Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Spore Production
2.2. Orange and Juice Samples
2.3. Sample Inoculation
2.4. UV-C Device and Inactivation Treatments
2.5. Microbial Analysis
2.6. Curve Fitting and Statistical Analysis
3. Results
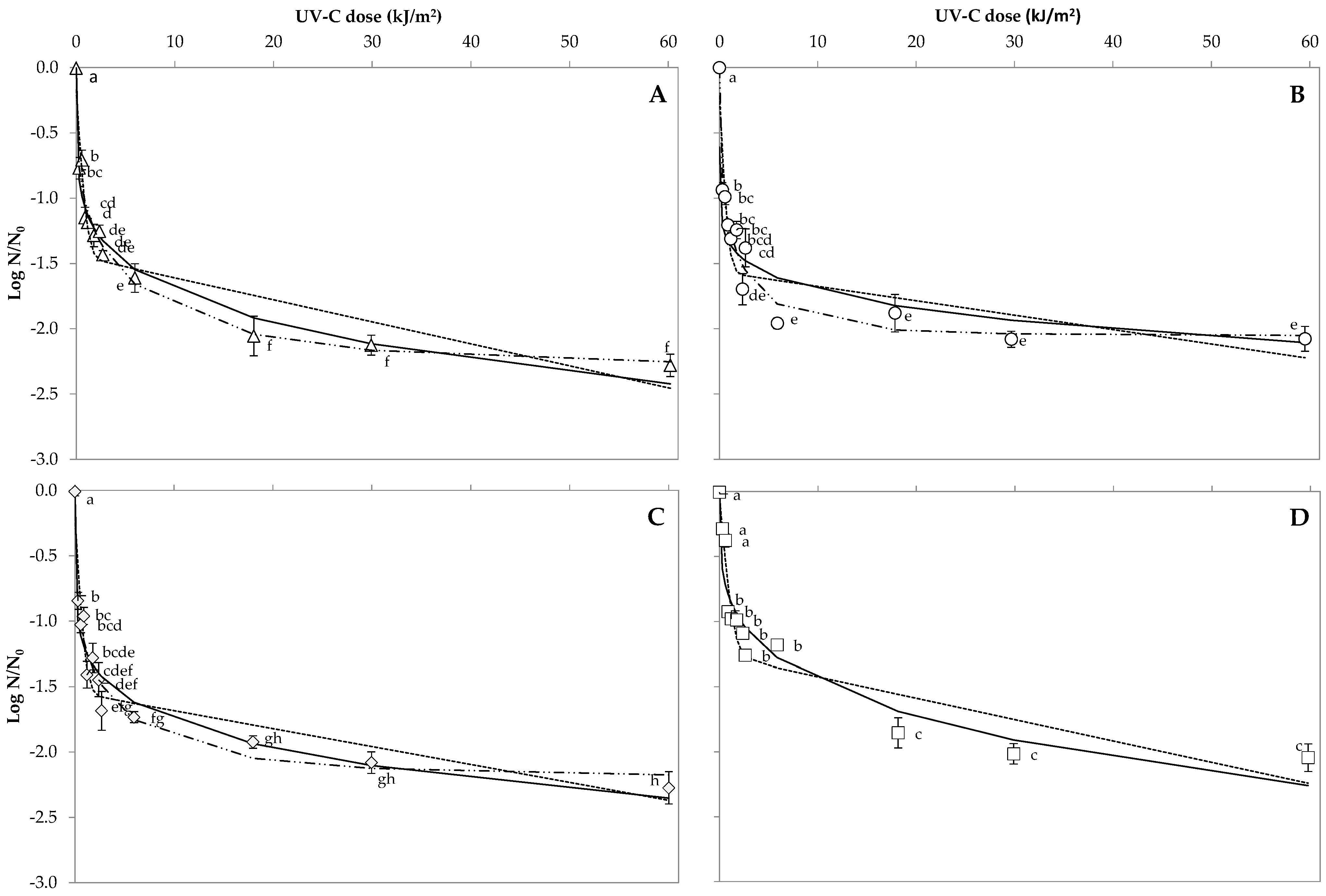
3.1. Bacterial Spore Inactivation on Orange Peel
3.2. Bacterial Spore Inactivation in Orange Juice
3.3. Predictive Microbial Models and Their Comparison on Orange Peel
3.4. Predictive Microbial Models and Their Comparison in Orange Juice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima Tribst, A.A.; de Souza Sant’Ana, A.; de Massaguer, P.R. Review: Microbiological quality and safety of fruit juices—past, present and future perspectives. Crit. Rev. Microbiol. 2009, 35, 310–339. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, B.; Dixon, B.; Couture, H.; Farber, J. Qualitative microbiological risk assessment of unpasteurized fruit juice and cider. Int. Food Risk Anal. J. 2013, 3, 6. [Google Scholar] [CrossRef][Green Version]
- Splittstoesser, D.F. Microbiology of fruit products. In Processing Fruits: Science and Technology, Biology, Principles and Applications; Somogyi, L.P., Ramaswamy, H.S., Hui, Y.H., Eds.; Technomic Publishing Co: Lancaster, PA, USA, 1996; pp. 261–292. [Google Scholar]
- Vasantha Rupasinghe, H.P.; Yu, L.J. Emerging Preservation Methods for Fruit Juices and Beverages. In Food Additive; El-Samragy, Y., Ed.; IntechOpen: London, UK, 2012; pp. 65–82. [Google Scholar]
- Vercammen, A.; Vivijs, B.; Lurquin, I.; Michiels, C.W. Germination and inactivation of Bacillus coagulans and Alicyclobacillus acidoterrestris spores by high hydrostatic pressure treatment in buffer and tomato sauce. Int. J. Food Microbiol. 2012, 152, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Smelt, J.P.P.M. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 1998, 9, 152–158. [Google Scholar] [CrossRef]
- Chang, S.-S.; Kang, D.-H. Alicyclobacillus spp. in the fruit juice industry: History, characteristics, and current isolation/detection procedures. Crit. Rev. Microbiol. 2004, 30, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Cerny, G.; Hennlich, W.; Poralla, K. Spoilage of fruit juice by bacilli: Isolation and characterization of the spoiling microorganism. Z. Leb. Forsch. 1984, 179, 224–227. [Google Scholar] [CrossRef]
- Snyder, A.B.; Worobo, R.W. The incidence and impact of microbial spoilage in the production of fruit and vegetable juices as reported by juice manufacturers. Food Control 2018, 85, 144–150. [Google Scholar] [CrossRef]
