1. Introduction
Production and consumption of bison (
Bison bison) has increased significantly since they were hunted to near extinction in North America during the late 1800s [
1,
2]. Currently it is estimated that there are approximately 400,000 bison in North America, including private, state, federal, and tribal herds [
3]. Despite growing popularity, meat quality and consumer preferences for bison are not well characterized, which limits opportunities to expands markets. In addition, both grain- and grass-finishing systems are utilized within the industry, contributing to product variation.
Research describing the influence of finishing system on bison carcass traits and meat quality are lacking. Results from beef studies have generally shown that forage finishing results in leaner carcasses compared with grain finishing when cattle are harvested at similar ages [
4,
5,
6]. Several beef studies have also reported that finishing system can impact meat quality [
7,
8,
9,
10,
11,
12], since the nutrient composition of the feed and amount of dietary energy available to the animal can modify carcass composition [
13], including the amount of intramuscular fat and the fatty acid profile. Changes in intramuscular fat and fatty acid profile are known to influence the eating quality and flavor of beef [
14,
15,
16,
17,
18]. Grain-finished beef is considered to have more acceptable flavor than forage-finished beef [
16,
19,
20,
21]. Changes in fatty acid profile can also impact nutritional quality. Food products containing increased ratios (greater than 0.45) of polyunsaturated fatty acids (PUFA) to saturated fatty acids (SFA) and lower ratios (less than 4.0) of n-6 to n-3 fatty acids may reduce the incidence of coronary artery disease [
22]. Forage-finished beef has been reported to have an increased PUFA to SFA ratio and an improved n-6 to n-3 ratio [
23,
24].
Currently there is very limited research on the carcass characteristics produced across the bison industry, or the effects of common finishing systems on product outcomes. The objective of this study was to characterize the influence of finishing system (grain-finished or grass-finished) on carcass characteristics, meat quality, nutritional composition, and consumer preference for bison meat.
2. Materials and Methods
2.1. Animals, Carcass Evaluation, and Striploin Collection
Prior to treatment allocation, bison heifers (Bison bison; n = 208) were allowed to graze native range near central South Dakota [common vegetation included: western wheatgrass (Pascopyrum smithii), blue grama (Bouteloua gracilis), needle-and-thread (Hesperostipa comata), and green needlegrass (Nassella viridula)]. When heifers were approximately 24 months of age, they were randomly assigned to one of two finishing treatments: Grain- (n = 108) or grass- (n = 93). Grain-finished heifers were placed in an open lot (9290 m2; allowing approximately 93 m2 per animal) and provided ad libitum access to prairie grass and alfalfa hay bales placed in hay rings, as well as a concentrate mixture (83% corn, 17% dried distillers grain) placed in feed bunks for 130 days prior to slaughter. Grass-finished heifers were allowed to continue grazing native range pasture until harvest. Both finishing treatments had access to a custom loose mineral and vitamin supplement [Custom Mineral Mix: Product numbers: 602713 and 603652 (included Rabon for fly control May-October, 2018) Furst-McNess, Freeport, IL, USA].
