In Vitro Study of Two Edible Polygonoideae Plants: Phenolic Profile, Cytotoxicity, and Modulation of Keap1-Nrf2 Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material, Extracts Preparation, and Chemical Analysis
2.3. Human Cell Line
2.4. Cytotoxicity and Drug Synergism Analysis
2.5. Flow Cytometry Analysis of Apoptosis and Cell Cycle Phase Distribution
2.6. Real-Time Quantitative PCR (qRT-PCR) Analysis
2.7. Statistical Analysis
3. Results
3.1. Identification of Compounds in the Extracts
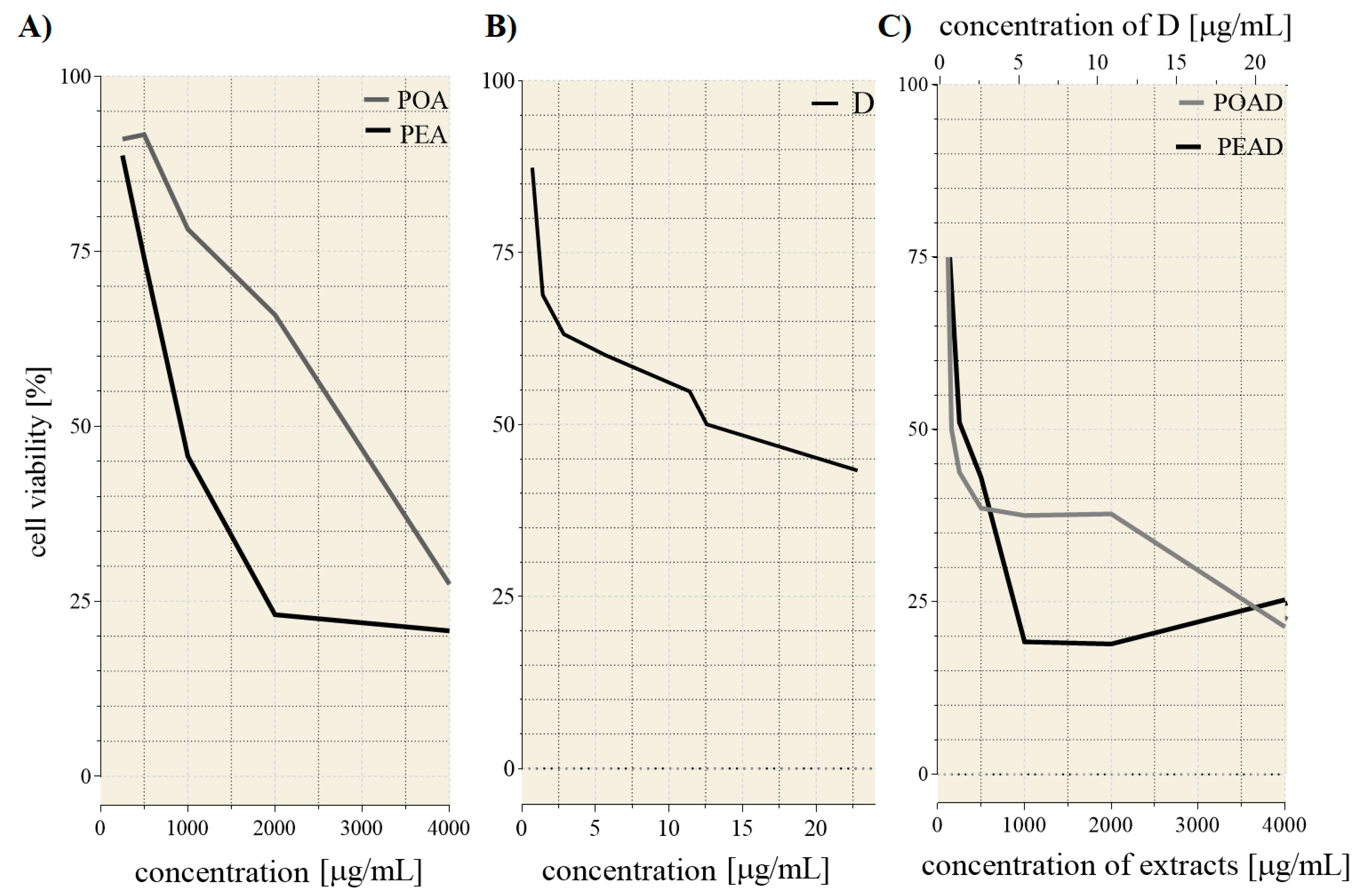
3.2. Cytotoxicity and Drug Synergism Analysis of Herbal Extracts and Doxorubicin
3.3. Effect of Herbal Extracts and Doxorubicin on Apoptosis and Cell Cycle
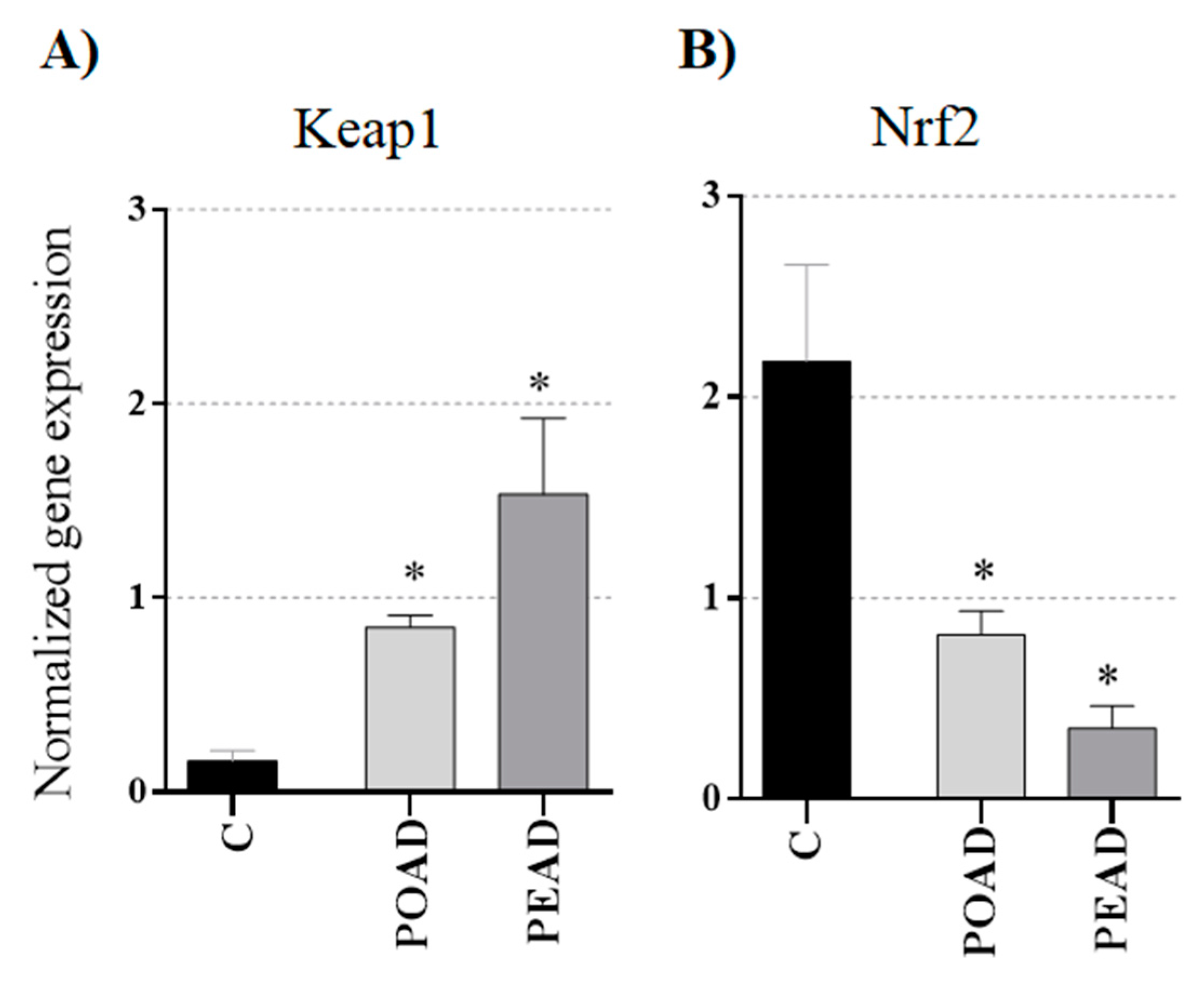
3.4. Effect of Herbal Extracts and Doxorubicin on Keap1 and Nrf2 Genes Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nugroho, A.; Kim, E.J.; Choi, J.S.; Park, H.J. Simultaneous quantification and peroxynitrite-scavenging activities of flavonoids in Polygonum aviculare L. herb. J. Pharm. Biomed. Anal. 2014, 89, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Özbay, H.; Alim, A. Antimicrobial activity of some water plants from the northeastern Anatolian region of Turkey. Molecules 2009, 14, 321–328. [Google Scholar] [CrossRef]
- Chon, S.U.; Heo, B.G.; Park, Y.S.; Cho, J.Y.; Gorinstein, S. Characteristics of the leaf parts of some traditional Korean salad plants used for food. J. Sci. Food Agric. 2008, 88, 1963–1968. [Google Scholar] [CrossRef]
- Costea, M.; Tardif, F.J. The biology of Canadian weeds. 131. Polygonum aviculare L. Can. J. Plant Sci. 2005, 85, 481–506. [Google Scholar] [CrossRef]
- Thu, N.N.; Sakurai, C.; Uto, H.; Van Chuyen, N.; Do, T.K.; Yamamoto, S.; Ohmori, R.; Kondo, K. The polyphenol content and antioxidant activities of the main edible vegetables in northern Vietnam. J. Nutr. Sci. Vitaminol. 2004, 50, 203–210. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Habibi, R.M.; Mohammadi, R.A.; Delazar, A.; Halabian, R.; Soleimani, R.J.; Mehdipour, A.; Bagheri, M.; Jahanian-Najafabadi, A. Effects of Polygonum aviculare herbal extract on proliferation and apoptotic gene expression of MCF-7. Daru J. Pharm. Sci. 2011, 19, 326. [Google Scholar]
