Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Pre-Treatments Applied to the “Rocha” Pear
2.3. Drying Experiments
2.4. Modeling of Drying Kinetics
2.5. Quality Parameters Evaluation
2.5.1. Water Activity
2.5.2. Color Properties
2.5.3. Sample Extraction
2.5.4. Total Phenolic Content
2.5.5. DPPH Radical Scavenging Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Color Evaluation
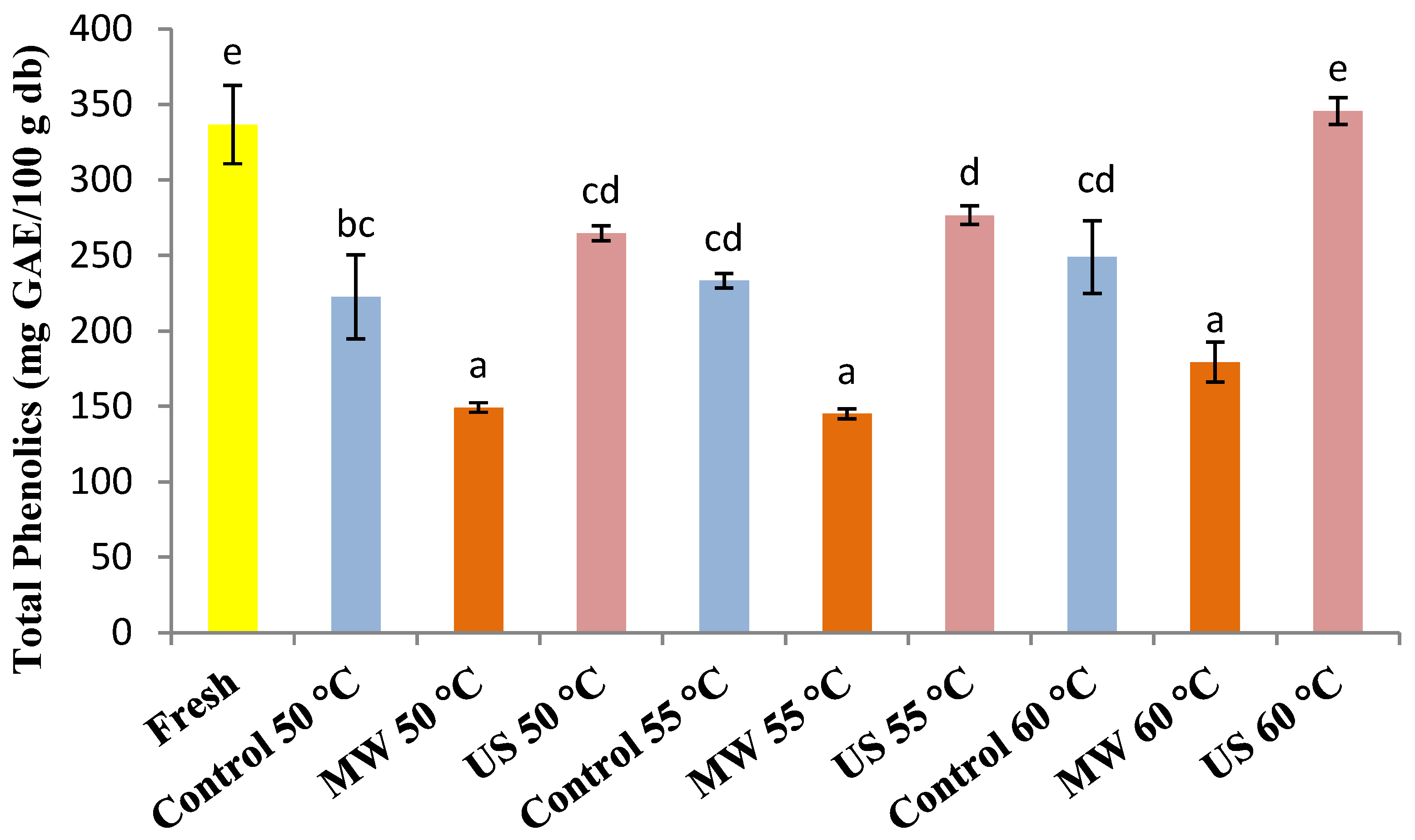
3.2. Total Phenolic Content (TPC)
3.3. DPPH Radical Scavenging Activity
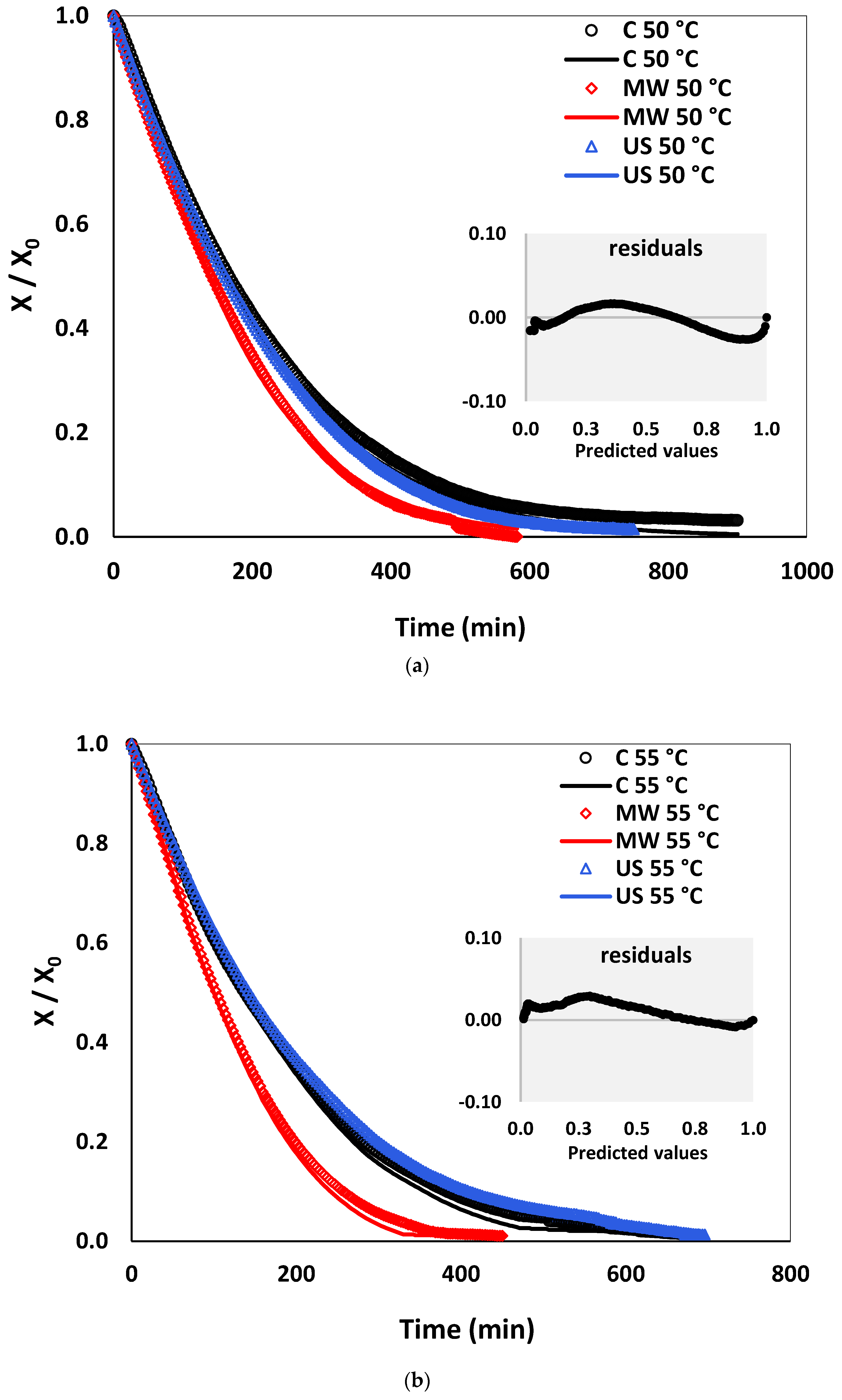
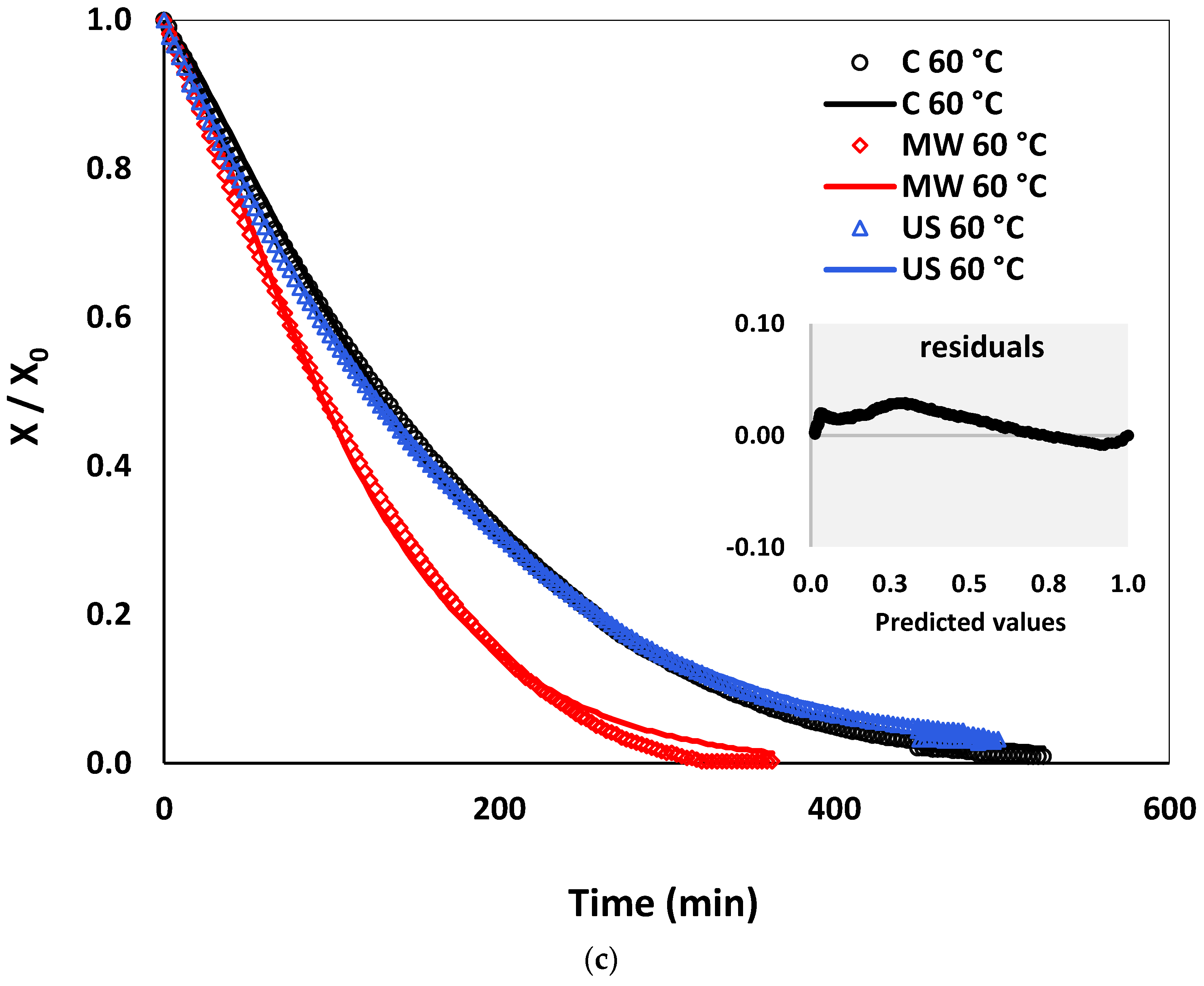
3.4. Drying Kinetics: Experiments and Empirical Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. Commission Regulation (EC) No 492/2003 of 18 March 2003; Publications Office of the EU: Luxembourg, 2003. [Google Scholar]
- Coelho, I.; Matos, A.S.; Teixeira, R.; Nascimento, A.; Bordado, J.; Donard, O.; Castanheira, I. Combining multielement analysis and chemometrics to trace the geographical origin of Rocha pear. J. Food. Compost. Anal. 2019, 77, 1–8. [Google Scholar] [CrossRef]
- Deuchande, T.; Carvalho, S.M.P.; Giné-Bordonaba, J.; Vasconcelos, M.W.; Larrigaudière, C. Transcriptional and biochemical regulation of internal browning disorder in ‘Rocha’ pear as affected by O2 and CO2 concentrations. Postharvest Biol. Technol. 2017, 132, 15–22. [Google Scholar] [CrossRef]
- Fundo, J.F.; Galvis-Sanchez, A.; Madureira, A.R.; Carvalho, A.; Feio, G.; Silva, C.LM.; Quintas, M.A.C. NMR water transwerse relaxation time approach to understand storage stability of fresh-cut ‘Rocha’ pear. LWT-Food Sci. Technol. 2017, 74, 280–285. [Google Scholar] [CrossRef]
- Arzoo, P.M. Why is pear so dear. Int. J. Res. Ayurveda Pharm. 2016, 7, 108–113. [Google Scholar]
- Proietti, N.; Adiletta, G.; Russo, P.; Buonocore, R.; Mannina, L.; Crescitelli, A.; Capitani, D. Evolution of physicochemical properties of pear during drying by conventional techniques, portable-NMR, and modelling. J. Food Eng. 2018, 230, 82–98. [Google Scholar] [CrossRef]
- Saquet, A.A. Storage of pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Singh, V.; Jawendha, S.K.; Gill, P.P.S.; Gill, M.S. Suppression of fruits softening and extension of shelf life of pear by putresche application. Sci. Hortic. 2019, 256, 108623. [Google Scholar] [CrossRef]
- Deuchande, T.; Carvalho, S.M.P.; Guterres, U.; Fidalgo, F.; Isidoro, N.; Larrigaudiere, C.; Vasconcelos, M.W. Dynamic controlled atmosphere for prevention of internal browning disorders in ‘Rocha’ pear. LWT-Food Sci. Technol. 2016, 65, 725–730. [Google Scholar] [CrossRef]