At approximately 28 months of age, all heifers were transported (~720 km) to a commercial harvest facility and harvested over a two-day period. On the first day of slaughter, 47 head of grass-finished heifers and 54 head of grain-finished were slaughtered. On the second day of slaughter, 46 head of grass-finished and 54 head of grain-finished were slaughtered. After an approximately 20 h chilling period, carcasses were ribbed between the 12th and 13th rib and ribeye area, backfat thickness, marbling score, skeletal maturity, lean maturity, and external fat color were determined by United States Department of Agriculture (USDA) graders. Skeletal maturity was subjectively scored based on the ossification percentage of the thoracic cartilage buttons, and assigned a number (11, 7, 5, or −5) that corresponded with ossification percentages as follows: 0–24% (slight, 11), 25–49% (moderate, 7), 50–99% (hardbone, 5), or 100–200 (extreme hardbone, −5). Lean maturity was subjectively scored based on the lean color of the exposed ribeye, and assigned a number (11, 7, 5, 3, 1, or 0) corresponding to a color description as follows: bright red (11), moderately bright red (7), slightly bright red (5), red (3), pale red (1), or dark cutter (0). Fat color was subjectively scored based on the external fat color, and assigned a number (11, 7, 5, 3, or 1) that corresponded to fat color as follows: white (11), moderately white (7), slightly white (5), moderately yellow (3), and yellow (1). Additionally, objective color (L*, a*, b*) of the exposed ribeye area and the subcutaneous fat of the carcass surface opposite the ribeye were recorded using a handheld Minolta colorimeter (Model CR-310, Minolta Corp., Ramsey, NJ, USA; 50 mm diameter measuring space; D65 illuminant). A subsample (n = 60; 30 carcasses closest to the average hot carcass weight (HCW) for each harvest date per treatment) was selected and transported to a commercial processing facility. Striploins (M. longissimus lumborum) were removed from one side of each carcass, vacuum packaged, and transported in a refrigerated trailer (maintained at approximately 4 °C) back to the South Dakota State University (SDSU) Meat Laboratory.
2.2. Striploin Fabrication and pH
Striploin samples arrived at the SDSU Meat Laboratory at 2- or 3-days postmortem. Upon arrival, all striploins were removed from vacuum packages and trimmed of external fat. Ultimate pH was recorded at the posterior end of the striploin using a hand-held pH meter (Thermo-Scientific Orion Star, Beverly, MA, USA, Model# A221 and Star A321 Portable pH Probe). An approximately 1.27-cm slice was removed from the anterior end of each striploin to create an even surface. The remaining striploin was fabricated into 2.54-cm steaks, all of which were individually vacuum packaged and assigned for analysis. One steak was designated for proximate analysis, analysis of cholesterol content and fatty acid profile and was frozen immediately. Five steaks were designated for Warner-Bratzler shear force (WBSF). One steak was stored for 14 days at 4 °C and sheared without freezing (fresh). Four additional steaks were assigned to a 4-, 7-, 14-, or 21-day aging period, then frozen for approximately three months at −10 °C prior to shear force analysis. The 14-day aged fresh and frozen samples were utilized to compare the influence of freezing on bison steak tenderness. Two steaks designated for a consumer sensory panel were aged for 14 days and frozen.
2.3. Proximate Analysis
To determine proximate nutrient composition of the
M. longissimus lumborum steaks collected from the anterior end of each striploin (at the junction between the 14th thoracic vertebrae and the 1st lumbar vertebrae) were thawed slightly and trimmed of excess external fat and accessory muscles, minced with a knife, submerged in liquid nitrogen, and powdered using a stainless-steel blender (Waring Products Division, Model# 51BL32, Lancaster, PA, USA). Homogenized samples were stored at −20 °C in plastic bags (Whirlpack, Nasco, Fort Atkinson, WI, USA) until chemical composition analyses. Percent crude fat and moisture were determine using ether extraction as described by Mohrhauser et al. [
25]. Powdered samples were weighed (~5 g) into dried aluminum tins (FisherBrand, Pittsburgh, PA, USA, Cat.# 08-732-101), covered with dried filter papers (Whatman, Buckinghamshire, UK, Cat.# 1001-1055) and dried in an oven (Precision Scientific, Winchester, VA, USA, Cat.# 51220159) at 101 °C for 24 h. Dried samples were then placed into a desiccator (Scienceware, Wayne, NJ, USA, Cat.# 420320000) and samples were reweighed after cooling for at least 1 h. Proximate moisture content was calculated as the difference between pre- and post- drying sample weights and expressed as percent of the pre-drying sample weight. Dried samples were then extracted with petroleum ether in a side-arm Soxhlet extractor (ThermoFisher Scientific, Rockville, MD, USA) for a 60-h reflux period followed by evaporation under the laboratory hood at room temperature for 4 h and subsequent drying in an oven at 101 °C for 4 h. Dried, extracted samples were placed in desiccators to cool for 1 h and then reweighed. Proximate intramuscular fat content was calculated as the difference between pre- and post-extraction sample weight and expressed as a percent of the pre-extraction sample weight.
