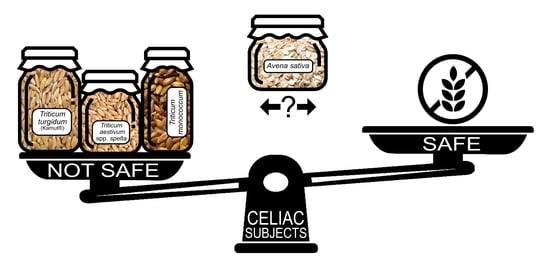
Ancient and Modern Cereals as Ingredients of the Gluten-Free Diet: Are They Safe Enough for Celiac Consumers?
Abstract
1. Introduction
2. Methods
3. Results and Discussion
3.1. Triticum monococcum
3.2. Triticum aestivum ssp. spelta
3.3. Kamut®
3.4. Avena sativa L.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tovoli, F. Clinical and diagnostic aspects of gluten related disorders. World J. Clin. Cases 2015, 3, 257–284. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef]
- Catassi, C. Gluten sensitivity. Ann. Nutr. Metab. 2015, 67, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Green, P.H.R.; Fasano, A. Extraintestinal manifestations of coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac disease, inflammation and oxidative damage: A nutrigenetic approach. Nutrients 2012, 4, 243–257. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P. The biochemical basis of celiac disease. Cereal Chem. 2008, 85, 1–13. [Google Scholar] [CrossRef]
- Monteiro, J.S.; Farage, P.; Zandonadi, R.P.; Botelho, R.B.A.; de Oliveira, L.d.L.; Raposo, A.; Shakeel, F.; Alshehri, S.; Mahdi, W.A.; Araújo, W.M.C. A Systematic Review on Gluten-Free Bread Formulations Using Specific Volume as a Quality Indicator. Foods 2021, 10, 614. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Rizzello, C. Consumers’ demand. Foods 2021, 10, 462. [Google Scholar] [CrossRef]
- Kosová, K.; Leišová-Svobodová, L.; Dvořáček, V. Oats as a Safe Alternative to Triticeae Cereals for People Suffering from Celiac Disease? A Review. Plant. Foods Hum. Nutr. 2020, 75, 131–141. [Google Scholar] [CrossRef]
- Silano, M.; Penas Pozo, E.; Uberti, F.; Manferdelli, S.; Del Pinto, T.; Felli, C.; Budelli, A.; Vincentini, O.; Restani, P. Diversity of oat varieties in eliciting the early inflammatory events in celiac disease. Eur. J. Nutr. 2014, 53, 1177–1186. [Google Scholar] [CrossRef]
- Silano, M.; Di Benedetto, R.; Maialetti, F.; De Vincenzi, A.; Calcaterra, R.; Cornell, H.J.; De Vincenzi, M. Avenins from different cultivars of oats elicit response by coeliac peripheral lymphocytes. Scand. J. Gastroenterol. 2007, 42, 1302–1305. [Google Scholar] [CrossRef]
- Ballabio, C.; Uberti, F.; Manferdelli, S.; Vacca, E.; Boggini, G.; Redaelli, R.; Catassi, C.; Lionetti, E.; Peñas, E.; Restani, P. Molecular characterisation of 36 oat varieties and in vitro assessment of their suitability for coeliacs’ diet. J. Cereal Sci. 2011, 54, 110–115. [Google Scholar] [CrossRef]
- Purdue University Center for New Crops and Plant Products Crop Index. Available online: https://hort.purdue.edu/newcrop/default.html (accessed on 19 February 2021).
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 19 February 2021).
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Romão, B.; Falcomer, A.L.; Palos, G.; Cavalcante, S.; Botelho, R.B.A.; Nakano, E.Y.; Raposo, A.; Shakeel, F.; Alshehri, S.; Mahdi, W.A.; et al. Glycemic Index of Gluten-Free Bread and Their Main Ingredients: A Systematic Review and Meta-Analysis. Foods 2021, 10, 506. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU). No. 828/2014 of 30 July 2014 on the requirements for the provision of information to consumers on the absence or reduced presence of gluten in food. Off. J. Eur. Union 2014, 228, 5–8. [Google Scholar]
- Escarnot, E.; Gofflot, S.; Sinnaeve, G.; Dubois, B.; Bertin, P.; Mingeot, D. Reactivity of gluten proteins from spelt and bread wheat accessions towards A1 and G12 antibodies in the framework of celiac disease. Food Chem. 2018, 268, 522–532. [Google Scholar] [CrossRef]
- Dubois, B.; Bertin, P.; Mingeot, D. Molecular diversity of α-gliadin expressed genes in genetically contrasted spelt (Triticum aestivum ssp. spelta) accessions and comparison with bread wheat (T. aestivum ssp. aestivum) and related diploid Triticum and Aegilops species. Mol. Breed. 2016, 36, 152. [Google Scholar] [CrossRef]
- Dubois, B.; Bertin, P.; Hautier, L.; Muhovski, Y.; Escarnot, E.; Mingeot, D. Genetic and environmental factors affecting the expression of α-gliadin canonical epitopes involved in celiac disease in a wide collection of spelt (Triticum aestivum ssp. spelta) cultivars and landraces. BMC Plant. Biol. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Di Stasio, L.; Picascia, S.; Auricchio, R.; Vitale, S.; Gazza, L.; Picariello, G.; Gianfrani, C.; Mamone, G. Comparative Analysis of in vitro Digestibility and Immunogenicity of Gliadin Proteins From Durum and Einkorn Wheat. Front. Nutr. 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; Van Veelen, P.; Drijfhout, J.W.; Jonker, H.; Van Soest, L.; Smulders, M.J.M.; Bosch, D.; Gilissen, L.J.W.J.; Koning, F. Natural variation in toxicity of wheat: Potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 2005, 129, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Asledottir, T.; Rehman, R.; Mamone, G.; Picariello, G.; Devold, T.G.; Vegarud, G.E.; Røseth, A.; Lea, T.E.; Halstensen, T.S.; Ferranti, P.; et al. Ancestral Wheat Types Release Fewer Celiac Disease Related T Cell Epitopes than Common Wheat upon Ex Vivo Human Gastrointestinal Digestion. Foods 2020, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Vincentini, O.; Maialetti, F.; Gazza, L.; Silano, M.; Dessi, M.; De Vincenzi, M.; Pogna, N.E. Environmental factors of celiac disease: Cytotoxicity of hulled wheat species Triticum monococcum, T. turgidum ssp. dicoccum and T. aestivum ssp. spelta. J. Gastroenterol. Hepatol. 2007, 22, 1816–1822. [Google Scholar] [CrossRef]