- Zlatković, B.K.; Bogosavljević, S.S.; Radivojević, A.R.; Pavlović, M.A. Traditional use of the native medicinal plant resource of Mt. Rtanj (Eastern Serbia): Ethnobotanical evaluation and comparison. J. Ethnopharmacol. 2014, 151, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Bolotova, Y.V. Aquatic plants of the Far East of Russia: A review on their use in medicine, pharmacological activity. BJMS 2015, 14, 9–13. [Google Scholar] [CrossRef]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y. Antioxidant activity of extract from Polygonum aviculare L. Biol. Res. 2006, 39, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Prota, N.; Mumm, R.; Bouwmeester, H.J.; Jongsma, M.A. Comparison of the chemical composition of three species of smart-weed (genus Persicaria) with a focus on drimane sesquiterpenoids. Phytochemistry 2014, 108, 129–136. [Google Scholar] [CrossRef]
- Orbán-Gyapai, O.; Lajter, I.; Hohmann, J.; Jakab, G.; Vasas, A. Xanthine oxidase inhibitory activity of extracts prepared from Polygonaceae species. Phytother. Res. 2015, 29, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Yoon, T.; Yang, W.K.; Kim, S.J.; Kim, D.S.; Kim, H.K. The antiobesity effect of Polygonum aviculare L. ethanol extract in high-fat diet-induced obese mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 626397. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Li, X.; Wang, B.; Cao, S.; Zhang, J.; Zhang, H.; Zhao, Y.; Ni, J. Pharmacological and other Bioactivities of the Genus Polygonum—A Review. Trop. J. Pharm. Res. 2014, 13, 1749–1759. [Google Scholar]
- Zhu, A.X. Systemic therapy of advanced hepatocellular carcinoma: How hopeful should we be? Oncologist 2006, 11, 790–800. [Google Scholar] [CrossRef]
- Tu, D.G.; Chyau, C.C.; Chen, S.Y.; Chu, H.L.; Wang, S.C.; Duh, P.D. Antiproliferative Effect and Mediation of Apoptosis in Human Hepatoma HepG2 Cells Induced by Djulis Husk and Its Bioactive Compounds. Foods 2020, 9, 1514. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metab-olites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef]
- Gao, A.M.; Ke, Z.P.; Shi, F.; Sun, G.C.; Chen, H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 2013, 206, 100–108. [Google Scholar] [CrossRef]
- Shafa, M.H.; Jalal, R.; Kosari, N.; Rahmani, F. Efficacy of metformin in mediating cellular uptake and inducing apoptosis activity of doxorubicin. Regul. Toxicol. Pharmacol. 2018, 99, 200–212. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, D.M.; Lee, S.H. Nrf2 expression and apoptosis in quercetin-treated malignant mesothelioma cells. Mol. Cells 2015, 38, 416. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef]