To determine ash percentage of each sample, duplicate powdered samples were weighed (approximately 3 g) into dried ceramic crucibles (COORSTEK, Golden, CO, USA, Cat. #60109) and placed into an oven at 101 °C for 24 h. Dried samples were then placed into a glass desiccator and samples were reweighed after cooling for at least 1 h, and then placed into a muffle furnace (Fisher Scientific Co., Pittsburgh, PA, USA, Model Series# 10-650) at 500 °C and ashed for 24 h. Ashed samples were removed and placed into a desiccator once the furnace cooled down to approximately 150 °C. Ashed samples were cooled in the desiccator for at least 1 h then reweighed. Proximate ash content was calculated as the difference between pre- and post-ashed sample weights and expressed as percent of the pre-ashed sample weight.
To determine protein content, duplicate powdered samples were weighed (approximately 250 mg) into crucibles and were subjected to dumas combustion by a nitrogen analyzer (Rapid Max N Exceed, Elementar, Hanau, Germany, Serial# 29161032). Percent protein content was determined based on the protein factor (6.25) multiplied by the percent nitrogen detected for each sample.
2.4. Cholesterol Determination
To determine cholesterol content of the
M. longissimus lumborum muscle steaks collected from the anterior end of each striploin (at the junction between the 14th thoracic vertebrae and the 1st lumbar vertebrae) were thawed slightly and trimmed of excess external fat and accessory muscles, minced, submerged in liquid nitrogen, and powdered using a stainless-steel blender (Waring Products Division, Model# 51BL32). Homogenized samples were held at −80 °C in plastic bags (Whirlpack, Nasco) until used for cholesterol determination. The AOAC Official Method 994.10, Cholesterol in Foods, Direct Saponification-Gas Chromatographic Method [
26] was used with modifications described by Dinh et al. [
27]. Cholesterol standards were prepared at concentrations of 0.0125, 0.025, 0.05, and 0.1 mg/mL to construct a standard curve for cholesterol determination. An internal standard, 5α-cholestane (ACROS Organics, NJ, USA, Cat.# AC165602500), was used as a correction factor to standardize injection errors. All standards were diluted in high-grade toluene (ACROS Organics, Lot# B052366, UN1294), and were subjected to gas chromatographic system (GC) analysis before and after sequential sample analysis to obtain an average curve. Frozen steak samples were accurately weighed to 1.000 (to the nearest 0.001 g) and placed into 125-mL flat-bottom boiling flasks, followed by the addition of 2 mL of 50% potassium hydroxide (KOH) in water and 10 mL of 95% ethanol. Flasks were placed onto heated magnetic stir plates and the mixtures were boiled, stirred, and refluxed for at least 25 min, or until mixture was clear. Flasks were removed from the stir plates and allowed to cool to room temperature (~25 °C). Mixed solutions were transferred from the boiling flasks to separatory funnels, followed by the addition of 10 mL high-grade toluene and 1.0 N aqueous KOH. Funnels were shaken vigorously for at least 10 s. Mixtures were allowed to stand until the toluene layer was distinctly separated from the bottom aqueous layer. The bottom aqueous layer was discarded, and 5 mL of 0.5 N aqueous KOH was added, gently mixed, and allowed to stand until a clear separation of layers occurred. The bottom aqueous layer was again discarded. The remaining toluene layer was purified by four washes of 5 mL of deionized water. After each wash of deionized water, the solution was mixed, and allowed to stand for complete separation of layers, which allowed the bottom aqueous layer to be discarded before the next wash. The final toluene layer, which could be cloudy, was poured into a 50 mL test tube containing approximately 3 g of anhydrous sodium sulfate. Test tubes were shaken for approximately 5 s to remove excess moisture associated with the toluene. The mixture was allowed to stand until a visibly clear toluene solution appeared, with the anhydrous remaining as a white gelatinous bottom layer. Additional anhydrous was added if the final toluene layer remained cloudy after shaking and allowed to settle. The final purified extract was stored in test tubes with Teflon-lined caps under refrigeration. Prior to mixing, all solutions were brought to room temperature. In a 2.0 mL GC vial (Agilent Technologies, Santa Clara, CA, USA, Part No., 5188–6592, Batch No., GTG023112229), 0.5 mL of the clear toluene solution containing the extracted cholesterol was mixed with 0.5 mL of internal standard and subjected to GC analysis.