- Iacomino, G.; Di Stasio, L.; Fierro, O.; Picariello, G.; Venezia, A.; Gazza, L.; Ferranti, P.; Mamone, G. Protective effects of ID331 Triticum monococcum gliadin on in vitro models of the intestinal epithelium. Food Chem. 2016, 212, 537–542. [Google Scholar] [CrossRef]
- Mamone, G.; Iacomino, G. Comparison of the in vitro toxicity of ancient Triticum monococcum varieties ID331 and Monlis. Int. J. Food Sci. Nutr. 2018, 69, 954–962. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Luchetti, R.; Giovannini, C.; Pogna, N.E.; Saponaro, C.; Galterio, G.; Gasbarrini, G. In vitro toxicity testing of alcohol-soluble proteins from diploid wheat triticum monococcum in celiac disease. J. Biochem. Toxicol. 1996, 11, 313–318. [Google Scholar] [CrossRef]
- Gianfrani, C.; Maglio, M.; Aufiero, V.R.; Camarca, A.; Vocca, I.; Iaquinto, G.; Giardullo, N.; Pogna, N.; Troncone, R.; Auricchio, S.; et al. Immunogenicity of monococcum wheat in celiac patients. Am. J. Clin. Nutr. 2012, 96, 1339–1345. [Google Scholar] [CrossRef]
- Šuligoj, T.; Gregorini, A.; Colomba, M.; Ellis, H.J.; Ciclitira, P.J. Evaluation of the safety of ancient strains of wheat in coeliac disease reveals heterogeneous small intestinal T cell responses suggestive of coeliac toxicity. Clin. Nutr. 2013, 32, 1043–1049. [Google Scholar] [CrossRef]
- Gianfrani, C.; Camarca, A.; Mazzarella, G.; Di stasio, L.; Giardullo, N.; Ferranti, P.; Picariello, G.; Rotondi Aufiero, V.; Picascia, S.; Troncone, R.; et al. Extensive in vitro gastrointestinal digestion markedly reduces the immune-toxicity of Triticum monococcum wheat: Implication for celiac disease. Mol. Nutr. Food Res. 2015, 59, 1844–1854. [Google Scholar] [CrossRef]
- Molberg, Ø.; Uhlen, A.K.; Jensen, T.; Flæte, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.A.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 128, 393–401. [Google Scholar] [CrossRef]
- Pizzuti, D.; Buda, A.; D’Odorico, A.; D’Incà, R.; Chiarelli, S.; Curioni, A.; Martines, D. Lack of intestinal mucosal toxicity of Triticum monococcum in celiac disease patients. Scand. J. Gastroenterol. 2006, 41, 1305–1311. [Google Scholar] [CrossRef]
- Zanini, B.; Villanacci, V.; De Leo, L.; Lanzini, A. Triticum monococcum in patients with celiac disease: A phase II open study on safety of prolonged daily administration. Eur. J. Nutr. 2015, 54, 1027–1029. [Google Scholar] [CrossRef]
- Zanini, B.; Petroboni, B.; Not, T.; Di Toro, N.; Villanacci, V.; Lanzarotto, F.; Pogna, N.; Ricci, C.; Lanzini, A. Search for atoxic cereals: A single blind, cross-over study on the safety of a single dose of Triticum monococcum, in patients with celiac disease. BMC Gastroenterol. 2013, 13, 92. [Google Scholar] [CrossRef]
- Picascia, S.; Camarca, A.; Malamisura, M.; Mandile, R.; Galatola, M.; Cielo, D.; Gazza, L.; Mamone, G.; Auricchio, S.; Troncone, R.; et al. In Celiac Disease Patients the In Vivo Challenge with the Diploid Triticum monococcum Elicits a Reduced Immune Response Compared to Hexaploid Wheat. Mol. Nutr. Food Res. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Dubois, B.; Bertin, P.; Muhovski, Y.; Escarnot, E.; Mingeot, D. Development of TaqMan probes targeting the four major celiac disease epitopes found in α-gliadin sequences of spelt (Triticum aestivum ssp. spelta) and bread wheat (Triticum aestivum ssp. aestivum). Plant. Methods 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Röder, M.S.; Grausgruber, H.; Börner, A. A DNA fingerprinting-based taxonomic allocation of Kamut wheat. Plant. Genet. Resour. 2006, 4, 172–180. [Google Scholar] [CrossRef]
- Colomba, M.S.; Gregorini, A. Are ancient durum wheats less toxic to celiac patients? A study of α-Gliadin from Graziella Ra and Kamut. Sci. World J. 2012, 2012, 837416. [Google Scholar] [CrossRef]
- Forssell, F.; Wieser, H. [Spelt wheat and celiac disease]. Zeitschrift fur Lebensmittel-Untersuchung Und-Forschung 1995, 201, 35–39. [Google Scholar] [CrossRef]
- Van De Wal, Y.; Kooy, Y.M.C.; Van Veelen, P.; Vader, W.; August, S.A.; Drijfhout, J.W.; Peña, S.A.; Koning, F. Glutenin is involved in the gluten-driven mucosal T cell response. Eur. J. Immunol. 1999, 29, 3133–3139. [Google Scholar] [CrossRef]
- Vader, W.; Kooy, Y.; Van Veelen, P.; De Ru, A.; Harris, D.; Benckhuijsen, W.; Pea, S.; Mearin, L.; Drijfhout, J.W.; Koning, F. The Gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 2002, 122, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Gregorini, A.; Colomba, M.; Julia Ellis, H.; Ciclitira, P.J. Immunogenicity characterization of two ancient wheat α-gliadin peptides related to coeliac disease. Nutrients 2009, 1, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Malalgoda, M.; Ohm, J.B.; Simsek, S. Celiac antigenicity of ancient wheat species. Foods 2019, 8, 675. [Google Scholar] [CrossRef] [PubMed]