- Merle, J.; Montville, T.J. Alicyclobacillus acidoterrestris: The organism, the challenge, potential interventions. J. Food Process. Preserv. 2014, 38, 153–158. [Google Scholar] [CrossRef]
- Smit, Y.; Cameron, M.; Venter, P.; Witthuhn, R.C. Alicyclobacillus spoilage and isolation—A review. Food Microbiol. 2011, 28, 331–349. [Google Scholar] [CrossRef]
- Pornpukdeewattana, S.; Jindaprasert, A.; Massa, S. Alicyclobacillus spoilage and control—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 108–122. [Google Scholar] [CrossRef]
- Peng, J.; Mah, J.H.; Somavat, R.; Mohamed, H.; Sastry, S.; Tang, J. Thermal inactivation kinetics of Bacillus coagulans spores in tomato juice. J. Food Prot. 2012, 75, 1236–1242. [Google Scholar] [CrossRef]
- Mallidis, C.G.; Frantzeskakis, P.; Balatsouras, G.; Katsabotxakis, C. Thermal treatment of aseptically processed tomato paste. Int. J. Food Sci. Technol. 1990, 25, 442–448. [Google Scholar] [CrossRef]
- Anderson, R.E. Growth and Corresponding Elevation of Tomato Juice pH by Bacillus coagulans. J. Food Sci. 1984, 49, 647. [Google Scholar] [CrossRef]
- EFSA. Panel on Biological Hazards (BIOHAZ). Opinion of the Scientific Panel on biological hazards (BIOHAZ) on Bacillus cereus and other Bacillus spp. in foodstuffs. EFSA J. 2005, 3, 1–48. [Google Scholar] [CrossRef]
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Kumar, V.; Kaur, M. Microbes associated with freshly prepared juices of citrus and carrots. Int. J. Food Sci. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.U.; Nasreen, T.; Feroza, B.; Parveen, S. Microbiological Quality of Local Market Vended Freshly Squeezed Fruit Juices in Dhaka City, Bangladesh. Bangladesh J. Sci. Ind. Res. 1970, 44, 421–424. [Google Scholar] [CrossRef]
- Talarmin, A.; Noicand, E.; Doucet, M.; Fermanian, C.; Baylac, P.; Buisson, Y. Toxi-infection alimentaire collective à Bacillus cereus. Bull. Epidemiol. Hebd. 1993, 33, 154–155. [Google Scholar]
- Roig-Sagués, A.X.; Asto, E.; Engers, I.; Hernández-Herrero, M.M. Improving the efficiency of ultra-high pressure homogenization treatments to inactivate spores of Alicyclobacillus spp. in orange juice controlling the inlet temperature. LWT Food Sci. Technol. 2015, 63, 866–871. [Google Scholar] [CrossRef]
- Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. Application of ultraviolet radiation and ultrasound treatments for Alicyclobacillus acidoterrestris spores inactivation in apple juice. LWT Food Sci. Technol. 2017, 78, 138–142. [Google Scholar] [CrossRef]
- FDA 21CFR179.39. Irradiation in the Production, Processing and Handling of Food. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=179.39 (accessed on 15 February 2021).