Liberated cholesterol was quantified using the Agilent 6890N GC system and the DB-17 capillary column (30 m × 0.250 mm × 0.15 μm, Agilent Technologies Inc.). The DB-17 has mid-polarity and is suitable for analysis of free steroids. One microliter (1.0 µL) of analyte cholesterol mixture was injected into the GC system with split/splitless injector and flame ionization detector. The inlet temperature was 250 °C and split ratio was 50:1. The carrier gas was helium at 1.4 mL per min constant flow. The oven was programmed isothermally at 260 °C and held for 13 min. Total time for GC determination was 15 min. The detector was set at 350 °C with 450 mL per min airflow, 40 mL per min hydrogen flow, and 40 mL per min constant column and helium makeup flow.
2.5. Fatty Acid Composition Analysis
For fatty acid methyl ester (FAME) analyses of the
longissimus dorsi muscle samples were thawed slightly and trimmed of excess external fat and accessory muscles, minced, submerged in liquid nitrogen, and powdered using a stainless-steel blender (Waring Products Division, Model# 51BL32). Homogenized samples were held at approximately −80 °C in plastic bags (Whirlpack, Nasco) until FAME analyses. Frozen samples were accurately weighed to 1.000 (to the nearest 0.001 g) and processed to generate FAME according to procedures outlined by O’Fallon et al. [