- Willemijn Vader, L.; De Ru, A.; Van Der Wal, Y.; Kooy, Y.M.C.; Benckhuijsen, W.; Luisa Mearin, M.; Drijfhout, J.W.; Van Veelen, P.; Koning, F. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J. Exp. Med. 2002, 195, 643–649. [Google Scholar] [CrossRef]
- Lerner, A. The enigma of oats in nutritional therapy for celiac disease. Int. J. Celiac Dis. 2014, 2, 110–114. [Google Scholar] [CrossRef][Green Version]
- Fritz, R.D.; Chen, Y. Oat safety for celiac disease patients: Theoretical analysis correlates adverse symptoms in clinical studies to contaminated study oats. Nutr. Res. 2018, 60, 54–67. [Google Scholar] [CrossRef]
- Koerner, T.B.; Cléroux, C.; Poirier, C.; Cantin, I.; Alimkulov, A.; Elamparo, H. Gluten contamination in the Canadian commercial oat supply. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2011, 28, 705–710. [Google Scholar] [CrossRef]
- Thompson, T. Gluten contamination of commercial oat products in the United States. N. Engl. J. Med. 2004, 351, 2021–2022. [Google Scholar] [CrossRef]
- Hernando, A.; Mujico, J.R.; Juanas, D.; Méndez, E. Confirmation of the Cereal Type in Oat Products Highly Contaminated with Gluten. J. Am. Diet. Assoc. 2006, 106, 665. [Google Scholar] [CrossRef]
- Picarelli, A.; Di Tola, M.; Sabbatella, L.; Gabrielli, F.; Di Cello, T.; Anania, M.C.; Mastracchio, A.; Silano, M.; De Vincenzi, M. Immunologic evidence of no harmful effect of oats in celiac disease. Am. J. Clin. Nutr. 2001, 74, 137–140. [Google Scholar] [CrossRef][Green Version]
- Maglio, M.; Mazzarella, G.; Barone, M.V.; Gianfrani, C.; Pogna, N.; Gazza, L.; Stefanile, R.; Camarca, A.; Colicchio, B.; Nanayakkara, M.; et al. Immunogenicity of two oat varieties, in relation to their safety for celiac patients. Scand. J. Gastroenterol. 2011, 46, 1194–1205. [Google Scholar] [CrossRef]
- Comino, I.; Bernardo, D.; Bancel, E.; De Lourdes Moreno, M.; Sánchez, B.; Barro, F.; Šuligoj, T.; Ciclitira, P.J.; Cebolla, Á.; Knight, S.C.; et al. Identification and molecular characterization of oat peptides implicated on coeliac immune response. Food Nutr. Res. 2016, 60, 30324. [Google Scholar] [CrossRef]
- Arentz-Hansen, H.; Fleckenstein, B.; Molberg, Ø.; Scott, H.; Koning, F.; Jung, G.; Roepstorff, P.; Lundin, K.E.A.; Sollid, L.M. The molecular basis for oat intolerance in patients with celiac disease. PLoS Med. 2004, 1, 84–95. [Google Scholar] [CrossRef]
- Comino, I.; Real, A.; De Lorenzo, L.; Cornell, H.; López-Casado, M.Á.; Barro, F.; Lorite, P.; Torres, M.I.; Cebolla, Á.; Sousa, C. Diversity in oat potential immunogenicity: Basis for the selection of oat varieties with no toxicity in coeliac disease. Gut 2011, 60, 915–922. [Google Scholar] [CrossRef]
- Hollén, E.; Högberg, L.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.E. Antibodies to oat prolamines (avenins) in children with coeliac disease. Scand. J. Gastroenterol. 2003, 38, 742–746. [Google Scholar] [CrossRef]
- Vainio, E.; Varjonen, E. Antibody response against wheat, rye, barley, oats and corn: Comparison between gluten-sensitive patients and monoclonal antigliadin antibodies. Int. Arch. Allergy Immunol. 1995, 106, 134–138. [Google Scholar] [CrossRef]
- Guttormsen, V.; Løvik, A.; Bye, A.; Bratlie, J.; Mørkrid, L.; Lundin, K.E.A. No induction of anti-avenin IgA by oats in adult, diet-treated coeliac disease. Scand. J. Gastroenterol. 2008, 43, 161–165. [Google Scholar] [CrossRef]
- Tjellström, B.; Stenhammar, L.; Sundqvist, T.; Fälth-Magnusson, K.; Hollén, E.; Magnusson, K.E.; Norin, E.; Midtvedt, T.; Högberg, L. The effects of oats on the function of gut microflora in children with coeliac disease. Aliment. Pharmacol. Ther. 2014, 39, 1156–1160. [Google Scholar] [CrossRef]
- Sjöberg, V.; Hollén, E.; Pietz, G.; Magnusson, K.E.; Fälth-Magnusson, K.; Sundström, M.; Holmgren Peterson, K.; Sandström, O.; Hernell, O.; Hammarström, S.; et al. Noncontaminated dietary oats may hamper normalization of the intestinal immune status in childhood celiac disease. Clin. Transl. Gastroenterol. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Lundin, K.E.A.; Nilsen, E.M.; Scott, H.G.; Løberg, E.M.; Gjøen, A.; Bratlie, J.; Skar, V.; Mendez, E.; Loøvik, A.; Kett, K. Oats induced villous atrophy in coeliac disease. Gut 2003, 52, 1649–1652. [Google Scholar] [CrossRef]
- Janatuinen, E.K.; Kemppainen, T.A.; Julkunen, R.J.K.; Kosma, V.M.; Mäki, M.; Heikkinen, M.; Uusitupa, M.I.J. No harm from five year ingestion of oats in coeliac disease. Gut 2002, 50, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Janatuinen, E.; Pikkarainen, P.; Kemppainen, T.; Kosma, V.-M.; Järvinen, R.; Uusitupa, M.; Julkunen, R. A comparison of diets with and without oats in adults with celiac disease. Eur. J. Gastroenterol. Hepatol. 1995, 12, 1232. [Google Scholar] [CrossRef]
- Sey, M.S.L.; Parfitt, J.; Gregor, J. Prospective study of clinical and histological safety of pure and uncontaminated canadian oats in the management of celiac disease. J. Parenter. Enter. Nutr. 2011, 35, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Collin, P.; Huhtala, H.; Mäki, M. Long-term consumption of oats in adult celiac disease patients. Nutrients 2013, 5, 4380–4389. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.Y.; Tye-Din, J.A.; Stewart, J.A.; Schmitz, F.; Dudek, N.L.; Hanchapola, I.; Purcell, A.W.; Anderson, R.P. Ingestion of oats and barley in patients with celiac disease mobilizescross-reactive T cells activated by avenin peptides andimmuno-dominant hordein peptides. J. Autoimmun. 2015, 56, 56–65. [Google Scholar] [CrossRef]