- Dong, L.; Han, X.; Tao, X.; Xu, L.; Xu, Y.; Fang, L.; Yin, L.; Qi, Y.; Li, H.; Peng, J. Protection by the total flavonoids from Rosa laevigata Michx fruit against lipopolysaccharide-induced liver injury in mice via modulation of FXR signaling. Foods 2018, 7, 88. [Google Scholar] [CrossRef]
- Šibul, F.S.; Orčić, D.Z.; Svirčev, E.; Mimica-Dukić, N.M. Optimization of extraction conditions for secondary biomolecules from various plant species. Hem. Ind. 2016, 70, 473–483. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Wu, L.; Zhang, J.; Li, X.; Wu, W. Chemical characterization and antioxidant properties of ethanolic extract and its fractions from sweet potato (Ipomoea batatas L.) leaves. Foods 2020, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Beara, I.N.; Lesjak, M.M.; Jovin, E.Đ.; Balog, K.J.; Anackov, G.T.; Orcic, D.Z.; Mimica-Dukic, N.M. Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. J. Agric. Food Chem. 2009, 57, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative Determination of Plant Phenolics in Urtica dioica Extracts by High-Performance Liquid Chromatography Coupled with Tandem Mass Spec-trometric Detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- Jovanović, M.; Srdić-Rajić, T.; Svirčev, E.; Jasnić, N.; Nikolić, B.; Bojić, S.; Stević, T.; Knežević-Vukčević, J.; Mitić-Ćulafić, D. Evaluation of anticancer and antimicrobial activities of the Polygonum maritimum ethanol extract. Arch. Biol. Sci. 2018, 70, 665–673. [Google Scholar] [CrossRef]
- Vasilijević, B.; Knežević-Vukčević, J.; Mitić-Ćulafić, D.; Orčić, D.; Francišković, M.; Srdic-Rajic, T.; Jovanović, M.; Nikolić, B. Chemical characterization, antioxidant, genotoxic and in vitro cytotoxic activity assessment of Juniperus communis var. sax-atilis. Food Chem. Toxicol. 2018, 112, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Srdic-Rajic, T.; Nikolic, K.; Cavic, M.; Djokic, I.; Gemovic, B.; Perovic, V.; Veljkovic, N. Rilmenidine suppresses proliferation and promotes apoptosis via the mitochondrial pathway in human leukemic K562 cells. Eur. J. Pharm. Sci. 2016, 81, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kaisarevic, S.; Dakic, V.; Hrubik, J.; Glisic, B.; Lübcke-von Varel, U.; Pogrmic-Majkic, K.; Fa, S.; Teodorovic, I.; Brack, W.; Kovacevic, R. Differential expression of CYP1A1 and CYP1A2 genes in H4IIE rat hepatoma cells exposed to TCDD and PAHs. Environ. Toxicol. Pharmacol. 2015, 39, 358–368. [Google Scholar] [CrossRef]
