Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
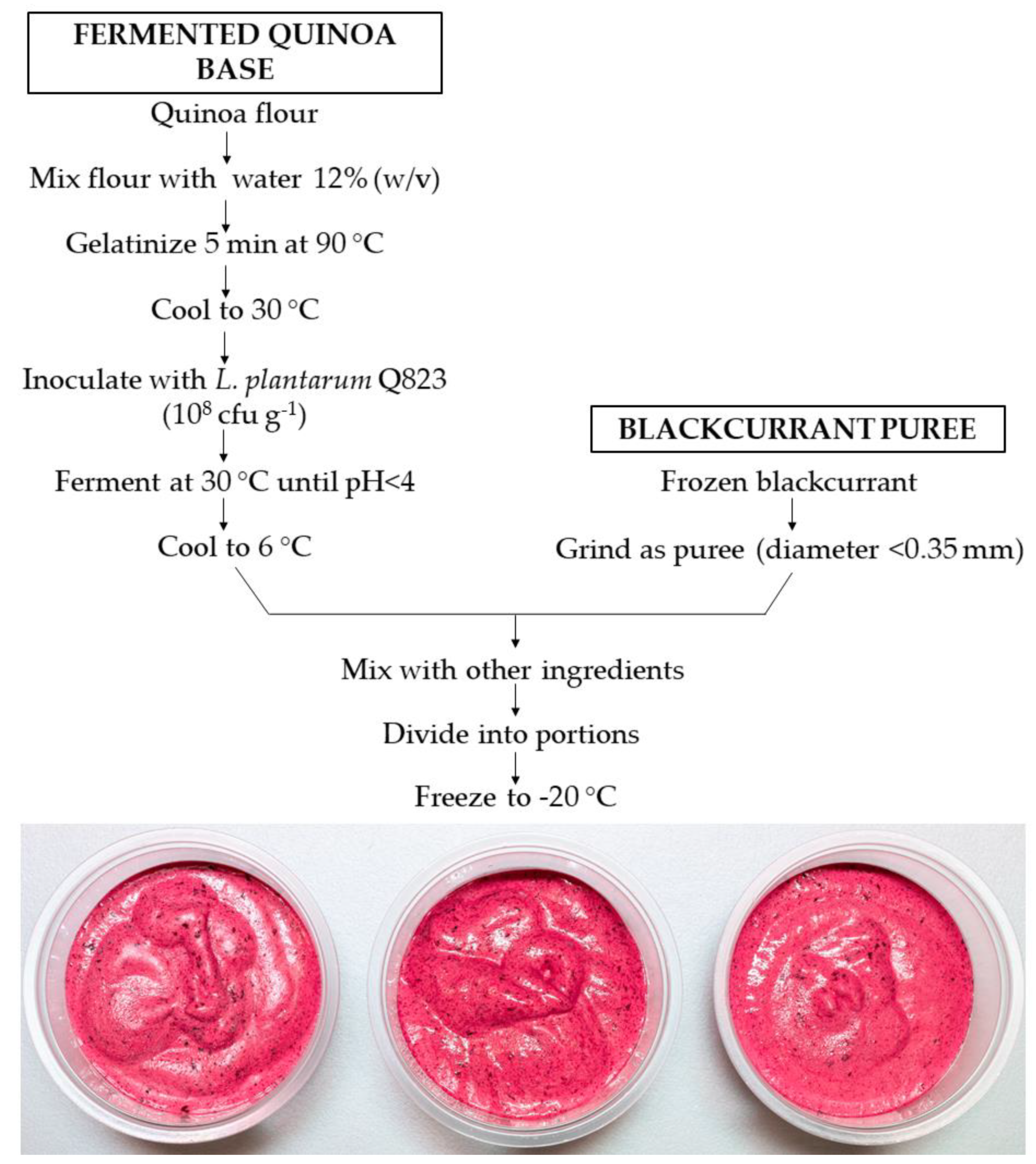
2.2. Manufacturing Process
2.3. Physicochemical Analysis
2.4. Consumer Sensory Evaluation
2.5. Viable Cell Counts during the 90-Day Storage
2.6. In Vitro Survival of L. plantarum Q823
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Analysis
3.2. Consumer Sensory Evaluation
3.3. Viable Cell Counts during the 90-Day Storage
3.4. In Vitro Survival of L. plantarum Q823
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Background Information | n = 71 | % |
|---|---|---|
| Gender | ||
| Male | 10 | 14.1 |
| Female | 60 | 84.5 |
| Other | 1 | 1.4 |
| Age | ||
| 18–24 | 22 | 31.0 |
| 25–30 | 14 | 19.7 |
| 31–40 | 14 | 19.7 |
| 41–50 | 8 | 11.3 |
| 51–65 | 13 | 18.3 |
| Job description | ||
| Working | 38 | 53.5 |
| Student | 31 | 43.7 |
| Other | 2 | 2.8 |
| Consumption of berries | ||
| Daily | 19 | 26.8 |
| Few times a week | 25 | 35.2 |
| Once a week | 9 | 12.7 |
| Few times a month | 16 | 19.7 |
| Rarely | 2 | 2.8 |
| Never | 0 | 0 |
| Do you look at a product’s nutritional information before buying it? | ||
| Yes | 14 | 19.7 |
| Yes, sometimes | 52 | 73.2 |
| No | 5 | 7.0 |
| Most important choosing factors when buying a product 1 | ||
| Package | 2 | 2.7 |
| Healthiness | 47 | 66.2 |
| Habit | 21 | 29.6 |
| Price | 48 | 67.6 |
| Organic or local product | 7 | 9.9 |
| Origin | 47 | 66.2 |
| Brand | 1 | 1.4 |
| Ecological aspect/Sustainability | 13 | 18.3 |
| Quality | 24 | 33.8 |
| Other | 1 | 1.4 |
Appendix B

Appendix C
| Unstandardized Coefficients | Standardized Coefficients | |||||
|---|---|---|---|---|---|---|
| Model | Variables in Model | β | Standard Error | β | t | Significance |
| 1 | (Constant) | 3.302 | 0.343 | 9.632 | 0.000 | |
| Sweetness | 0.563 | 0.045 | 0.652 | 12.476 | 0.000 | |
| 2 | (Constant) | 1.839 | 0.319 | 5.758 | 0.000 | |
| Sweetness | 0.414 | 0.040 | 0.479 | 10.307 | 0.000 | |
| Texture | 0.354 | 0.036 | 0.462 | 9.937 | 0.000 | |
| 3 | (Constant) | 1.578 | 0.305 | 5.173 | 0.000 | |
| Sweetness | 0.291 | 0.045 | 0.336 | 6.504 | 0.000 | |
| Texture | 0.309 | 0.035 | 0.403 | 8.914 | 0.000 | |
| Sourness | 0.210 | 0.040 | 0.275 | 5.232 | 0.000 | |
| 4 | (Constant) | 7.168 | 2.543 | 2.819 | 0.005 | |
| Sweetness | 0.292 | 0.044 | 0.338 | 6.601 | 0.000 | |
| Texture | 0.312 | 0.034 | 0.407 | 9.062 | 0.000 | |
| Sourness | 0.201 | 0.040 | 0.264 | 5.037 | 0.000 | |
| Protein | −7.360 | 3.324 | −0.090 | −2.214 | 0.028 | |
| 5 | (Constant) | 7.696 | 2.521 | 3.053 | 0.003 | |
| Sweetness | 0.274 | 0.044 | 0.317 | 6.169 | 0.000 | |
| Texture | 0.282 | 0.036 | 0.367 | 7.801 | 0.000 | |
| Sourness | 0.153 | 0.044 | 0.201 | 3.490 | 0.001 | |
| Protein | −8.405 | 3.311 | −0.103 | −2.539 | 0.012 | |
| Berryness | 0.126 | 0.051 | 0.141 | 2.483 | 0.014 |
References
- de Medeiros, A.C.; Filho, E.R.T.; Bolini, H.M.A. Impact of Natural and Artificial Sweeteners Compounds in the Sensory Profile and Preference Drivers Applied to Traditional, Lactose-Free, and Vegan Frozen Desserts of Chocolate Flavor. J. Food Sci. 2019, 84, 2973–2982. [Google Scholar] [CrossRef] [PubMed]