- Silva, F.J.P.; Gomes, M.H.; Rodrigues, J.A.; Almeida, D.P. Antioxidant properties and fruit quality during long-term storage of ‘Rocha’ pear: Effects of maturity and storage conditons. J. Food Qual. 2010, 33, 1–20. [Google Scholar] [CrossRef]
- Prusky, D. Reduction of incidence of postharvest quality losses, and future prospects. Food Secur. 2011, 3, 463–474. [Google Scholar] [CrossRef]
- Marques, L.C.; Prado, M.M.; Freire, J.T. Rehydration characteristics of freeze-dried tropical fruits. LWT-Food Sci. Technol. 2009, 42, 1232–1237. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, phsicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, I. Effect of citric acid and blanching pre-treatments on drying and rehydration of Amasya red apples. Food Bioprod. Process. 2010, 88, 124–132. [Google Scholar] [CrossRef]
- Kingsly, R.P.; Goyal, R.K.; ManiKaantan, M.R.; Ilyas, S.M. Effects of pre-treatments and drying air temperature on drying behaviour of peach slice. Int. J. Food Sci. Tecnol. 2007, 42, 65–69. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Chen, G. Recent advances of novel thermal combined hot air drying of agricultural crops. Trends Food Sci. Technol. 2016, 57, 132–145. [Google Scholar] [CrossRef]
- Bozkır, H.; Ergün, A.R.; Serdar, E.; Metin, G.; Baysal, T. Influence of ultrasound and osmotic dehydration pretreatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Sledz, M.; Witrowa-Rajchert, D. Ultrasound as a pretreatment method to improve drying kinetics and sensory properties of dried apple. J. Food Process Eng. 2016, 39, 256–265. [Google Scholar] [CrossRef]
- Miano, A.C.; Ibarz, A.; Augusto, P.E.D. Mechanisms for imroving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason. Sonochem. 2016, 29, 413–419. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, Y.; Kadam, S.U.; Han, Y.; Wang, J.; Zhou, J. Power ultrasound as a pre-treatment to convective drying of mulberry (Morus alba L.) leaves: Impact on drying kinetics and selected quality properties. Ultrason. Sonochem. 2016, 31, 310–318. [Google Scholar] [CrossRef]
- Silva, G.D.; Barros, Z.M.P.; Medeiros, R.A.B.; Carvalho, C.B.O.; Brandão, S.C.R.; Azoubel, P.M. Pre-treatments for melon drying implementing ultrasound and vacuuum. LWT-Food Sci. Technol. 2016, 74, 14–119. [Google Scholar]