- Voelker, D.; Vess, C.; Tillmann, M.; Nagel, R.; Otto, G.W.; Geisler, R.; Schirmer, K.; Scholz, S. Differential gene expression as a toxicant-sensitive endpoint in zebrafish embryos and larvae. Aquat. Toxicol. 2007, 81, 355–364. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, S.; Wang, H.; Chen, S. Simultaneous determination of eight flavonoids in Polygonum aviculare L. by RP-HPLC-UV. J. Chin. Pharm. Sci. 2014, 23, 170–176. [Google Scholar] [CrossRef]
- Smolarz, H.D. Flavonoid glycosides in nine Polygonum L. taxons. Acta Soc. Bot. Pol. 2002, 71, 29–33. [Google Scholar] [CrossRef][Green Version]
- Smolarz, H.D. Comparative study on the free flavonoid aglycones in herbs of different species of Polygonum L. Acta Pol. Pharm. 2002, 59, 145–148. [Google Scholar] [PubMed]
- Cong, H.J.; Zhang, S.W.; Zhang, C.; Huang, Y.J.; Xuan, L.J. A novel dimeric procyanidin glucoside from Polygonum aviculare. Chin. Chem. Lett. 2012, 23, 820–822. [Google Scholar] [CrossRef]
- Yunuskhodzhaeva, N.A.; Eshbakova, K.A.; Abdullabekova, V.N. Flavonoid composition of the herb Polygonum aviculare. Chem. Nat. Compd. 2010, 46, 803–804. [Google Scholar] [CrossRef]
- Avula, B.; Joshi, V.C.; Wang, Y.H. Simultaneous Identification and Quantification of Anthraquinones, Polydatin, and Resveratrol in Polygonum multiflorum, Various Polygonum Species, and Dietary Supplements by Liquid Chromatography and Microscopic Study of Polygonum Species. J. AOAC Int. 2007, 90, 1532–1538. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kanomata, T.; Yoshitama, K. Flavonoids in the Leaves of Twenty-Eight Polygonaceous Plants. Bot. Mag. Shokubutsu-gaku-zasshi 1986, 99, 63–74. [Google Scholar] [CrossRef]
- Al-Hazimi, H.M.A.; Haque, S.N. A new naphthoquinone from Polygonum aviculare. Nat. Prod. Lett. 2002, 16, 115–118. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, E.R.; Park, H.A. Novel Lignan and Flavonoids from Polygonum aviculare. J. Nat. Prod. 1994, 57, 581–586. [Google Scholar] [CrossRef]