- Størsrud, S.; Olsson, M.; Arvidsson Lenner, R.; Nilsson, L.Å.; Nilsson, O.; Kilander, A. ORIGINAL COMMUNICATION Adult coeliac patients do tolerate large amounts of oats. Eur. J. Clin. Nutr. 2003, 57, 163–169. [Google Scholar] [CrossRef]
- Kemppainen, T.; Janatuinen, E.; Holm, K.; Kosma, V.M.; Heikkinen, M.; Mäki, M.; Laurila, K.; Uusitupa, M.; Julkunen, R. No observed local immunological response at cell level after five years of oats in adult coeliac disease. Scand. J. Gastroenterol. 2007, 42, 54–59. [Google Scholar] [CrossRef]
- Hoffenberg, E.J.; Haas, J.; Drescher, A.; Barnhurst, R.; Osberg, I.; Bao, F.; Eisenbarth, G. A trial of oats in children with newly diagnosed celiac disease. J. Pediatr. 2000, 137, 361–366. [Google Scholar] [CrossRef]
- Holm, K.; Mäki, M.; Vuolteenaho, N.; Mustalahti, K.; Ashorn, M.; Ruuska, T.; Kaukinen, K. Oats in the treatment of childhood coeliac disease: A 2-year controlled trial and a long-term clinical follow-up study. Aliment. Pharmacol. Ther. 2006, 23, 1463–1472. [Google Scholar] [CrossRef]
- Högberg, L.; Laurin, P.; Fâlth-Magnusson, K.; Grant, C.; Grodzinsky, E.; Jansson, G.; Ascher, H.; Browaldh, L.; Hammersjö, J.Å.; Lindberg, E.; et al. Oats to children with newly diagnosed coeliac disease: A randomised double blind study. Gut 2004, 53, 649–654. [Google Scholar] [CrossRef]
- Hollén, E.; Forslund, T.; Högberg, L.; Laurin, P.; Stenhammar, L.; Fälth-Magnusson, K.; Magnusson, K.E.; Sundqvist, T. Urinary nitric oxide during one year of gluten-free diet with or without oats in children with coeliac disease. Scand. J. Gastroenterol. 2006, 41, 1272–1278. [Google Scholar] [CrossRef]
- Koskinen, O.; Villanen, M.; Korponay-Szabo, I.; Lindfors, K.; Mäki, M.; Kaukinen, K. Oats do not induce systemic or mucosal autoantibody response in children with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 559–565. [Google Scholar] [CrossRef]
- Lionetti, E.; Gatti, S.; Galeazzi, T.; Caporelli, N.; Francavilla, R.; Cucchiara, S.; Roggero, P.; Malamisura, B.; Iacono, G.; Tomarchio, S.; et al. Safety of Oats in Children with Celiac Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Pediatr. 2018, 194, 116–122. [Google Scholar] [CrossRef]
- Aaltonen, K.; Laurikka, P.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. The long-term consumption of oats in celiac disease patients is safe: A large cross-sectional study. Nutrients 2017, 9, 611. [Google Scholar] [CrossRef]
| Allowed | Not Allowed |
|---|---|
| Cereals | |
| Corn | Wheat (Spelt, semolina, durum) |
| Rice | Rye |
| Sorghum | Barley |
| Oat * | Kamut® |
| Pseudocereals | |
| Buckwheat | |
| Quinoa | |
| Amaranth | |
| Test | Outcome |
|---|---|
| K562(S) cells | Agglutination test: K562(S) cells agglutinated after contact with gluten. The agglutination ratio is strongly correlated with cereal toxicity |
| Caco-2 cells | Inhibition of cell growth, correlated with toxic cereals |
| Activation of cell apoptosis | |
| Nitric oxide (NO) release: Cao-2 cells produce NO after exposure to toxic peptides | |
| Alteration of transepithelial electrical resistance (TEER): Toxic cereals influence the barrier integrity with a consequent decrease in TEER | |
| Expression of tissue transglutaminase (TG II): increasing amounts of TG II are associated with toxic cereal exposure | |
| Cytoskeleton reorganization: gliadin-derived peptides induce early actin reorganization | |
| Zonulin release: Gliadin peptides induce zonulin release correlated with the alteration of epithelial permeability | |
| Biopsy specimens from the duodenum are processed for ex vivo organ culture | Conventional histology (e.g., enterocyte height) |
| Immunochemical studies (e.g., expression of HLA-DR, number of CD3+ T cells; Interferon-γ secretion) | |
| T cell line (generated from duodenal biopsies) | Cell proliferation test: Evaluates the immune stimulatory properties of cereal samples |
| Immunochemical studies (Interferon-γ secretion) |
| Key Words | Celiac Disease | Gluten | Total |
|---|---|---|---|
| Spelt or Triticum aestivum ssp. spelta | 17 (6) | 27 (0) | 6 |
| Kamut® or Triticum turgidum | 7 (4) | 4 (0) | 4 |
| Einkorn or Triticum monococcum | 20 (12) | 28 (2) | 12 |
| Oat or Avena sativa | 70 (11) | 162 (19) | 30 |
| Objective of the Study | Methods | Main Outcomes | Ref. |
|---|---|---|---|
| Lower toxicity | |||
| To evaluate the toxicity of gliadin peptides from 10 varieties of T. monococcum. | The peptic–tryptic digestion products (PTd) of alcohol-soluble proteins from samples were tested for their agglutinating activities in K562(S) cells. | No agglutination was observed in K562(S) cells. | [29] |
| To evaluate the T-cell stimulatory capacity of different wheat species (including T. monococcum). | Gluten was extracted from different ancient wheat species and screened for T-cell stimulatory gluten peptides. | The intestinal T-cell response was different for the diploid species considered. The sequence of gliadin 33 mer (the most toxic) was not observed in diploid einkorn. | [33] |