- Romero, C.A.J.; Yépez, B.D.V. Ultrasound as pretreatment to convective drying of Andean blackberry (Rubus glaucus Benth). Ultrason. Sonochem. 2015, 22, 205–210. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, L.; Li, D.; Liu, C.; Ma, R.; Song, J.; Zhao, J. Low intensity ultrasound as a pre-treatment to drying of daylilies: Impact on enzyme inactivation, color changes and nutrition quality parameters. Ultrason. Sonochem. 2017, 36, 50–58. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Ricce, C.; Rojas, M.L.; Miano, A.C.; Siche, R.; Augusto, P.E.D. Ultrasound pre-treatment enhances the carrot drying and rehydration. Food Res. Int. 2016, 89, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, X.; Yagoub, A.E.A.; Xu, B.; Wu, L.; Zhou, C. Vacuum pretreatment coupled to ultrasound assisted osmotic dehydration as a novel method for garlic-slices dehydration. Ultrason. Sonochem. 2019, 50, 363–372. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci Tech. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S.; Zahedi, Y.; Shaddel, R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind. Crop. Prod. 2019, 128, 527–533. [Google Scholar] [CrossRef]
- Chahbani, A.; Fakhfakh, N.; Balti, M.A.; Mabrouk, M.; El-Hatmi, H.; Zouari, N.; Kechaou, N. Microwave drying effects on drying kinetics, bioactive compounds and antioxidant activity of green peas (Pisum sativum L.). Food Biosci. 2018, 25, 32–38. [Google Scholar] [CrossRef]
- Mothibe, K.J.; Zhang, M.; Mujumdar, A.S.; Wang, Y.C.; Cheng, X. Effects of ultrasound and microwave pretreatments of apple before spouted bed drying on rate of dehydration and physical properties. Dry. Technol. 2014, 32, 1848–1856. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A.; Wang, S. Trends is microwave-related drying of fruits and vegetables. Trends Food Sci Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Sabry, Z.A.; Bahlol, H.E.; El-Desouky, A.I.; Assous, M.T. Effect of microwave pre-treatment and drying methods on the carrot powder quality. Middle East J. Appl. Sci. 2016, 6, 349–356. [Google Scholar]
- Brasiello, A.; Adiletta, G.; Russo, P.; Crescitelli, S.; Albanese, D.; Di Matteo, M. Mathematical modeling of eggplant drying: Shrinkage effect. J. Food Eng. 2013, 114, 99–105. [Google Scholar] [CrossRef]
- Kowalski, S.J.; Mierzwa, D. Numerical analysis of drying kinetics for shrinkable products such as fruits and vegetables. J. Food Eng. 2013, 114, 522–529. [Google Scholar] [CrossRef]