28]. Analysis of FAME was conducted by GC using an HP-88 capillary column (30m × 0.25 mm × 0.20 µm; Agilent Technologies, Palo Alto, CA, USA) and a flame ionization detector (FID). One microliter of sample was injected with a split ratio of 50:1. The oven method was as follows: 120 °C held for 1 min, increased to a temperature of 170 °C at the rate of 15 °C per min, held for 2 min, then increased to a temperature of 200 °C at the rate of 3 °C per min, held for 1 min, and finally increased to a temperature of 235 °C at a rate of 20 °C per min and held for 1 min. Hydrogen was used as the carrier gas. The FID was operated at 300 °C. Fatty acid methyl esters were identified and quantified by use of authentic standards (Supelco 37 Component FAME mix, Sigma-Aldrich, St. Louis, MO, USA). Concentrations of fatty acids were calculated and expressed on both a raw wet-weight, and percentage of total fatty acid basis.
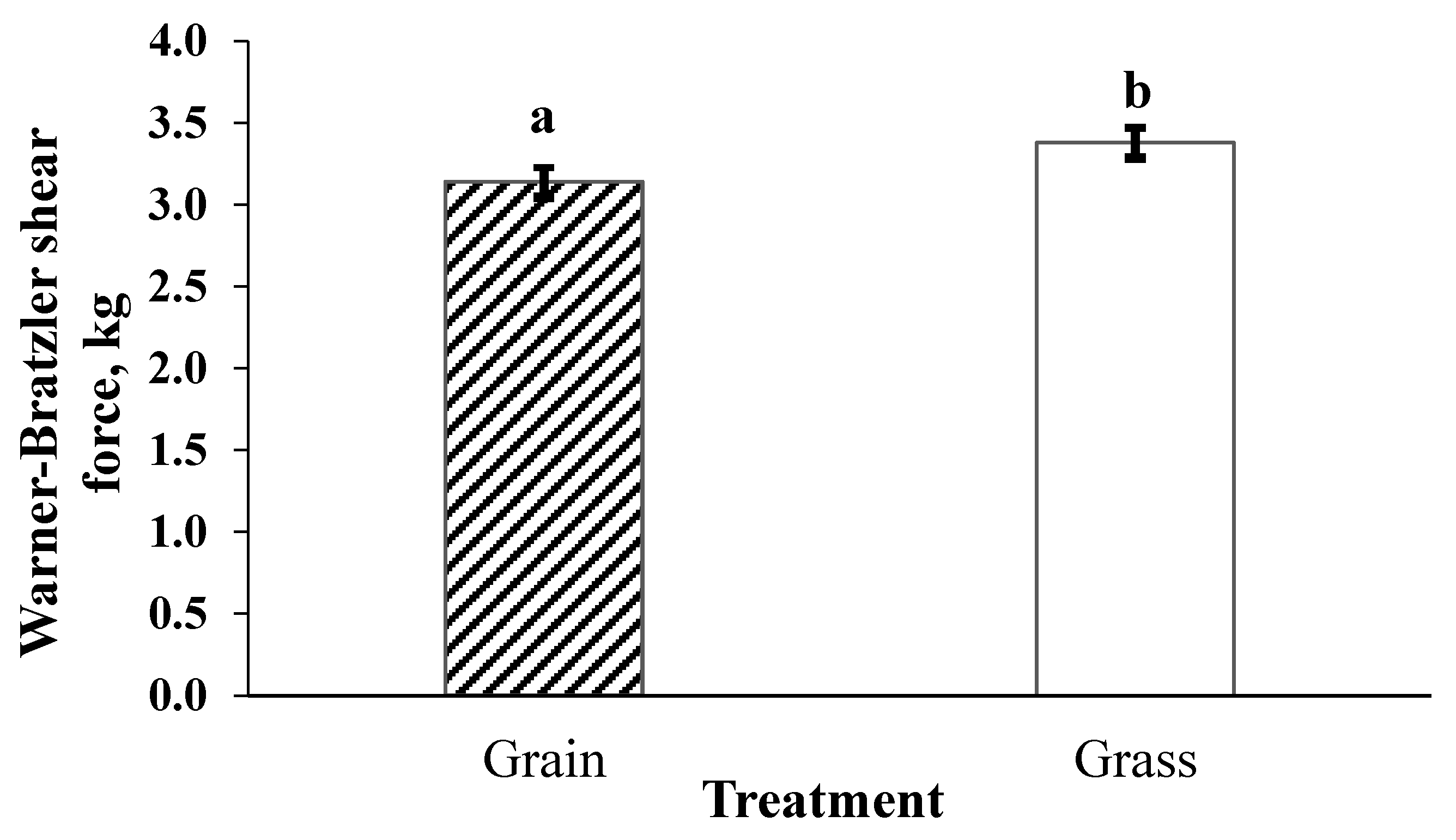
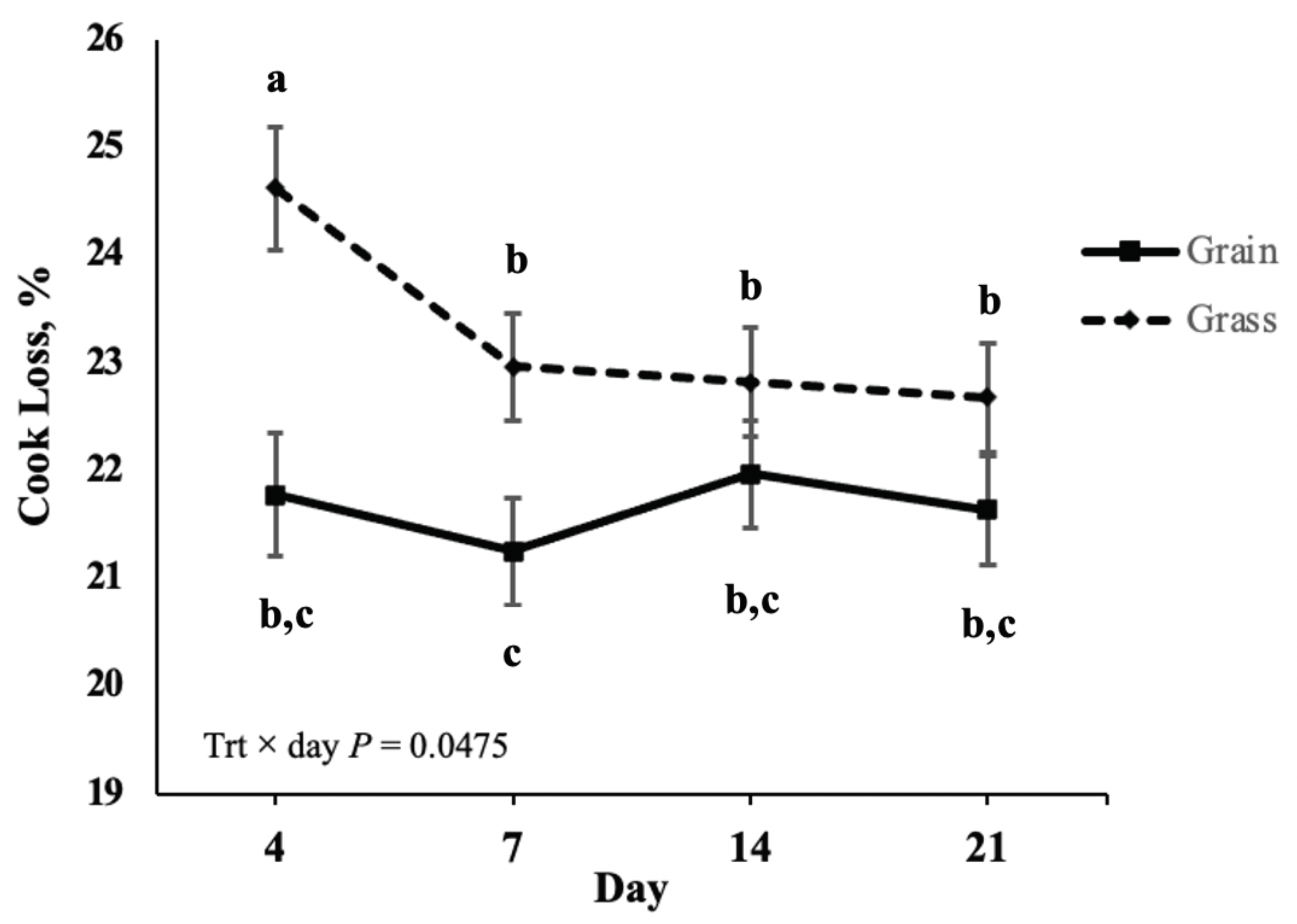
2.6. Warner-Bratzler Shear Force and Cook Loss
Warner-Bratzler Shear Force was utilized to compare the tenderness of grass- and grain-finished bison, the influence of postmortem aging on tenderness of striploin steaks from grain- and grass-finished bison, and the influence of storage conditions (fresh versus frozen) on tenderness of bison striploin steaks. In preparation for WBSF, frozen steaks were thawed at 4 °C for 24 h before cooking. All steaks were weighed prior to cooking to an internal temperature of 71 °C. Steaks were cooked on an electric clamshell grill (George Forman 9 Serving Classic Plate Grill, Model GR2144P, Middleton, WI, USA). Internal temperature was monitored using a digital thermometer (Cooper-Atkins, Middlefield, CT, Model# 41-983430-5) placed near the geometric center of each steak. After cooking, all steaks were allowed to cool to room temperature before they were reweighed to determine cook loss. Cook loss was reported as a percentage of the raw weight using the following equation: [(raw weight − cooked weight)/raw weight] × 100. Cooked steaks were cooled at 4 °C for 24 h before removing 5 to 6 cores (1.27 cm in diameter) parallel to the muscle fiber orientation. Each core was sheared once perpendicular to the muscle fiber orientation and peak force was recorded [
29]. A texture analyzer (Shimadzu Scientific Instruments Inc., Lenexa, KS, USA, Model EZ-SX) with a Warner-Bratzler attachment was used to determine peak force required to shear each core. An average shear peak force value was then reported for each steak.