- Nikolaeva, G.G.; Lavrent’Eva, M.V.; Nikolaeva, I.G. Phenolic compounds from several Polygonum species. Chem. Nat. Compd. 2009, 45, 735–736. [Google Scholar] [CrossRef]
- Granica, S. Quantitative and qualitative investigations of pharmacopoeial plant material polygoni avicularis herba by UHPLC-CAD and UHPLC-ESI-MS methods. Phytochem. Anal. 2015, 26, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Piwowarski, J.P.; Popławska, M.; Jakubowska, M.; Borzym, J.; Kiss, A.K. Novel insight into qualitative standardization of Polygoni avicularis herba (Ph. Eur.). J. Pharm. Biomed. Anal. 2013, 72, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Granica, S.; Czerwińska, M.E.; Zyżyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glu-curonides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, L.; Lin, X.; Hu, X.; Wang, L. Phenolic profiles and screening of potential α-glucosidase inhibitors from Polygonum aviculare L. leaves using ultra-filtration combined with HPLC-ESI-qTOF-MS/MS and molecular docking analysis. Ind. Crop. Prod. 2020, 154, 112673. [Google Scholar] [CrossRef]
- Smolarz, H.D.; Budzianowski, J.; Bogucka-Kocka, A.; Kocki, J.; Mendyk, E. Flavonoid glucuronides with anti-leukaemic activity from Polygonum amphibium L. Phytochem. Anal. 2008, 19, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, M.; Alfazari, M.; Al-Naqeb, G.; Krishnan Selvarajan, K.; Hazizul Hasan, M.; Adam, A. Apoptosis induction by Polygonum minus is related to antioxidant capacity, alterations in expression of apoptotic-related genes, and S-phase cell cycle arrest in HepG2 cell line. BioMed. Res. Int. 2014, 2014, 539607. [Google Scholar]
- Hu, B.; An, H.M.; Shen, K.P.; Song, H.Y.; Deng, S. Polygonum cuspidatum extract induces anoikis in hepatocarcinoma cells associated with generation of reactive oxygen species and downregulation of focal adhesion kinase. Evid.-Based Compl. Alt. 2012, 2012, 607675. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Ramya, I.A. Comprehensive review on Polygonum glabrum. IJOP 2017, 8, 457–467. [Google Scholar]
- Chiu, Y.J.; Chou, S.C.; Chiu, C.S.; Kao, C.P.; Wu, K.C.; Chen, C.J.; Tsai, J.C.; Peng, W.H. Hepatoprotective effect of the ethanol extract of Polygonum orientale on carbon tetrachloride-induced acute liver injury in mice. J. Food Drug Anal. 2017, 26, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Taylor, W.R. Analyzing the G2/M checkpoint. In Checkpoint Controls and Cancer; Humana Press: Totowa, NJ, USA, 2004; pp. 51–82. [Google Scholar]