- Radnitz, C.; Beezhold, B.; DiMatteo, J. Investigation of lifestyle choices of individuals following a vegan diet for health and ethical reasons. Appetite 2015, 90, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Sampson, H.A.; Bock, S.; Burks, A.; Harden, K.; Noone, S.; Martin, D.; Leung, S.; Wilson, G. Soy allergy in infants and children with IgE-associated cow’s milk allergy. J. Pediatr. 1999, 134, 614–622. [Google Scholar] [CrossRef]
- Annunziata, A.; Vecchio, R. European consumers acceptance of healthy food products: A review of functional foods. In Functional Foods: Sources, Biotechnology Applications, and Health Challenges; Emerson, D., Robinson, A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA; Hauppauge: New York, NY, USA, 2013; pp. 121–141. [Google Scholar]
- Erkaya, T.; Daǧdemir, E.; Sengül, M. Influence of Cape gooseberry (Physalis peruviana L.) addition on the chemical and sensory characteristics and mineral concentrations of ice cream. Food Res. Int. 2012, 45, 331–335. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Demirci, T.; Akın, N. Production of functional probiotic ice creams with white and dark blue fruits of Myrtus communis: The comparison of the prebiotic potentials on Lactobacillus casei 431 and functional characteristics. LWT 2018, 90, 339–345. [Google Scholar] [CrossRef]
- da Silva, J.M.; Klososki, S.J.; Silva, R.; Raices, R.S.L.; Silva, M.C.; Freitas, M.Q.; Barão, C.E.; Pimentel, T.C. Passion fruit-flavored ice cream processed with water-soluble extract of rice by-product: What is the impact of the addition of different prebiotic components? LWT 2020, 128, 109472. [Google Scholar] [CrossRef]
- Lankinen, M.; Uusitupa, M.; Schwab, U. Nordic Diet and Inflammation—A Review of Observational and Intervention Studies. Nutrients 2019, 11, 1369. [Google Scholar] [CrossRef]
- Ovaskainen, M.-L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary Intake and Major Food Sources of Polyphenols in Finnish Adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries, and anthocyanins: Promising functional food ingredients with postprandial glycaemia-lowering effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Lappi, J.; Raninen, K.; Väkeväinen, K.; Kårlund, A.; Törrönen, R.; Kolehmainen, M. Blackcurrant (Ribes nigrum) lowers sugar-induced postprandial glycaemia independently and in a product with fermented quinoa: A randomised crossover trial. Br. J. Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- del Bo’, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory Effects in Humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Natural Resources Institute Finland (LuKe). Statistics Database. Available online: http://statdb.luke.fi (accessed on 14 May 2020).
- Laaksonen, O.; Mäkilä, L.; Tahvonen, R.; Kallio, H.; Yang, B. Sensory quality and compositional characteristics of blackcurrant juices produced by different processes. Food Chem. 2013, 138, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Slining, M.M.; Popkin, B.M. Use of Caloric and Noncaloric Sweeteners in US Consumer Packaged Foods, 2005–2009. J Acad. Nutr. Diet. 2012, 112, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Avila, P.; Namiesnik, J.; Toledo, F.; Werner, E.; Martinez-Ayala, A.L.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Gorinstein, S. The influence of different time durations of thermal processing on berries quality. Food Control 2012, 26, 587–593. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blueberry Products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Cruz, A.G.; Antunes, A.E.C.; Sousa, A.L.O.P.; Faria, J.A.F.; Saad, S.M.I. Ice-cream as a probiotic food carrier. Food Res. Int. 2009, 42, 1233–1239. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Probiotics-From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. 2018. [Google Scholar] [CrossRef]
