 0.92 | 14.13 a ± 0.97 | 13.71 a ± 1.54 | 0.049 |
| C18:1 n-9 | 47.47 a,b ± 0.83 | 48.65 a ± 1.12 | 46.25 b ± 1.12 | 47.79 b ± 1.41 | 0.013 |
| C18:2 n-6 | 8.17 ± 0.67 | 7.68 ± 0.56 | 7.96 ± 0.71 | 7.57 ± 0.58 | 0.362 |
| C18:3 n-3 | 0.45 ± 0.05 | 0.42 ± 0.05 | 0.425 ± 0.05 | 0.39 ± 0.04 | 0.244 |
| C20:0 | 0.18 ± 0.02 | 0.21 ± 0.02 | 0.20 ± 0.02 | 0.22 ± 0.03 | 0.107 |
| C20:1 n-9 | 1.18 ± 0.20 | 1.13 ± 0.11 | 1.18 ± 0.10 | 1.23 ± 0.18 | 0.743 |
| SFA | 39.89 b ± 1.39 | 39.23 b ± 0.94 | 41.65 a ± 1.24 | 40.53 a,b ± 1.63 | 0.031 |
| MUFA | 51.48 a,b ± 0.90 | 52.65 a ± 1.46 | 49.97 b ± 1.19 | 51.50 a,b ± 1.80 | 0.026 |
| PUFA | 8.62 ± 0.71 | 8.11 ± 0.60 | 8.38 ± 0.76 | 7.96 ± 0.61 | 0.358 |
| PUFA/SFA | 0.22 ± 0.02 | 0.21 ± 0.01 | 0.20 ± 0.21 | 0.20 ± 0.02 | 0.333 |
| n-6/n-3 | 17.33 a ± 0.67 | 17.23 a ± 0.56 | 18.89 a,b ± 0.70 | 20.00 b ± 0.00 | 0.010 |
| G1 | G2 | G3 | G4 | p | |
|---|---|---|---|---|---|
| C12:0 | 0.14 a ± 0.047 | 0.08 b ± 0.01 | 0.09 b ± 0.01 | 0.09 b ± 0.03 | 0.023 |
| C14:0 | 1.51 ± 0.09 | 1.45 ± 0.11 | 1.46 ± 0.09 | 1.49 ± 0.01 | 0.618 |
| C14:1 n-5 | 0.03 b ± 0.01 | 0.03 b ± 0.00 | 0.03 a ± 0.00 | 0.04 a ± 0.01 | 0.000 |
| C15:0 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.253 |
| C16:0 | 25.76 ± 1.73 | 25.86 ± 0.08 | 25.51 ± 0.79 | 25.75 ± 0.58 | 0.889 |
| C16:1 n-7 | 4.16 ± 0.98 | 3.97 ± 0.32 | 4.16 ± 0.38 | 3.99 ± 0.34 | 0.836 |
| C17:0 | 0.14 ± 0.022 | 0.15 ± 0.032 | 0.16 ± 0.04 | 0.14 ± 0.013 | 0.466 |
| C18:0 | 12.47 ± 1.60 | 12.29 ± 0.69 | 11.62 ± 0.47 | 12.35 ± 0.57 | 0.199 |
| C18:1 n-7 | 3.87 ± 0.72 | 3.85 ± 0.24 | 4.08 ± 0.17 | 3.85 ± 0.26 | 0.529 |
| C18:1 n-9 | 45.82 ± 1.68 | 46.59 ± 0.89 | 46.40 ± 1.18 | 45.85 ± 0.96 | 0.396 |
| C18:2 t10-c12 | 0.09 a ± 0.012 | 0.09 a ± 0.01 | 0.08 a ± 0.01 | 0.08 b ± 0.01 | 0.074 |
| C18:2 n-6 | 3.46 ± 0.61 | 3.09 ± 0.31 | 3.62 ± 0.76 | 3.63 ± 0.63 | 0.175 |
| C18:3 n-3 | 0.15 ± 0.026 | 0.13 ± 0.011 | 0.14 ± 0.022 | 0.15 ± 0.025 | 0.176 |
| C18:3 c9-c11-c13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.13 ± 0.00 | 0.249 |
| C18:3 n-6 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.140 |
| C20:0 | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.18 ± 0.01 | 0.19 ± 0.02 | 0.309 |
| C20:1 n-9 | 0.93 ± 0.06 | 0.97 ± 0.09 | 0.97 ± 0.07 | 0.93 ± 0.08 | 0.564 |
| C20:2 n-6 | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.19 ± 0.03 | 0.19 ± 0.02 | 0.323 |
| C20:3 n-6 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.02 ± 0.02 | 0.103 |
| C20:4 n-3 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.20 ± 0.00 | 0.02 ± 0.00 | 0.3628 |
| C20:4 n-6 | 0.46 a,b ± 0.18 | 0.43 b ± 0.11 | 0.58 a,b ± 0.22 | 0.61 a ± 0.19 | 0.088 |
| C20:5 n-3 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.448 |
| C21:0 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.413 |