- Koç, B.; Eren, İ.; Kaymak-Ertekin, F. Modelling bulk density, porosity and shrinkage of quince during drying: The effect of drying method. J. Food Eng. 2008, 85, 340–349. [Google Scholar] [CrossRef]
- Doymaz, İ.; İsmail, O. Drying characteristics of sweet cherry. Food Bioprod. Process. 2011, 89, 31–38. [Google Scholar] [CrossRef]
- Adiletta, G.; Russo, P.; Senadeera, W.; Di Matteo, M. Drying characteristics and quality of grape under physical pretreatment. J. Food Eng. 2016, 172, 9–18. [Google Scholar] [CrossRef]
- Johnson, A.C.; Ali Al Mukhaini, E.M. Drying studies on peach and strawberry slices. Cogent Food Agric. 2016, 2, 1141654. [Google Scholar] [CrossRef]
- Senadeera, W.; Adiletta, G.; Önal, B.; Di Matteo, M.; Russo, P. Influence of different hot air drying temperatures on drying kinetics, shrinkage, and colour of persimmon slices. Foods 2020, 9, 101. [Google Scholar] [CrossRef]
- AOAC. Ofiicial Methods of Analysis, 16th ed.; Association of Analytical Chemist: Arlington, VA, USA, 1998. [Google Scholar]
- Fernandes, F.A.N.; Linhares, F.E.; Rodrigues, S. Ultrasound as pre-treatment for drying of pineapple. Ultrason. Sonochem. 2008, 15, 1045–1054. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Ramos, I.N.; Brandao, T.R.S.; Silva, C.L.M. Effect of air-drying temperature on the quality and bioactive characteristics of dried galega kale (Brassica oleracea L. Var. Acephala). J. Food Process. Preserv 2015, 2485–2496. [Google Scholar] [CrossRef]
- Jayes, W. The Sugar Engineers. Available online: http://www.sugartech.co.za/psychro (accessed on 1 July 2019).
- Diamente, L.M.; Munro, P.A. Mathematical modelling of the thin layer solar drying of sweet potato slices. Sol. Energy 1993, 51, 271–276. [Google Scholar] [CrossRef]
- Bala, B.K.; Woods, J.I. Simulation of the indirect natural-convection solar drying of rough rice. Sol. Energy 1994, 53, 259–266. [Google Scholar] [CrossRef]
- Ramos, I.N.; Brandao, T.R.S.; Silva, C.L.M. Effect of pre-treatments on solar drying kinetics of red seedless. Int. J. Food Stud. 2014, 3, 239–247. [Google Scholar] [CrossRef]
- Doymaz, I. Sun drying of figs: An experimental study. J. Food Eng. 2005, 71, 403–407. [Google Scholar] [CrossRef]
- Yaldiz, O.; Ertekin, C.; Uzun, H.I. Mathematical modeling of thin layer solar drying of sultana grapes. Energy 2001, 26, 457–465. [Google Scholar] [CrossRef]