- Karimi, R.; Mortazavia, A.M.; Da Cruz, A.G. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Sci. Technol. 2011, 91, 283–308. [Google Scholar] [CrossRef]
- Akalın, A.S.; Kesenkas, H.; Dinkci, N.; Unal, G.; Ozer, E.; Kınık, O. Enrichment of probiotic ice cream with different dietary fibers: Structural characteristics and culture viability. J. Dairy Sci. 2018, 101, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Vera-Pingitore, E.; Jimenez, M.E.; Dallagnol, A.; Belfiore, C.; Fontana, C.; Fontana, P.; von Wright, A.; Vignolo, G.; Plumed-Ferrer, C. Screening and characterization of potential probiotic and starter bacteria for plant fermentations. LWT 2016, 71, 288–294. [Google Scholar] [CrossRef]
- Urquizo, F.E.; García Torres, S.M.; Tolonen, T.; Jaakkola, M.; Pena-Niebuhr, M.G.; Von Wright, A.; Repo-Carrasco-Valencia, R.; Korhonen, H.; Plumed-Ferrer, C. Development of a fermented quinoa- based beverage. Food Sci. Nutr. 2016, 5, 602–608. [Google Scholar] [CrossRef]
- Väkeväinen, K.; Ludena-Urquizo, F.; Korkala, E.; Lapveteläinen, A.; Peräniemi, S.; von Wright, A.; Plumed-Ferrer, C. Potential of quinoa in the development of fermented spoonable vegan products. LWT 2020, 120, 108912. [Google Scholar] [CrossRef]
- Rogeauz, M. Improving team tasting in the food industry. In Rapid Sensory Profiling Techniques and Related Methods—Applications in New Product Development and Consumer Research, 1st ed.; Delarue, J., Lawlor, J., Rogeaux, M., Eds.; Woodhead: Cambridge, UK, 2015; pp. 345–362. [Google Scholar]
- Rolon, L.M.; Bakke, A.J.; Coupland, J.N.; Hayes, J.E.; Roberts, R.F. Effect of fat content on the physical properties and consumer acceptability of vanilla ice cream. J. Dairy Sci. 2017, 100, 5217–5227. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- ISO. ISO 8589:2007 (E). In Sensory Analysis—General Guidance for the Design of Test Rooms; The International Organization of Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Foods—Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- dos Santos Cruxen, C.E.; Hoffmann, J.F.; Zandoná, G.P.; Fiorentini, Â.M.; Rombaldi, C.V.; Chaves, F.C. Probiotic butiá (Butia odorata) ice cream: Development, characterization, stability of bioactive compounds, and viability of Bifidobacterium lactis during storage. LWT 2017, 75, 379–385. [Google Scholar] [CrossRef]
- Mäkilä, L.; Laaksonen, O.; Kallio, H.; Yang, B. Effect of processing technologies and storage conditions on stability of black currant juices with special focus on phenolic compounds and sensory properties. Food Chem. 2017, 221, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Prot 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA Gene Sequence Analysis and Multiplex PCR Assay with recA Gene-Derived Primers Downloaded from. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson new international edition; Pearson: Harlow, UK, 2014. [Google Scholar]