- Lee, T.; Lau, T.; Ng, I. Doxorubicin-induced apoptosis and chemosensitivity in hepatoma cell lines. Cancer Chemother. Pharmacol. 2002, 49, 78–86. [Google Scholar] [CrossRef]
- Shieh, D.E.; Chen, Y.Y.; Yen, M.H.; Chiang, L.C.; Lin, C.C. Emodin-induced apoptosis through p53-dependent pathway in human hepatoma cells. Life Sci. 2004, 74, 2279–2290. [Google Scholar] [CrossRef]
- Lee, B.H.; Huang, Y.Y.; Wu, S.C. Hepatoprotective activity of fresh Polygonum multiflorum against HepG2 hepatocarcinoma cell proliferation. J. Food Drug Anal. 2011, 19, 26–32. [Google Scholar]
- Ow, Y.Y.; Stupans, I. Gallic acid and gallic acid derivatives: Effects on drug metabolizing enzymes. Curr. Drug Metab. 2003, 4, 241–248. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, E.Y.; Kim, D.J.; Kim, H.J.; Park, H.R. Quercetin inhibits cell survival and metastatic ability via the EMT-mediated pathway in oral squamous cell carcinoma. Molecules 2020, 25, 757. [Google Scholar] [CrossRef] [PubMed]
- Pinmai, K.; Chunlaratthanabhorn, S.; Ngamkitidechakul, C.; Soonthornchareon, N.; Hahnvajanawong, C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: Doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J. Gastroenterol. 2008, 14, 1491. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Kong, A.N. Anticarcinogenesis by dietary phytochemicals: Cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-κB and AP-1 in abnormal cancer cells. Food Chem. Toxicol. 2008, 46, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Sun, Z.; Villeneuve, N.F.; Zhang, S.; Zhao, F.; Li, Y.; Chen, W.; Yi, X.; Zheng, W.; Wondrak, G.T.; et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 2008, 29, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
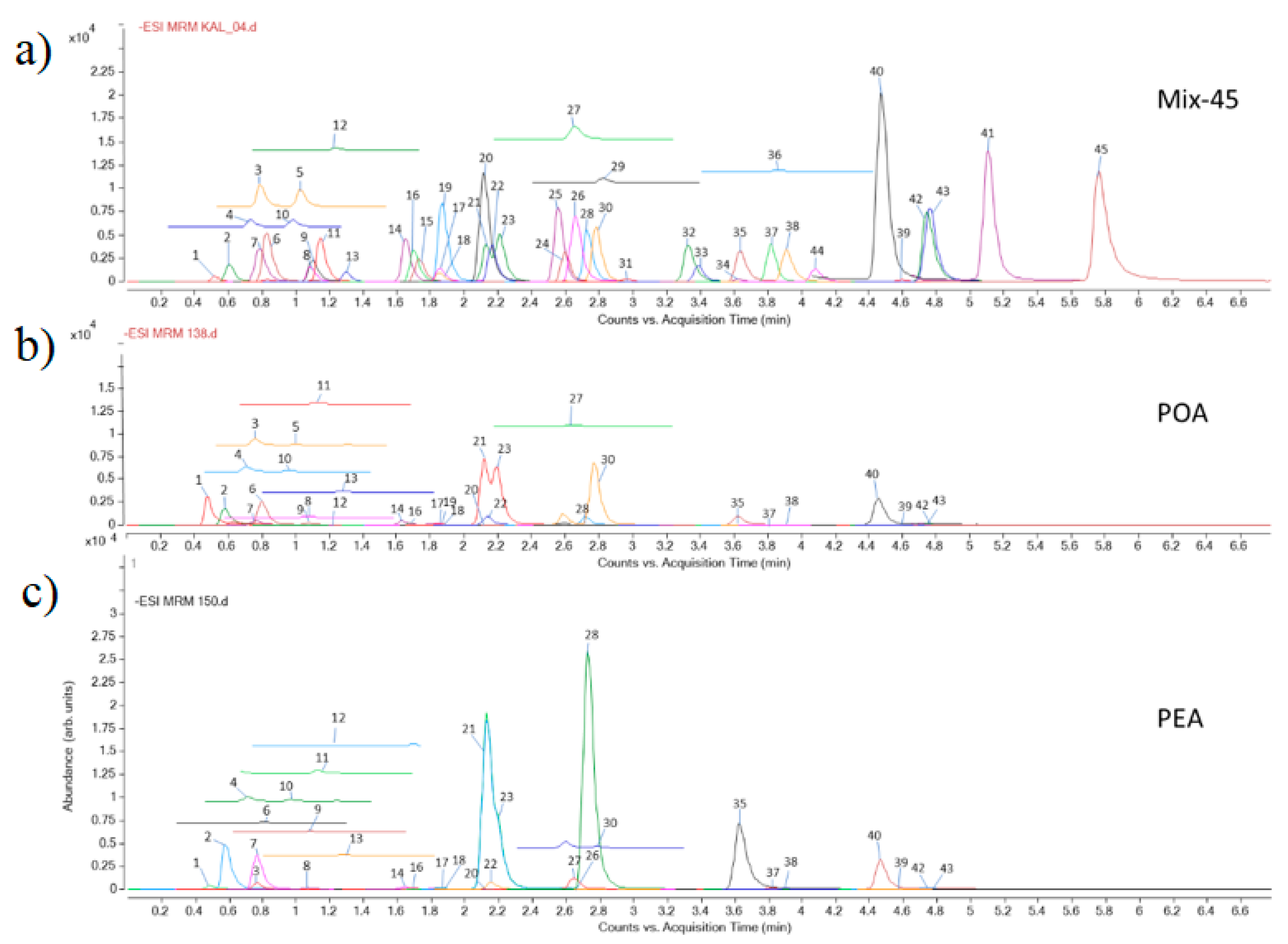

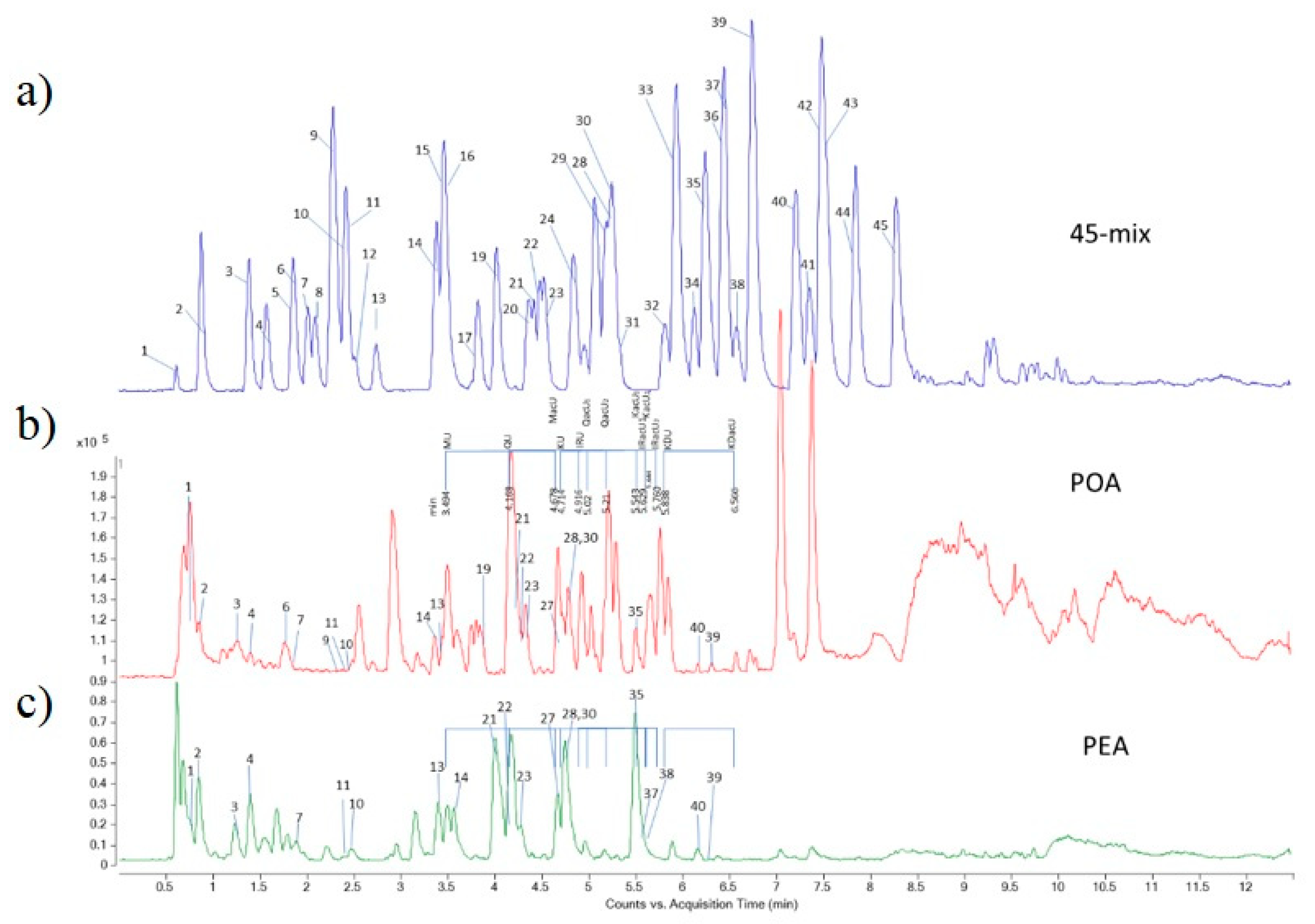
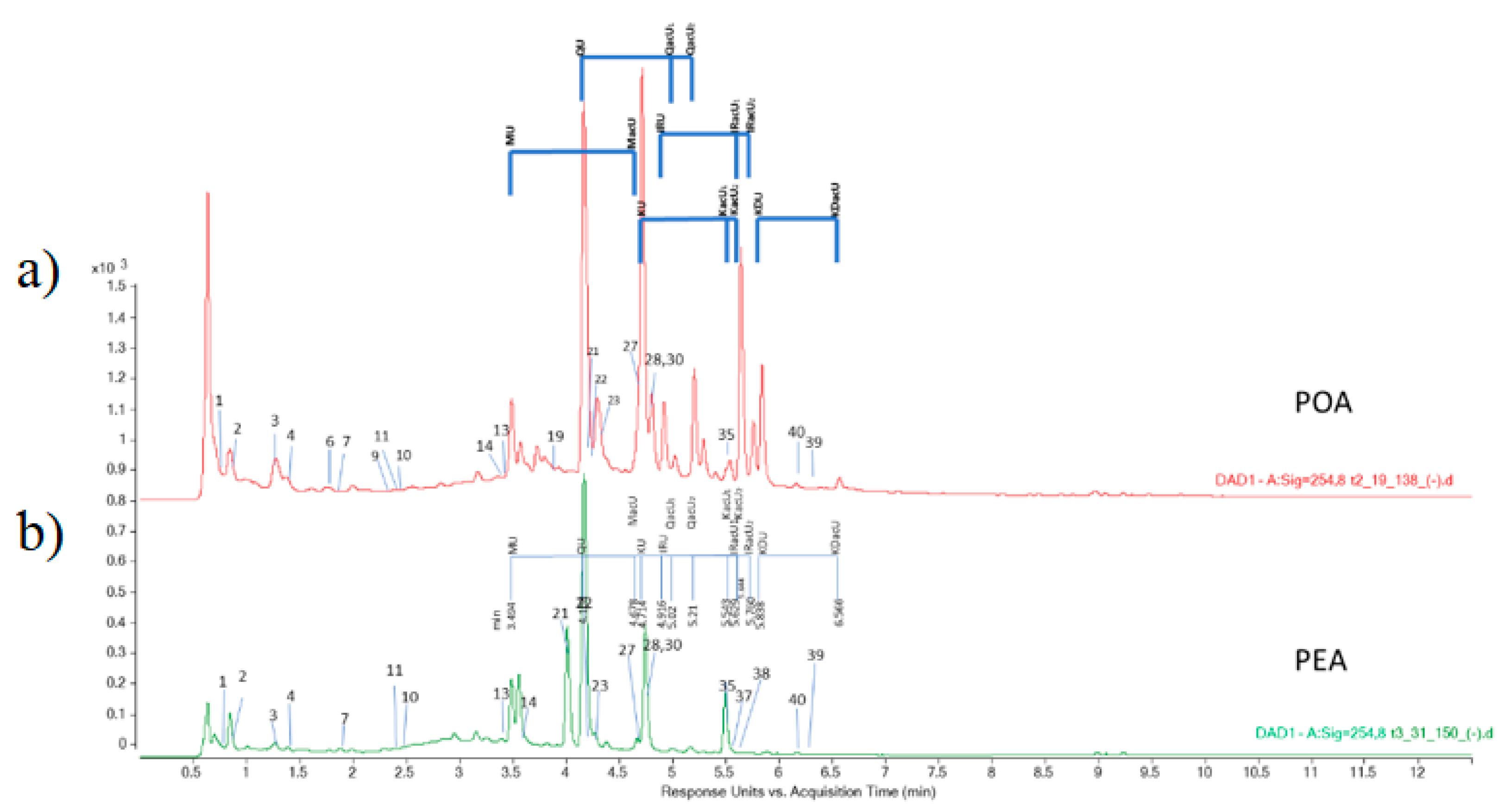
| Class of Secondary Metabolites | Compound | No a | Rt b [min] | LoQ c [μg/g de] | Content [μg/g dw] d | |
|---|---|---|---|---|---|---|
| POA | PEA | |||||
| Cyclohexanecarboxylic acids | Quinic acid | 1 | 0.52 | 5.0 | (8.7 ± 0.9) 103 | (8.8 ± 0.9) 102 |
| Hydroxybenzoic acids | Gallic acid | 2 | 0.58 | 10 | (9.5 ± 0.8) 102 | (3.5 ± 0.3) 103 |
| Protocatechuic acid | 3 | 0.79 | 2.0 | (2.3 ± 0.2) 102 | (1.9 ± 0.1) 101 | |