- Mahmutoglu, T.; Emir, F.; Saygi, Y.B. Sun/solar drying of differently treated grapes and storage stability of dried grapes. J. Food Eng. 1996, 29, 289–300. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chen, W.; Wenga, Y.M.; Tsenga, C.Y. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003, 83, 85–92. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef]
- Salta, J.; Martins, A.; Santos, R.G.; Neng, N.R.; Nogueira, J.M.F.; Justino, J.; Rauter, A.P. Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars–A comparative study. J. Funct. Foods 2010, 2, 153–157. [Google Scholar] [CrossRef]
- Önal, B.; Adiletta, G.; Crescitelli, A.; Di Matteo, M.; Russo, P. Optimization of hot air drying temperature combined with pre-treatment to improve physico-chemical and nutritional quality of ‘Annurca’ apple. Food Bioprod. Process. 2019, 115, 87–99. [Google Scholar] [CrossRef]
- Adiletta, G.; Petriccione, M.; Liguori, L.; Pizzolongo, F.; Romano, R.; Di Matteo, M. Study of pomological traits and physico-chemical quality of pomegranate (Punica granatum L.) genotypes grown in Italy. Eur. Food Res. Technol. 2018, 244, 1427–1438. [Google Scholar] [CrossRef]
- Deng, L.Z.; Yang, X.H.; Mujumdar, A.S.; Zhao, J.H.; Wang, D.; Zhang, Q.; Wang, J.; Gao, Z.J.; Xiao, H.W. Red pepper (Capsicum annuum L.) drying: Effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure. Dry. Technol. 2018, 36, 893–907. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.H.; Mujumdar, A.S.; Fang, X.M.; Zhang, Q.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Effects of high humidity hot air impingement blanching (HHAIB) pre-treatment on the change of antioxidant capacity, the degradation kinetics of red pigment, ascorbic acid in dehydrated red peppers during storage. Food Chem. 2018, 269, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experiments an İntroduction to Design, Data Analysis and Model Building; John Wiley and Sons: New York, NY, USA, 1978. [Google Scholar]
- Krokida, M.K.; Kiranoudis, C.T.; Maroulis, Z.B.; Marinos-Kouris, D. Effect of pre-treatment on color of dehydration products. Dry. Technol. 2000, 18, 1239–1250. [Google Scholar] [CrossRef]
- Wojdylo, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of convective and vacuum-microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess. Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Horuz, E.; Bozkurt, H.; Karatas, H.; Maskan, M. Effect of hybrid (microwave-convectional) and convectional drying on drying kinetics, total phenolics, antioxidant capacity, vitamin C, color and rehydration capacity of sour cherries. Food Chem. 2017, 230, 295–305. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D.S. Changes in quality of microwave-treated agricultural products–A Review. Biosyst. Eng. 2007, 98, 1–16. [Google Scholar] [CrossRef]