- Koutchma, T.; Forney, L.J.; Moraru, C.I. Ultraviolet Light in Food Technology: Principles and Applications; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Baysal, A.H.; Molva, C.; Unluturk, S. UV-C light inactivation and modeling kinetics of Alicyclobacillus acidoterrestris spores in white grape and apple juices. Int. J. Food Microbiol. 2013, 166, 494–498. [Google Scholar] [CrossRef]
- Fundo, J.F.; Miller, F.A.; Mandro, G.F.; Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. UV-C light processing of Cantaloupe melon juice: Evaluation of the impact on microbiological, and some quality characteristics, during refrigerated storage. Lwt- Food Sci. Technol. 2019, 103, 247–252. [Google Scholar] [CrossRef]
- Do Prado, D.B.; Szczerepa, M.M.d.A.; Capeloto, O.A.; Astrath, N.G.C.; dos Santos, N.C.A.; Previdelli, I.T.S.; Nakamura, C.V.; Mikcha, J.M.G.; de Abreu Filho, B.A. Effect of ultraviolet (UV-C) radiation on spores and biofilms of Alicyclobacillus spp. in industrialized orange juice. Int. J. Food Microbiol. 2019, 305, 108238. [Google Scholar] [CrossRef] [PubMed]
- Gayán, E.; Álvarez, I.; Condón, S. Inactivation of bacterial spores by UV-C light. Innov. Food Sci. Emerg. Technol. 2013, 19, 140–145. [Google Scholar] [CrossRef]
- Groenewald, W.H.; Gouws, P.A.; Witthuhn, R.C. Isolation, identification and typification of Alicyclobacillus acidoterrestris and Alicyclobacillus acidocaldarius strains from orchard soil and the fruit processing environment in South Africa. Food Microbiol. 2009, 26, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Pettigrew, C.A.; Tremblay, M.E.; Roselle, B.J.; Scouten, A.J. Lethality of chlorine, chlorine dioxide, and a commercial fruit and vegetable sanitizer to vegetative cells and spores of Bacillus cereus and spores of Bacillus thuringiensis. J. Food Prot. 2004, 67, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Ghafoor, K.; Kim, J.U.; Kim, S.; Jung, B.; Lee, D.U.; Park, J. Inactivation of Escherichia coli O157:H7 on orange fruit surfaces and in juice using photocatalysis and high hydrostatic pressure. J. Food Prot. 2015, 78, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–114. [Google Scholar] [CrossRef]
- Albert, I.; Mafart, P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005, 100, 197–211. [Google Scholar] [CrossRef]
- Cerf, O. A Review. Tailing of Survival Curves of Bacterial Spores. J. Appl. Bacteriol. 1977, 42, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, W.D.; Esty, J.R. The thermal death point in relation to time of typical thermophilic organisms. J. Infect. Dis. 1920, 27, 602–617. [Google Scholar] [CrossRef]