| To determine the toxicity of T. monococcum on small intestinal mucosa through an organ culture system. | 29 distal duodenum biopsies (12 from treated celiac patients, 17 from control subjects) were cultured for 24 h with 1 mg/mL of gliadin from T. aestivum (bread) or from T. monococcum. Tests included conventional histological examination, immunohistochemical detection of CD3 + IELs, HLA-DR and the IFN-γ. | T. monococcum gliadin did not determine any significant morphological changes in celiac subjects, HLA-DR overexpression in the crypt epithelium, increased number of CD3 + IELs nor significant IFN-γ response. | [34] |
| To evaluate the cytotoxicity of prolamins for celiac disease in different species from ancient wheat (T. aestivum ssp. spelta, T. monococcum and T. turgidum ssp. dicoccum). | Cytotoxicity was evaluated in term of activation of apoptosis, inhibition of cell growth, release of nitric oxide, alteration of TEER and detection of TG II on Caco-2/Tc7 and K562(S) cell agglutination. | The PTd from T. monoccum wheat did not show any negative effects on Caco-2/TC7 and K562(S) cells. | [26] |
| To investigate the toxicity of T. monococcum and T. aestivum cultivars after treatment with the products from PTd or extensive digestion with brush border membrane enzymes. | T-cell lines or jejunal biopsies from celiac patients were tested to evaluate the immunostimulatory properties of digested samples. | The T-cell response profiles due to PTd products from T. monococcum and T. aestivum were comparable. However, extensive gastrointestinal treatment drastically reduced the immune stimulatory properties of T. monococcum gliadin. MS-based analysis showed that several T. monococcum peptides were hydrolyzed during gastrointestinal digestion. | [32] |
| To investigate the biological effects of T. monococcum on human Caco-2 intestinal epithelial cells. | The effects of gliadin-derived peptides from T. monococcum (ID331) were tested on epithelial permeability, zonulin release, viability, and cytoskeleton reorganization. Triticum aestivum was used as a positive control. | ID331 gliadin did not alter the parameters evaluated in the Caco-2 cell monolayers. Some ID331 peptides showed a protective action, reducing the damage due to Triticum aestivum gliadin on cytoskeleton reorganization and cell viability. | [27] |
| To compare the immunological properties of gliadins from two T. monococcum cultivars (Hammurabi and Norberto-ID331) versus Triticum durum (Adamello). | The product from in vitro digestion with brush border membrane enzymes was tested for its effect on IFN-γ production in T-cell lines from celiac disease subjects. | The ability of gliadins from T. monococcum of both cultivars to activate T cells was reduced by gastrointestinal digestion, determining a lower toxicity in celiac patients (p < 0.05). | [23] |
| To evaluate the toxicity of two ancient T. monococcum varieties (ID331 and Monlis) for consumers with gluten related disorders. | The effect of peptides from digestion on Caco-2 cells was evaluated. T. aestivum was used as a positive control. | Differences between the varieties included were observed: (1) ID331 did not enhanced cell permeability and did not induced zonulin release in Caco-2 monolayers, (2) Monlis showed detectable toxicity. | [28] |
| To identify peptides stimulating T cells after ex vivo digestion of ancestral (including T. monococcum) and common wheat using human gastrointestinal juices. | Wheat porridge from ancestral and common cereals was digested using a static ex vivo model (240 min) and analyzed with high-performance liquid chromatography/ electrospray ionization tandem mass spectrometry (HPLC-ESI MS/MS). | Ancestral wheat, compared to common wheat, released fewer immunogenic peptides after ex vivo digestion. However, ancestral wheat was still highly toxic for celiac patients. | [25] |
| Toxic response | |||
| To compare the immunological properties of T. monococcum versus T. aestivum. | PTd products from samples were tested for their effects on IFN-γ production and proliferation of intestinal gliadin-specific T cell lines and clones. The effects of PTd products from gliadin on innate and adaptive immune response were evaluated in organ cultures of jejunal biopsies from 28 celiac patients by immunohistochemistry. | T. monococcum samples induced IFN-γ production and proliferation in celiac mucosal T cells. A different activation of innate immune pathways was observed between the two lines of T. monococcum tested but both were toxic for celiac patients. | [30] |
| To study the toxicity of Triticum accessions with different origin (ancient/modern) and ploidy (di-, tetra-hexaploid). | T-cell lines, generated from 13 celiac patients, were tested with wheat samples in proliferation assays. | All varieties of wheat, regardless of ploidy or ancient/modern origin, determined heterogeneous responses considering a wide range of stimulation indices. | [31] |
| Study Details | Objective of the Study | Protocol | Main Outcomes | Ref. |
|---|---|---|---|---|