- Mothibe, K.J.; Zhang, M.; Nsor-atindana, J.; Wang, Y.-C. Use of ultrasound pre-treatment in drying of fruits: Drying rates, quality attributes and shelf life extension. Dry. Technol. 2011, 29, 1611–1621. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.; Fraooq, U.; Abbas, S.; Jia, C.; Zhong, F.; Zhang, J. Efffect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
- Robinson, J.P.; Kingman, S.W.; Sanpe, C.E.; Shang, H.; Barronco, R.; Seeid, A. Separation of polyaromatic hydrocarbons from contamined soils using microwave heating. Sep. Purif. Technol. 2009, 62, 249–254. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, W.; Mezrigui, S.; Badwak, L.; Bejar, A.K.; Perez, C.T.; Kechaou, N. Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason. Sonochem. 2017, 36, 286–300. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rynak, K.; Vitrowa-Rajchert, D. The influence of immersion and contact ultrasound treatment on selected properties of apple tissue. Appl. Acoust. 2016, 103, 136–142. [Google Scholar] [CrossRef]
- Di Scala, K.; Vega-Gálvez, A.; Uribe, E.; Oyanadel, R.; Miranda, M.; Veragara, J.; Quispe, I. Changes of quality characteristics of pepino fruit (Solanum muricatum Ait) during convective drying. Int. J. Food Sci. 2011, 46, 746–753. [Google Scholar] [CrossRef]
- Ek, P.; Araújo, A.C.; Oliveira, S.M.; Ramos, I.N.; Brandão, T.R.S.; Silva, C.L.M. Assessment of nutritional quality and colour parameters of convective dried watercress(Nasturtiumofficinale). J. Food Process. Preserv. 2017, 42, 1–9. [Google Scholar]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capcicum annuum, L. var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Foods Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Kamiloğlu, S.; Capanoglu, E. Polyphenol content in figs (Ficus carica L.): Effect of sun drying. Int. J. Food Prop. 2015, 18, 521–535. [Google Scholar] [CrossRef]
- Rahman, N.F.A.; Shamsudin, R.; Ismail, A.; Shah, N.N.A.K.; Varith, J. Effects of drying methods on total phenolic contents and antioxidant capacity of pomelo (Citrus grandis (L.) Osbeck) peels. Innov. Food Sci. Emerg. Technol. 2018, 50, 217–225. [Google Scholar] [CrossRef]
- Doymaz, İ. Evaluation of some thin-layer drying models of persimmon slices (Diospyros kaki L.). Energy Convers. Manag. 2012, 56, 199–205. [Google Scholar] [CrossRef]