| C22:1 n-9 | 0.01 b ± 0.00 | 0.01 b ± 0.00 | 0.02 a ± 0.00 | 0.02 a ± 0.00 | 0.004 |
| C22:4 n-6 | 0.08 a,b ± 0.02 | 0.07 b ± 0.01 | 0.10 a ± 0.03 | 0.10 a ± 0.0229 | 0.024 |
| C22:5 n-3 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.07 ± 0.02 | 0.07 ± 0.018 | 0.119 |
| C22:6 n-3 | 0.02 b ± 0.00 | 0.01 b ± 0.00 | 0.02 a ± 0.01 | 0.02 a ± 0.01 | 0.026 |
| SFA | 40.31 ± 3.19 | 40.16 ± 1.45 | 39.15 ± 1.54 | 40.12 ± 1.00 | 0.516 |
| MUFA | 54.98 ± 3.24 | 55.63 ± 1.04 | 55.85 ± 1.28 | 54.86 ± 1.07 | 0.587 |
| PUFA | 4.67 ± 0.92 | 4.20 ± 0.47 | 4.99 ± 1.12 | 5.01 ± 0.89 | 0.157 |
| n-6/ n-3 | 15.35 b ± 0.38 | 15.49 b ± 0.45 | 16.03 a ± 0.62 | 16.49 a ± 0.43 | 0.000 |
| G1 | G2 | G3 | G4 | p | |
|---|---|---|---|---|---|
| Moisture (%) | 66.75 ± 2.51 | 66.57 ± 1.77 | 67.81 ± 2.41 | 68.63 ± 2.25 | 0.159 |
| Fat (%) | 11.53 a ± 2.77 | 10.84 a ± 2.28 | 9.66 a,b ± 3.08 | 8.14 b ± 2.19 | 0.047 |
| Protein (%) | 20.51 ± 0.73 | 20.83 ± 0.6 | 20.92 ± 0.76 | 21.01 ± 0.93 | 0.498 |
| Lightness (L*) | 69.59 ± 3.46 | 69.86 ± 2.12 | 69.46 ± 1.62 | 69.96 ± 1.17 | 0.96 |
| Redness index (a*) | 6.19 ± 0.73 | 6.71 ± 1.05 | 5.94 ± 0.54 | 6.12 ± 0.8 | 0.183 |
| Yellowness index (b*) | 8.92 ± 1.32 | 9.22 ± 0.73 | 8.65 ± 1.09 | 8.96 ± 1.22 | 0.546 |
| pH | 5.74 ± 0.04 | 5.73 ± 0.039 | 5.71 ± 0.054 | 5.75 ± 0.04 | 0.307 |
| Cooking losses (%) | 15.90 a,b ± 1.9 | 14.23 b ± 3.45 | 15.87 a,b ± 2.11 | 18.84 a ± 2.56 | 0.003 |
| Warner–Bratzler shear force (kg) | 3.2 ± 1.08 | 3.23 ± 0.98 | 3.65 ± 0.65 | 3.89 ± 1.13 | 0.333 |
| G1 | G2 | G3 | G4 | p | |
|---|---|---|---|---|---|
| External appreciation of fresh meat | |||||
| Color | 2.80 ± 0.71 | 2.83 ± 0.71 | 2.83 ± 0.82 | 2.95 ± 0.83 | 0.925 |
| Marbling | 3.75 a,b ± 0.66 | 4.28 a ± 0.57 | 4.00 a,b ± 0.63 | 3.30 b ± 0.81 | 0.002 |
| Fat color | 1.07 ± 0.0.24 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.293 |
| Fat distribution | 3.95 a,b ± 0.68 | 4.23 a ± 0.69 | 4.16 a ± 0.40 | 3.50 b ± 0.83 | 0.010 |
| Characteristics of cooked meat | |||||
| Color | 1.77 ± 0.69 | 1.66 ± 0.48 | 2.25 ± 0.61 | 11.87 ± 0.60 | 0.228 |
| Odor Intensity | 3.12 ± 0.60 | 3.11 ± 0.76 | 3.50 ± 0.54 | 3.98 ± 0.82 | 0.476 |
| Tenderness | 3.77 a ± 0.69 | 3.67 a ± 1.02 | 3.17 a,b ± 0.41 | 3.12 b ± 0.65 | 0.036 |
| Chewiness | 2.85 a ± 0.81 | 2.66 a ± 0.77 | 2.83 a,b ± 0.41 | 2.43 b ± 0.67 | 0.014 |
| Juiciness | 3.52 a ± 0.50 | 3.50 a,b ± 0.56 | 3.03 b ± 0.63 | 2.95 b ± 0.60 | 0.002 |
| Flavor intensity | 3.45 ± 0.51 | 3.44 ± 0.51 | 3.333 ± 0.52 | 3.37 ± 0.66 | 0.949 |
| Flavor quality | 3.65 ± 0.67 | 3.78 ± 0.55 | 3.33 ± 0.52 | 3.35 ± 0.58 | 0.117 |
| Overall liking | 3.53 a,b ± 0.749 | 3.72 a ± 0.46 | 3.17 a,b ± 0.41 | 3.05 b ± 0.39 | 0.016 |
| Microbial Counts (Log CFU/g) | Pig Diet | D0 | D4 | D8 | D11 | D16 | D21 | Days p-Value |
|---|---|---|---|---|---|---|---|---|