| Single blind, cross-over study; 12 celiac patients (mean age: 40.9 years) on GFD for at least 12 months. | To investigate the safety of a single dose of gluten from Tm. | Follow up: day 0, 14 and 28. Dose: 2.5 g of Tm, rice or pure gluten (Amygluten). End-points: Changes in intestinal permeability (measured with the urinary lactulose/rhamnose ratio (L/R ratio) and the occurrence of adverse gastrointestinal events (World Health Organization (WHO) scale). | The oral challenge with the three cereals did not determined changes in urinary L/R ratio. In all cases, occurrence of gastrointestinal events was graded as: (1) “mild” or “moderate” with Tm and rice, (2) “severe” or “disabling” with Amygluten (n = 4). | [36] |
| Intervention study; 5 patients (F/M: 4/1, age: 19–44 years). | To evaluate the safety of chronic daily intake of Tm. | Protocol: Administration of 100 g/day Tm biscuits for 60 days. End-points: Symptoms (recorded with the Gastrointestinal Symptom Rating Scale questionnaire—GSRS), CD-related serology (T0, T30 and T60 days) and duodenal biopsy (T0 and T60). | No difference in GSRS score were observed at T0 and T60. All patients had Marsh II lesion at T0; 4 had Marsh III and 1 had recurrence of dermatitis herpetiformis at T60. The antibodies CD related to converted from negative to positive at T60 in 3 patients. | [35] |
| Oral challenge; 17 subjects with CD (median age: 13 years). | To evaluate the gluten-reactive T-cells elicited by diploid and hexaploid wheat in CD subjects after short oral challenge. | Protocol: For 3 days, patients consumed sandwiches made with Tm, or Ta flour, corresponding to 12 gr of gluten/day. End-points: Quantification of IFN-γ-secreting T-cells subjects using EliSpot and the expression of inflammatory cytokines/receptors (IL-12A, IL-15, IL-18RAP, IFN-γ) by qPCR. | Tm (p > 0.05) compared to Ta did not induce significant cell mobilization (p > 0.05). The group consuming Ta showed an increased mRNA expression for IL-12A and IFN-γ compared to the group consuming Tm ( p < 0.05). | [37] |
| Aim of the Study | Methods | Main Outcomes | Ref. |
|---|---|---|---|
| To evaluate the toxicity of spelt wheat for celiac subjects. | Spelt wheat and T. aestivum L. were compared by analyzing α-gliadin N-terminal portions. | The identity of spelt and bread wheat was confirmed by the N-terminal sequences of alpha-gliadins (from position 3 to 56). | [41] |
| To investigate and compare the genetic diversity of gliadin transcripts from spelt and bread wheat. | Genetic constitution data from 85 spelt transcripts were used to select 11 accessions, from which genes of alpha-gliadin were copied and sequenced. | High variations among accessions were observed. | [21] |
| To develop a tool to evaluate the immunogenic content of spelt and bread wheat gliadins. | The epitope expression levels in eleven different spelt accessions and three bread wheat accessions were measured with probes. | A wide variability in the epitope expression and accessions was observed. | [38] |
| To investigate gliadin epitope expression in spelt accessions and the effect of environmental factors. | 121 spelt accessions were studied. | The epitope expression correlated with celiac disease varied among the spelt accessions included in the study and was not associated with environmental factors. | [22] |
| To detect epitope-containing peptides in T cells after ancestral (among which spelt) and common wheat ex vivo digestion using human gastrointestinal juices. | Wheat porridge of ancestral and common cereals was digested using a static ex vivo model (240 min) and analyzed with High Performance Liquid Chromatography coupled with Mass Spectrometry (ESI MS/MS). | Ex vivo digestion delivered fewer T-cell epitope-containing peptides from the ancestral wheat varieties compared to the common wheat varieties. However, ancestral wheat is still highly toxic for celiac patients. | [25] |
| Study Details | Objective of the Study | Cereals Included | Main Outcomes | Ref. |
|---|---|---|---|---|
| Intervention trial, 19 adult CD patients (2 M) on a GFD. | To study the clinical setting after the consumption of oat by CD patients. | Patients received fifty g/day of oat for twelve weeks. The oats used in this trial was from a single manufacturer. The products were tested using different techniques (ELISA, western blot and mass spectrometry) resulting free of gluten contamination. | (1) Most patients tolerated oat well (apart from initial abdominal soreness and bloating); (2) one patient developed villous atrophy and dermatitis after oat consumption; (3) in 5 patients were detected positive levels of interferon γ mRNA after challenge. | [62] |
| Randomized, double-blind, intervention trial (from 11.3 to 14.9 months); 28 patients (age:0.7–14.2 years). | To investigate the influence of oat on the immune status of intestinal mucosa of CD patients. | Patients received either of two dietetic treatments: standard gluten free diet (GFD-std; n = 13) and uncontaminated gluten free diet containing oat (GFD-oat; n = 15). Median intake of oat was twenty g (range 3–43 g). The oat samples used in the study were specially grown, powdered, and packaged so that they were not contaminated with other toxic cereals. | A group of pediatric CD patients were not tolerant to oat. In these patients, oat affected the immune condition of intestinal mucosa: the mRNA profile suggested the presence of activated cytotoxic lymphocytes, regulatory T-cell and a stressed epithelium with altered tight junctions. | [61] |