- Corrêa, J.L.G.; Rasia, M.C.; Mulet, A.; Cárcel, J.A. Influence of ultrasound application on both the osmotic pre-treatment and subsequent convective drying of pineapple (Ananas comosus). Innov. Food Sci. Emerg. Technol. 2017, 41, 284–291. [Google Scholar] [CrossRef]
- Nascimento, E.M.C.G.; Mulet, A.; Ascheri, J.L.R.; Carvalho, C.W.P.; Cárcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016, 170, 108–118. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, J.; Jiang, S.; Xu, Y.; Show, P.L.; Han, Y.; Ye, X.; Ye, M. Contacting ultrasound enhanced hot air convective drying of garlic slices: Mass transfer modelling and quality evaluation. J. Food Eng. 2018, 235, 79–88. [Google Scholar] [CrossRef]
- Ju, H.-Y.; Law, C.-L.; Fang, X.-M.; Xiao, H.-W.; Liu, Y.-H.; Gao, Z.-J. Drying kinetics and evolution of the sample’s core temperature and moisture distribution of yam slices (Dioscorea alata L.) during convective hot-air drying. Dry. Technol. 2016, 34, 1297–1306. [Google Scholar] [CrossRef]
- Dehghannya, J.; Kadkhadaei, S.; Heshmati, M.K.; Ghanbarzadeh, B. Ultrasound-assisted intensification of a hybrid intermittent microwave-hot air drying process of potato: Quality aspect and energy consumption. Ultrasonics 2019, 96, 104–122. [Google Scholar] [CrossRef] [PubMed]
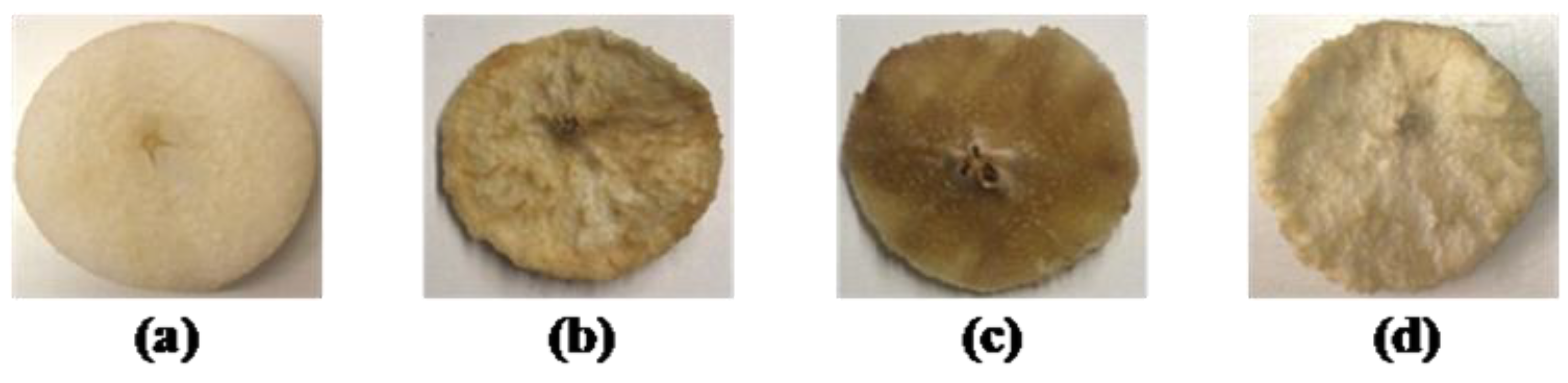

| Sample | L* | WI | ∆E |
|---|---|---|---|
| Fresh | 78.60 ± 0.90 e | 72.11 ± 1.00 f | - |
| C50 °C | 71.35 ± 1.98 c | 61.21 ± 1.21 c | 11.57 ± 0.71 d |
| MW50 °C | 55.77 ± 2.99 a | 48.35 ± 1.17 a | 23.41 ± 2.18 f |
| US50 °C | 73.77 ± 0.89 cd | 63.23 ± 0.88 cd | 9.80 ± 0.71 cd |
| C55 °C | 72.46 ± 1.66 c | 63.03 ± 0.60 cd | 10.23 ± 0.78 cd |
| MW55 °C | 58.35 ± 0.84 ab | 51.38 ± 1.15 b | 21.99 ± 0.92 f |
| US55 °C | 77.15 ± 0.42 de | 68.00 ± 0.48 e | 6.11 ± 0.32 ab |
| C60 °C | 75.06 ± 0.80 cde | 64.96 ± 0.67 d | 8.13 ± 0.71 bc |
| MW60 °C | 60.53 ± 0.68 b | 52.88 ± 0.95 b | 14.91 ± 0.81 e |
| US60 °C | 79.05 ± 0.25 e | 70.96 ± 0.21 f | 3.86 ± 0.23 a |
| Model Name | Temperature | Parameters | Control | Microwaved | Ultrasound |
|---|---|---|---|---|---|
| Newton | 50 °C | k × 102 (1/min) | 0.446 ± 0.005 | 0.558 ± 0.024 | 0.478 ± 0.009 |
| 55 °C | k × 102 (1/min) | 0.578 ± 0.015 | 0.808 ± 0.043 | 0.546 ± 0.023 | |
| 60 °C | k × 102 (1/min) | 0.604 ± 0.013 | 0.851 ± 0.071 | 0.608 ± 0.014 | |
| Henderson & Pabis | 50 °C | a | 1.061 ± 0.007 | 1.074 ± 0.019 | 1.068 ± 0.010 |