| Total viable counts (TVC) | G1 | 3.9 a,A | 3.9 a,A | 4.2 a,b,A | 4.4 A | 6.2 B | 7.4 B | 0.000 |
| G2 | 3.3 b,A | 2.9 b,A | 3.5 A,b | 5.2 B | 5.0 B | 7.0 C | 0.000 | |
| G3 | 4.1 a,A | 3.4 a,b,A | 4.6 a,B | 4.7 B | 6.3 C | 7.3 D | 0.000 | |
| G4 | 3.4 b,A | 3.5 a,b,A | 3.4 b,A | 4.8 B | 5.7 B | 6.9 C | 0.000 | |
| Pig diet p-value | 0.006 | 0.075 | 0.074 | 0.288 | 0.254 | 0.721 | ||
| Enterobacteria | G1 | 1.0 a,A | 1.4 A | 1.7 A,B | 3.0 B | 3.5 B | 5.8 a,b,C | 0.000 |
| G2 | 1.0 a,A | 1.6 A | 2.0 A,B | 3.0 B | 3.8 B | 5.2 b,C | 0.000 | |
| G3 | 1.0 a,A | 1.4 A | 1.7 A | 3.0 B | 4.4 B | 5.1 b,C | 0.000 | |
| G4 | 1.5 b,A | 1.6 b,A | 1.7 A | 3.0 B | 4.0 B | 6.0 a,C | 0.002 | |
| Pig diet p-value | 0.063 | 0.596 | 0.916 | 0.839 | 0.921 | 0.086 | ||
| Lactic acid bacteria (LAB) | G1 | 1.7 A | 2.4 A,B | 2.7 AB | 3.2 B | 5.7 a,C | 6.4 C | 0.000 |
| G2 | 1.9 A | 1.8 A | 2.6 A,B | 3.4 B | 3.8 b,B | 5.4 C | 0.000 | |
| G3 | 2.0 A | 2.0 A | 3.2 B | 4.2 B2 | 5.6 a,B,C | 6.4 C | 0.000 | |
| G4 | 2.0 A | 2.0 A | 2.3 A | 4.1 B | 4.5 b,B | 5.9 C | 0.000 | |
| Pig diet p-value | 0.665 | 0.438 | 0.630 | 0.254 | 0.063 | 0.129 | ||
| Pseudomonas spp. | G1 | 2.0 A | 2.2 A | 3.0 a,B | 3.4 a,B | 4.4 a,B,C | 6.5 C | 0.000 |
| G2 | 2.0 A | 2.0 A | 2.0 b,A | 2.0 b,A | 2.6 b,A,B | 6.4 B | 0.000 | |
| G3 | 2.0 A | 2.0 A | 3.2 a,B | 3.9 a,A,B | 4.7 a,B | 6.0 C | 0.000 | |
| G4 | 2.2 A | 2.2 A | 2.0 b,A | 2.9 b,A | 2.0 b,A | 6.4 B | 0.000 | |
| Pig diet p-value | 0.441 | 0.596 | 0.007 | 0.096 | 0.005 | 0.559 | ||
| Brochothrix thermosphacta | G1 | 2.2 A | 2.2 A | 2.9 A,B | 4.1 A,B | 4.8 a,b,B | 5.5 B | 0.003 |
| G2 | 2.0 A | 2.0 A | 2.5 A | 2.4 A | 2.0 b,A | 4.2 B | 0.002 | |
| G3 | 2.0 A | 2.0 A | 2.5 A,B | 3.6 B | 5.7 a,C | 5.7 C | 0.000 | |
| G4 | 2.0 | 2.0 | 2.0 | 2.2 | 2.9 b | 3.7 | 0.449 | |
| Pig diet p-value | 0.441 | 0.441 | 0.223 | 0.111 | 0.012 | 0.449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, C.; Sarmiento-García, A.; García, J.-J.; Rubio, B.; Martínez, B. Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet. Foods 2021, 10, 985. https://doi.org/10.3390/foods10050985
Vieira C, Sarmiento-García A, García J-J, Rubio B, Martínez B. Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet. Foods. 2021; 10(5):985. https://doi.org/10.3390/foods10050985
Chicago/Turabian StyleVieira, Ceferina, Ainhoa Sarmiento-García, Juan-José García, Begoña Rubio, and Beatriz Martínez. 2021. "Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet" Foods 10, no. 5: 985. https://doi.org/10.3390/foods10050985
APA StyleVieira, C., Sarmiento-García, A., García, J.-J., Rubio, B., & Martínez, B. (2021). Quality and Shelf Life of Fresh Meat from Iberian Pigs as Affected by a New Form of Presentation of Oleic Acid and an Organic-Acid Mix in the Diet. Foods, 10(5), 985. https://doi.org/10.3390/foods10050985