| Randomized, double-blind study, 116 children with recent celiac disease diagnosis (age: 0.7–17.2 years). | To define fecal short chain fatty acids (SCFAs) profiles in children with newly diagnosed CD who received GFD with or without oat for one year. | The impact of a gluten free diet containing oat was compared to a “standard” GFD (GFD-oat, n = 57; GFD-std, n = 59). Daily oat intake (strictly gluten free): 25–50 g. | Fecal SCFAs are produced by the gut microbiota. High fecal SCFA levels in children affected by celiac disease suggest gut microflora metabolism alteration. The GFD-std group had a significantly lower total fecal short chain fatty acids levels after one year compared with 0 months ( p < 0.05). On the other hand, total short chain fatty acids in GFD-oat patients maintained high levels after 12 months on the gluten free diet. | [60] |
| Study Details | Objective of the Study | Cereals Included | Main Outcomes | Ref. |
|---|---|---|---|---|
| Randomized intervention trial. 52 adults with CD in remission; followed for 6 months (oat group: 9 M and 17 F, 48 ± 12 years; control group: 8 M and 18 F, 42 ± 10 years). Total of 40 adults with newly diagnosed CD followed for 12 months (oat group: 7 M and 12 F, 42 ± 14 years; control group: 5 M and 16 F, 48 ± 11 years). | To compare the effects of GFD with and without oat. | The consumption of oat in treated group was 50–70 g per day, taken with wheat-starch flour, muesli (60% of oat) and breakfast cereal. Oat contamination not tested. | There were not significantly differences between groups in (1) symptoms, (2) nutritional status, (3) laboratory measures. Regardless of diet, patients in remission did not show worsening architecture of the duodenal villi or increased mononuclear-cell infiltration. Except for one (control group), at one year all the newly diagnosed patients were in remission. | [64] |
| Self-controlled, open-labeled. Duration: 6 months. 10 children with newly diagnosed CD (5 M and 5 F); age: 6.8 ± 4.0 years. | To evaluate the safety of oat in children with newly diagnosed CD. | Patients consumed commercial oat breakfast cereal product (24 g of oat cereal/d, or 1.2 ± 0.9 g/kg/d). The gliadin content was tested using ELISA Kit. The outcomes at the end of the trial were compared with the initial evaluations (T0), without control group. | ↓ biopsy score ( p < 0.01), ↓ intra-epithelial lymphocyte count ( p < 0.005), ↓ anti-tissue transglutaminase IgA antibody titer ( p < 0.01), ↓ number of symptoms ( p < 0.01). | [70] |
| Randomized trial. Oat group: n = 35 (13 M and 22 F, mean age 53 years); control group (conventional GFD): n = 28 (10 M and 18 F, mean age 52 (10) years). | To evaluate the safety of long-term inclusion of oat in celiac patients’ diets. | Both groups followed a GFD for 5 years. Treated group were allowed to eat oat freely. The oat products were gluten-free. | No significant differences between groups in (1) duodenal villous architecture, (2) inflammatory cell infiltration of the duodenal mucosa, (3) antibody profile. | [63] |
| 2-year intervention study. 20 adult patients (age: 22–71 years) (5 drop-out during the study). | To investigate the safety of the long-term inclusion of oat in the diet of CD adult patients. | Median daily intake of oat: 93 g. The gluten contamination of oat products (rolled oat) was tested using ELISA Kit. | No adverse effects were observed in (1) small bowel histology, (2) serology, (3) nutritional status. | [68] |
| 1-year randomized intervention trial, 116 CD children. | To evaluate the possible negative effects of oat in some CD patients. | Patients were randomized to 1) GFD-std: a standard GFD, 2) GFD-oat: a GFD supplemented with oat. The urinary nitrite/nitrate concentrations were monitored at 0, 3, 6, 9 and 12 months. | No significant differences were observed. | [73] |
| 1-year double blind multicenter study. 116 children (mean age: 6.5 years, range 8 months–17.5 years; M:F distribution 1:1.4); 92 participants complete the study. | To evaluate if children with CD tolerated oat in their GFD. | Subjects were randomized in two groups (1) GFD-std (n = 50): follow a standard GFD, (2) GFD-oat (n = 42): follow a GFD with additional oat products (wheat free). Median of oat intake was 15 g/day. The oats used were specially grown, milled, and packaged and the gluten contamination was tested using ELISA Kit. | No significant differences between groups in (1) serological markers, (2) small bowel mucosal architecture, (3) numbers of intraepithelial lymphocytes. | [72] |