| k × 102 (1/min) | 0.481 ± 0.006 | 0.598 ± 0.026 | 0.508 ±0.010 | ||
| 55 °C | a | 1.068 ± 0.012 | 1.098 ± 0.025 | 1.058 ± 0.010 | |
| k × 102 (1/min) | 0.615 ± 0.01 | 0.881 ± 0.046 | 0.577 ± 0.012 | ||
| 60 °C | a | 1.074 ± 0.018 | 1.090 ± 0.077 | 0.632 ± 0.017 | |
| k × 102 (1/min) | 0.646 ± 0.026 | 0.925 ± 0.031 | 1.041 ± 0.011 | ||
| Page | 50 °C | k × 102 (1/minN) | 0.169 ± 0.004 | 0.154 ± 0.015 | 0.165 ± 0.008 |
| N | 1.184 ± 0.005 | 1.240 ± 0.021 | 1.191 ± 0.009 | ||
| 55 °C | k × 102 (1/minN) | 0.213 ± 0.014 | 0.170 ± 0.018 | 0.282 ± 0.029 | |
| N | 1.186 ± 0.013 | 1.310 ± 0.023 | 1.124 ± 0.022 | ||
| 60 °C | k × 102 (1/minN) | 0.184 ± 0.062 | 0.177 ± 0.029 | 0.312 ± 0.019 | |
| N | 1.224 ± 0.019 | 1.319 ± 0.031 | 1.126 ± 0.01 |
| Model Name | Temperature | Correlation Coefficients | Control | Microwaved | Ultrasound |
|---|---|---|---|---|---|
| Newton | 50 °C | R2 | 0.995 | 0.984 | 0.991 |
| s | 0.1168 | 0.1734 | 0.1622 | ||
| 55 °C | R2 | 0.991 | 0.978 | 0.993 | |
| s | 0.1477 | 0.1925 | 0.1321 | ||
| 60 °C | R2 | 0.986 | 0.974 | 0.994 | |
| s | 0.2038 | 0.1999 | 0.1282 | ||
| Henderson & Pabis | 50 °C | R2 | 0.997 | 0.989 | 0.995 |
| s | 0.0835 | 0.1432 | 0.1263 | ||
| 55 °C | R2 | 0.994 | 0.986 | 0.996 | |
| s | 0.1202 | 0.153 | 0.1001 | ||
| 60 °C | R2 | 0.991 | 0.983 | 0.996 | |
| s | 0.1612 | 0.1733 | 0.1071 | ||
| Page | 50 °C | R2 | 1 | 0.997 | 0.999 |
| s | 0.0255 | 0.0595 | 0.0481 | ||
| 55 °C | R2 | 0.999 | 0.998 | 0.998 | |
| s | 0.0541 | 0.0534 | 0.0776 | ||
| 60 °C | R2 | 0.999 | 0.997 | 0.999 | |
| s | 0.065 | 0.0737 | 0.0499 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Önal, B.; Adiletta, G.; Di Matteo, M.; Russo, P.; Ramos, I.N.; Silva, C.L.M. Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics. Foods 2021, 10, 853. https://doi.org/10.3390/foods10040853
Önal B, Adiletta G, Di Matteo M, Russo P, Ramos IN, Silva CLM. Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics. Foods. 2021; 10(4):853. https://doi.org/10.3390/foods10040853
Chicago/Turabian StyleÖnal, Begüm, Giuseppina Adiletta, Marisa Di Matteo, Paola Russo, Inês N. Ramos, and Cristina L. M. Silva. 2021. "Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics" Foods 10, no. 4: 853. https://doi.org/10.3390/foods10040853
APA StyleÖnal, B., Adiletta, G., Di Matteo, M., Russo, P., Ramos, I. N., & Silva, C. L. M. (2021). Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics. Foods, 10(4), 853. https://doi.org/10.3390/foods10040853