2.7. Consumer Preference
A consumer sensory panel was conducted at the University of Minnesota Sensory Laboratory to determine subjective meat quality characteristics of grain- and grass-finished bison striploin steaks. A sub-sample (n = 46; steaks from the 23 carcasses closest to the average marbling score for each treatment) was utilized for the sensory panel. Random participants (n = 113) were recruited from the student and staff population of the University of Minnesota and included anyone who expressed an interest in participating in sensory tests. Participants were 18 years or older, had no food allergies or sensitivities, were willing to consume bison meat, and must have consumed any type of meat at least once a year. Participants were compensated for their time. The University of Minnesota’s Institutional Review Board (IRB) approved all recruiting and experimental procedures (IRB #6792). Sample steaks, aged 14 days and kept in frozen storage conditions approximately 10 months prior to analysis, were wrapped in aluminum foil, and allowed to thaw for 48 h before they were placed in an electric oven set to 204 °C. Internal temperature was monitored using a digital thermometer (Cooper-Atkins, Model# DTT361-01) placed near the geometric center of each steak. Steaks were cooked until they reached an internal temperature of 71 °C. Cooked steaks were allowed an approximate 3 min rest time before they were trimmed of external fat, placed into a grid cutter, and cut into 1-cm × 1-cm × 2.5-cm sample cubes. Cubes were held in porcelain double boilers, lined with aluminum foil, and heated to approximately 60 °C to maintain temperature before allocation to individual sample cups. Samples were transferred to lidded, 4 oz. foam cups with random 3-digit codes specific to each treatment code. The foam cups were held until served inside a proofing cabinet (Win-Holt NSF ETL, Syosset, NY, USA, Model #NHPL-1836C) set to a temperature of 54–60 °C and a humidity setting of 9. Each participant received two samples per treatment (samples from each treatment were presented to panelists simultaneously) and were provided with distilled water.
Participants were first asked to assess aroma liking. They were instructed to evaluate sample aroma by partially opening the sample lid and observing the aroma of the sample. Participants were then instructed to taste one of the sample cubes and rate it for overall liking, liking of flavor, and liking of texture. Participants were then instructed to taste the second piece and rate tenderness, juiciness, and off-flavor intensity. Liking ratings were made on 120-point labeled affective magnitude scales, with the left most end labeled ‘greatest imaginable disliking’ and the right most end labeled ‘greatest imaginable liking’. Intensity ratings were made on 20-point line scales with the left most ends labeled ‘none’ and the right most ends labeled ‘extremely intense’ for off-flavor, ‘extremely juicy’ for juiciness, and ‘extremely tough’ for toughness. Participants who rated the off-flavor at an intensity of 10 or more were required to answer the following open-ended question: “Please describe, as specifically as you can, what this off-flavor was”.
2.8. Statistical Analysis
Live body weight, dressing percent, carcass measurements, shear force, cook loss, storage conditions (fresh vs. frozen for cook loss and shear force analyses), fatty acid profile, cholesterol content, and proximate analysis data were analyzed using the MIXED procedures of SAS (SAS Inst. Inc., Cary, NC, USA). Subjective carcass measurements, including fat color, lean, and skeletal maturity, and all USDA Yield Grade data were analyzed using the GLIMMEX procedures of SAS for the main effect of finishing treatment. Kill date was included as a random effect, and peak steak cooking temperature was included as a covariate for shear force and cook loss. The interaction of storage conditions with finishing treatment was initially included but was not significant for shear force or cook loss and therefore omitted from the final model. Cook loss and shear force samples were subjected to different postmortem aging periods before frozen and were analyzed as repeated measures using the ante-dependence covariance structure in the MIXED procedure of SAS for effects of finishing treatment, aging, and their interaction; peak temperature was included as a covariate. The interaction of postmortem aging period with finishing treatment was not significant for shear force and therefore omitted from the model. Consumer preference data was analyzed using the MIXED procedures of SAS for the main effects of finishing treatment and serving order; time and panelist were used as random effects. For all attributes except toughness and juiciness ratings, serving order was not significant and omitted from the final model. Separation of least-squares main effect means was performed using LSD with a Tukey’s adjustment and assuming an alpha level of 0.05. Carcass served as the experiment unit for all carcass and meat quality analyses, and the individual panelists served as the experimental unit for sensory analysis.