| 2-year controlled trial. 32 children with celiac disease: n = 13 oat challenge: 6 F, 7 M, median age 11 (9–17) years; n = 10 gluten challenge: 9 F, 1 M, median age 13 (7–15) years; n = 9 newly detected CD, GFD with oat, median age 12 (8–14) years. | To evaluate the long-term safety of oat in children with CD. | 23 children in remission were randomized either to oat (50 g/day) or gluten (20 g/day) group; when small bowel histological relapse was evident after gluten challenge, a GFD including oat was started. 9 newly detected celiac patients followed an oat-containing GFD. The rolled oat provided during the trial were tested with ELISA assay and polymerase chain reaction techniques and were free from contamination. | In celiac children in remission, oat had no detrimental effect on the parameters considered during the 2-year trial (intestinal histology or serology). On the contrary, the gluten-challenge group relapsed after 3–12 months. All patients (relapsed or newly detected) with an oat-containing GFD, showed a complete recovery from the disease. | [71] |
| 5-year follow-up intervention study. 42 CD patients, oat group: 22 patients (10 drop-out during the study), control group: 20 patients. | To clarify the long-term inclusion of oat in the GFD by analyzing local cellular immunological responses. | Median daily intake of oat: 30 g (range 10–70 g). The purity of the oat-products was confirmed during the trial. | Long-term consumption of oat does not stimulate an immunological response locally in the mucosa of the small intestine of CD patients. | [69] |
| 2-year follow-up intervention study. 23 CD patients (7 F; 7–18 years). | To study the toxicity of oat in CD children. | Patients were randomized to (1) oat challenge, (2) gluten challenge (grains with gluten and oat). Patients in gluten group continued only with oat after jejunal histological relapse was evident. The uncontaminated oat products (cultivar not indicated) were given to the patients (processed as rolled oats). | No significant change was observed in the intensity of TG2-targeted autoantibody deposits in the oat group, within 2 years. The intensity of deposits in the gluten containing grains group clearly decrease after the exclusion of gluten, despite oat consumption. | [74] |
| Prospective study. 15 adults with CD (age: 57 ± 9 years) | To test the safety of oat products manufactured under the Canadian Celiac Association guidelines. | Subjects were tested with 350 g/week of pure oat for 12 weeks. Pure, uncontaminated oats, tested with ELISA assay, were provided to patients. | During oat consumption no significant changes were observed in symptom scores, weight, hemoglobin, ferritin, or albumin and histology scores. tTG remained negative in all patients. | [65] |
| Cross-sectional follow-up study. 106 long-term treated CD adults. Oat group: n = 70 (median age: 59 (24–81) years), no oat group: n= 36 (median age: 54 (36–73) years). | To evaluate the long-term tolerability of oat for CD patients. | Median oat consumption: 20 g/d (1–100 g/d) for up to 8 years (oat market products). | Oat consumption was not determined: (1) small-bowel mucosal villous damage, (2) inflammation, (3) gastro-intestinal symptoms. | [66] |
| Intervention study. 58 F and 15 M with CD (aged 20–69 years, median 51). | To evaluate whether ingestion of oat stimulate an avenin specific T cell response in vivo. | Patients consumed for 3 days 100 g per day dry weight oat prepared as porridge. Three sources of oats were used. Two of them were labelled as having gluten content <3 ppm. | Avenin-specific responses were observed in 8% patients. In vitro, immunogenic avenin peptides were susceptible to digestive endopeptidases and showed a reduce HLA-DQ2.5 binding stability. The low rates of T-cell activation after an oat challenge (100 g/d) supports the safety of long-term oat consumption at the doses commonly consumed. | [67] |
| Randomized double-blind, placebo-controlled, crossover multicenter study. 15-month trial, 177 children (4–14 years of age) with CD, on a GFD for ≥2 years. | To evaluate the long-term safety of oat in the treatment of children with CD. | Children consumed gluten-free food containing an age-dependent amount (15–40 g) of either placebo or purified nonreactive varieties of oat (Irina and Potenza) for 2 consecutive 6-month periods separated by washout standard GFD for 3 months. | Direct treatment effect was not statistically significant for clinical, serologic, and intestinal permeability variables. | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, F.; Di Lorenzo, C.; Biella, S.; Bani, C.; Restani, P. Ancient and Modern Cereals as Ingredients of the Gluten-Free Diet: Are They Safe Enough for Celiac Consumers? Foods 2021, 10, 906. https://doi.org/10.3390/foods10040906
Colombo F, Di Lorenzo C, Biella S, Bani C, Restani P. Ancient and Modern Cereals as Ingredients of the Gluten-Free Diet: Are They Safe Enough for Celiac Consumers? Foods. 2021; 10(4):906. https://doi.org/10.3390/foods10040906
Chicago/Turabian StyleColombo, Francesca, Chiara Di Lorenzo, Simone Biella, Corinne Bani, and Patrizia Restani. 2021. "Ancient and Modern Cereals as Ingredients of the Gluten-Free Diet: Are They Safe Enough for Celiac Consumers?" Foods 10, no. 4: 906. https://doi.org/10.3390/foods10040906
APA StyleColombo, F., Di Lorenzo, C., Biella, S., Bani, C., & Restani, P. (2021). Ancient and Modern Cereals as Ingredients of the Gluten-Free Diet: Are They Safe Enough for Celiac Consumers? Foods, 10(4), 906. https://doi.org/10.3390/foods10040906