- Gündüz, G.T.; Juneja, V.K.; Pazir, F. Application of ultraviolet-C light on oranges for the inactivation of postharvest wound pathogens. Food Control 2015, 57, 9–13. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.N.; Tió-Coma, M.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Effect of single and combined UV-C and ultra-high pressure homogenisation treatments on inactivation of Alicyclobacillus acidoterrestris spores in apple juice. Innov. Food Sci. Emerg. Technol. 2020, 60, 102299. [Google Scholar] [CrossRef]
- Ferreira, T.V.; Mizuta, A.G.; de Menezes, J.L.; Dutra, T.V.; Bonin, E.; Castro, J.C.; Szczerepa, M.M.d.A.; Pilau, E.J.; Nakamura, C.V.; Mikcha, J.M.G.; et al. Effect of ultraviolet treatment (UV–C) combined with nisin on industrialized orange juice in Alicyclobacillus acidoterrestris spores. Lwt- Food Sci. Technol. 2020, 133. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule; EPA: Washington, DC, USA, 2003.
- Sastry, S.K.; Datta, A.K.; Worobo, R.W. Ultraviolet Light. J. Food Sci. 2000, 65, 90–92. [Google Scholar] [CrossRef]
- Tremarin, A.; Brandão, T.R.S.; Silva, C.L.M. Inactivation kinetics of Alicyclobacillus acidoterrestris in apple juice submitted to ultraviolet radiation. Food Control 2017, 73, 18–23. [Google Scholar] [CrossRef]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. Effect of pilot-scale UV-C light treatment assisted by mild heat on E. coli, L. plantarum and S. cerevisiae inactivation in clear and turbid fruit juices. Storage study of surviving populations. Int. J. Food Microbiol. 2020, 332. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Ramírez-Guadiana, F.H.; Barraza-Salas, M.; Ramírez-Ramírez, N.; Ortiz-Cortés, M.; Setlow, P.; Pedraza-Reyes, M. Alternative excision repair of ultraviolet B- and C-Induced DNA damage in dormant and developing spores of Bacillus subtilis. J. Bacteriol. 2012, 194, 6096–6104. [Google Scholar] [CrossRef]
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.I.; Chung, M.S. Bacillus spores: A review of their properties and inactivation processing technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef] [PubMed]

| Orange Peel Exposition | Orange Juice Exposition | ||
|---|---|---|---|
| Time (seconds) | UV 254 nm (kJ/m2) | Time (min) | UV 254 nm (kJ/m2) |
| 3 | 0.3 ± 0.0 | 5 | 31.4 ± 0.4 |
| 6 | 0.6 ± 0.0 | 10 | 62.8 ± 0.8 |
| 9 | 0.9 ± 0.0 | 15 | 94.4 ± 1.2 |
| 12 | 1.2 ± 0.0 | 20 | 125.9 ± 1.5 |
| 18 | 1.8 ± 0.0 | 25 | 157.2 ±2.0 |
| 24 | 2.4 ± 0.0 | 30 | 185.8 ± 2.3 |
| 27 | 2.7 ± 0.0 | 45 | 278.8 ± 2.8 |
| 60 | 5.9 ± 0.0 | 60 | 370.6 ± 5.5 |
| 180 | 18.0 ± 0.1 | ||
| 300 | 29.9 ± 0.4 | ||
| 600 | 59.9 ± 0.2 | ||
| Bacterial Spore | Statistical Indices | Weibull Model | Weibull Plus Tail Model | Biphasic Model |
|---|---|---|---|---|