| 2,5-dihydroxybenzoic acid | 5 | 1.03 | 3.5 | (3.0 ± 0.2) 101 | <LoQ | |
| p-Hydroxybenzoic acid | 8 | 1.08 | 4.0 | (3.1 ± 0.2) 101 | (4.8 ± 0.3) 101 | |
| Vanillic acid | 12 | 1.24 | 50 | (2.6 ± 0.8) 101 | (0.3 ± 0.1) 102 | |
| Syringic acid | 13 | 1.31 | 20 | (1.5 ± 0.3) 102 | (1.1 ± 0.2) 102 | |
| Phenylpropanoids | Cinnamic acid | 36 | 3.91 | 40 | <LoQ | <LoQ |
| Hydroxycinnamic acids | Caffeic acid | 11 | 1.18 | 3.0 | (2.2 ± 0.2) 101 | (6.2 ± 0.4) 101 |
| p-Coumaric acid | 14 | 1.69 | 2.0 | (5.1 ± 0.4) 101 | (3.7 ± 0.3) 101 | |
| Ferulic acid | 17 | 1.90 | 5.0 | (2.8 ± 0.3) 101 | (5.0 ± 0.5) 101 | |
| Sinapic acid | 18 | 1.92 | 20 | (1.6 ± 0.2) 101 | (3.9 ± 0.4) 101 | |
| o-coumaric acid | 24 | 2.62 | 3.0 | 1.3 ± 0.1 | <LoQ | |
| 3,4-dimethoxycinnamic acid | 31 | 2.99 | 25 | <LoQ | <LoQ | |
| Chlorogenic acids | 5-O-caffeoylquinic acid | 6 | 0.80 | 3.5 | (6.9 ± 0.3) 102 | 11.0 ± 0.5 |
| Flavan-3-ols | Catechin | 4 | 0.74 | 25 | (7.0 ± 0.7) 102 | (6.5 ± 0.6) 102 |
| Epicatechin | 10 | 0.95 | 30 | (8.8 ± 0.9) 101 | (2.3 ± 0.2) 102 | |
| Flavan-3-ol-derivatives | Epigallocatechin gallate | 7 | 0.81 | 50 | (1.5 ± 0.1) 102 | (1.3 ± 0.1) 103 |
| Coumarins | Esculetin | 9 | 1.13 | 3.0 | 5.1 ± 0.3 | 9.1 ± 0.5 |
| Umbelliferone | 15 | 1.73 | 5.0 | <LoQ | <LoQ | |
| Scopoletin | 16 | 1.77 | 3.5 | 1.0 ± 0.1 | 11.2 ± 0.9 | |
| Flavone glycosides | Luteolin-7-O-glucoside | 20 | 2.13 | 2.5 | (11.0 ± 0.3) 10−1 | 3.1 ± 0.1 |
| Vitexin | 19 | 1.90 | 2.0 | 3.2 ± 0.2 | <LoQ | |
| Apiin | 25 | 2.60 | 1.5 | <LoQ | <LoQ | |
| Apigenin-7-O-glucoside | 26 | 2.81 | 3.0 | <LoQ | (6.0 ± 0.3) 10−1 | |
| Baicalin | 32 | 3.4 | 10 | <LoQ | <LoQ | |
| Flavones | Luteolin | 38 | 4.03 | 2.0 | 6.4 ± 0.3 | 12.6 ± 0.6 |
| Apigenin | 39 | 4.71 | 5.0 | (8.5 ± 0.6) 101 | (4.0 ± 0.3) 101 | |
| Baicalein | 41 | 5.15 | 15 | <LoQ | <LoQ | |
| Chrysoeriol | 43 | 4.82 | 2.0 | (5.0 ± 0.1) 10−1 | (8.0 ± 0.4) 10−1 | |
| Biflavonoid | Amentoflavone | 45 | 5.78 | 2.5 | <LoQ | <LoQ |
| Flavonol-glycosides | Quercetin-3-O-galactoside | 21 | 2.16 | 3.0 | (3.0 ± 0.2) 103 | (11.9 ± 0.7) 103 |
| Quercetin-3-O-rutinoside | 22 | 2.33 | 1.5 | (26.2 ± 0.8) 101 | (20.5 ± 0.6) 101 | |
| Quercetin-3-O-glucoside | 23 | 2.25 | 2.0 | (13.8 ± 0.4) 102 | (14.9 ± 0.4) 102 | |
| Quercetin-3-O-L-rhamnoside | 28 | 2.75 | 1.5 | (1.6 ± 0.1) 102 | (9.8 ± 0.6) 103 | |
| Kaempferol-3-O-glucoside | 30 | 2.8 | 2.0 | (13.3 ± 0.5) 102 | (2.8 ± 0.1) 101 | |
| Flavonols | Myricetin | 27 | 2.67 | 50 | (11.1 ± 0.8) 101 | (8.6 ± 0.6) 102 |
| Quercetin | 35 | 3.74 | 50 | (0.4 ± 0.1) 103 | (5.5 ± 1.6) 103 | |
| Kaempferol | 40 | 4.55 | 3.0 | (12.0 ± 0.8) 101 | (13.4 ± 0.9) 101 | |
| Isorhamnetin | 42 | 4.79 | 10 | 5.1 ± 0.3 | 8.6 ± 0.5 | |
| Flavanones | Naringenin | 37 | 3.87 | 3.5 | 6.5 ± 0.4 | (1.6 ± 0.1) 101 |
| Isoflavones | Daidzein | 33 | 3.43 | 5.0 | <LoQ | <LoQ |
| Genistein | 44 | 4.12 | 3.0 | <LoQ | <LoQ | |
| Lignans | Secoisolariciresinol | 29 | 2.90 | 25 | <LoQ | <LoQ |
| Matairesinol | 34 | 3.66 | 50 | <LoQ | <LoQ | |
| TOTAL | 18,782 (1.88%) | 37,113 (3.71%) | ||||
| Individual Treatments | |||
| IC25 * | IC50* | ||
| D | 1.3 | 12.56 | |
| POA | 1250 | 2800 | |
| PEA | 500 | 910 | |
| IC25 * values of the co-treatments | |||
| POAD | PEAD | ||
| POA | D | PEA | D |
| 120 | 0.68 | 140 | 0.79 |
| IC50 * values of the co-treatments | |||
| POAD | PEAD | ||
| POA | D | PEA | D |
| 160 | 0.91 | 250 | 1.43 |
| CI | |||
| POAD | PEAD | ||
| IC25 | 0.62 | 0.89 | |
| IC50 | 0.13 | 0.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, M.; Tenji, D.; Nikolić, B.; Srdić-Rajić, T.; Svirčev, E.; Mitić-Ćulafić, D. In Vitro Study of Two Edible Polygonoideae Plants: Phenolic Profile, Cytotoxicity, and Modulation of Keap1-Nrf2 Gene Expression. Foods 2021, 10, 811. https://doi.org/10.3390/foods10040811
Jovanović M, Tenji D, Nikolić B, Srdić-Rajić T, Svirčev E, Mitić-Ćulafić D. In Vitro Study of Two Edible Polygonoideae Plants: Phenolic Profile, Cytotoxicity, and Modulation of Keap1-Nrf2 Gene Expression. Foods. 2021; 10(4):811. https://doi.org/10.3390/foods10040811
Chicago/Turabian StyleJovanović, Marina, Dina Tenji, Biljana Nikolić, Tatjana Srdić-Rajić, Emilija Svirčev, and Dragana Mitić-Ćulafić. 2021. "In Vitro Study of Two Edible Polygonoideae Plants: Phenolic Profile, Cytotoxicity, and Modulation of Keap1-Nrf2 Gene Expression" Foods 10, no. 4: 811. https://doi.org/10.3390/foods10040811
APA StyleJovanović, M., Tenji, D., Nikolić, B., Srdić-Rajić, T., Svirčev, E., & Mitić-Ćulafić, D. (2021). In Vitro Study of Two Edible Polygonoideae Plants: Phenolic Profile, Cytotoxicity, and Modulation of Keap1-Nrf2 Gene Expression. Foods, 10(4), 811. https://doi.org/10.3390/foods10040811