| A. acidoterrestris CECT 7094T | RMSE | 0.220 | 0.207 | 0.257 |
| R2-adj | 0.887 | 0.900 | 0.845 | |
| A. acidoterrestris TA-1.183 | RMSE | 0.324 | 0.223 | 0.284 |
| R2-adj | 0.717 | 0.866 | 0.782 | |
| B. cereus | RMSE | 0.250 | 0.241 | 0.2702 |
| R2-adj | 0.847 | 0.858 | 0.821 | |
| B. coagulans | RMSE | 0.264 | 0.266 | 0.257 |
| R2-adj | 0.840 | 0.838 | 0.849 |
| Model | Kinetic Parameters | A. acidoterrestris CECT 7094T | A. acidoterrestris TA-1.183 | B. cereus | B. coagulans |
|---|---|---|---|---|---|
| Weibull | δ | 0.54 (0.26) | 0.17 (0.12) | 0.27 (0.18) | 1.58 (0.77) |
| p | 0.19 (0.02) | 0.14 (0.01) | 0.16 (0.02) | 0.23 (0.02) | |
| Weibull plus tail | δ | 0.75 (0.25) | 0.44 (0.16) | 0.45 (0.19) | 1.58 (0.78) |
| p | 0.28 (0.03) | 0.30 (0.05) | 0.26 (0.04) | 0.23 (0.04) | |
| Biphasic | f | 0.94 (0.01) | 0.95 (0.01) | 0.95 (0.01) | 0.94 (0.01) |
| kmax1 | 2.80 (0.43) | 3.44 (0.55) | 2.94 (0.44) | 2.09 (0.33) | |
| kmax2 | 0.04 (0.00) | 0.03 (0.01) | 0.03 (0.00) | 0.04 (0.01) |
| Bacterial Spore | Statistical Indices | Log-Linear Model | Weibull Model |
|---|---|---|---|
| A. acidoterrestris CECT 7094T | RMSE | 0.236 | 0.234 |
| R2-adj | 0.932 | 0.933 | |
| A. acidoterrestris TA-1.183 | RMSE | 0.226 | 0.229 |
| R2-adj | 0.907 | 0.904 | |
| B. cereus | RMSE | 0.284 | 0.271 |
| R2-adj | 0.850 | 0.863 | |
| B. coagulans | RMSE | 0.252 | 0.209 |
| R2-adj | 0.902 | 0.933 |
| Model | Kinetic Parameters | A. acidoterrestris CECT 7094T | A. acidoterrestris TA-1.183 | B. cereus | B. coagulans |
|---|---|---|---|---|---|
| Log-linear | kmax | 0.02 (0.00) | 0.01 (0.00) | 0.01 (0.00) | 0.01 (0.00) |
| Weibull | δ | 147.5 (14.59) | 172.05 (19.07) | 201.38 (17.08) | 212.98 (12.45) |
| p | 1.11 (0.10) | 1.03 (0.11) | 1.37 (0.17) | 1.60 (0.15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colás-Medà, P.; Nicolau-Lapeña, I.; Viñas, I.; Neggazi, I.; Alegre, I. Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light. Foods 2021, 10, 855. https://doi.org/10.3390/foods10040855
Colás-Medà P, Nicolau-Lapeña I, Viñas I, Neggazi I, Alegre I. Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light. Foods. 2021; 10(4):855. https://doi.org/10.3390/foods10040855
Chicago/Turabian StyleColás-Medà, Pilar, Iolanda Nicolau-Lapeña, Inmaculada Viñas, Isma Neggazi, and Isabel Alegre. 2021. "Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light" Foods 10, no. 4: 855. https://doi.org/10.3390/foods10040855
APA StyleColás-Medà, P., Nicolau-Lapeña, I., Viñas, I., Neggazi, I., & Alegre, I. (2021). Bacterial Spore Inactivation in Orange Juice and Orange Peel by Ultraviolet-C Light. Foods, 10(4), 855. https://doi.org/10.3390/foods10040855