- Sofjan, R.P.; Hartel, R.W. Effects of overrun on structural and physical characteristics of ice cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Camire, M.E.; Dougherty, M.P.; Teh, Y.-H. Frozen Wild Blueberry-Tofu-Soymilk Desserts. J. Food Sci. 2006, 71, S119–S123. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Edmonds, L.; Wadhwa, S.S.; Wibisono, R. Producing ice cream using a substantial amount of juice from kiwifruit with green, gold or red flesh. Food Res. Int. 2013, 50, 647–656. [Google Scholar] [CrossRef]
- National Institute for Health and Welfare. Fineli—National Food Composition Database. Available online: https://fineli.fi/fineli/en/index (accessed on 5 December 2019).
- Faye, T.; Tamburello, A.; Vegarud, G.E.; Skeie, S. Survival of lactic acid bacteria from fermented milks in an in vitro digestion model exploiting sequential incubation in human gastric and duodenum juice. J. Dairy Sci 2012, 95, 558–566. [Google Scholar] [CrossRef]
- Ferraz, J.L.; Cruz, A.G.; Cadena, R.S.; Freitas, M.Q.; Pinto, U.M.; Carvalho, C.C.; Faria, J.A.; Bolini, H.M. Sensory Acceptance and Survival of Probiotic Bacteria in Ice Cream Produced with Different Overrun Levels. J. Food Sci. 2012, 77, S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Marino, M.; Maifreni, M.; Innocente, N. Potential application of monoglyceride structured emulsions as delivery systems of probiotic bacteria in reduced saturated fat ice cream. LWT 2018, 96, 329–334. [Google Scholar] [CrossRef]
- Ahmadi, A.; Milani, E.; Madadlou, A.; Mortazavi, S.A.; Mokarram, R.R.; Salarbashi, D. Synbiotic yogurt-ice cream produced via incorporation of microencapsulated Lactobacillus acidophilus (la-5) and fructooligosaccharide. J. Food Sci. Technol. 2014, 51, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, C.; Silva, H.; Cavalcanti, R.; Esmerino, E.; Cappato, L.; Abud, Y.; Moraes, J.; Andrade, M.; Freitas, M.; Sant’Anna, C.; et al. Prebiotics addition in sheep milk ice cream: A rheological, microstructural and sensory study. J. Funct. Foods 2017, 35, 564–573. [Google Scholar] [CrossRef]
 ), product flavored with sugar and vanilla (
), product flavored with sugar and vanilla ( ), and product flavored with sugar, vanilla, and lemon (
), and product flavored with sugar, vanilla, and lemon ( ). The sugar-only flavored blackcurrant product was significantly different from that of the product flavored with sugar and vanilla at an individual time point: * p < 0.05 (Tukey test). Results are means of triplicates.
). The sugar-only flavored blackcurrant product was significantly different from that of the product flavored with sugar and vanilla at an individual time point: * p < 0.05 (Tukey test). Results are means of triplicates.
 ), product flavored with sugar and vanilla (
), product flavored with sugar and vanilla ( ), and product flavored with sugar, vanilla, and lemon (
), and product flavored with sugar, vanilla, and lemon ( ). The sugar-only flavored blackcurrant product was significantly different from that of the product flavored with sugar and vanilla at an individual time point: * p < 0.05 (Tukey test). Results are means of triplicates.
). The sugar-only flavored blackcurrant product was significantly different from that of the product flavored with sugar and vanilla at an individual time point: * p < 0.05 (Tukey test). Results are means of triplicates.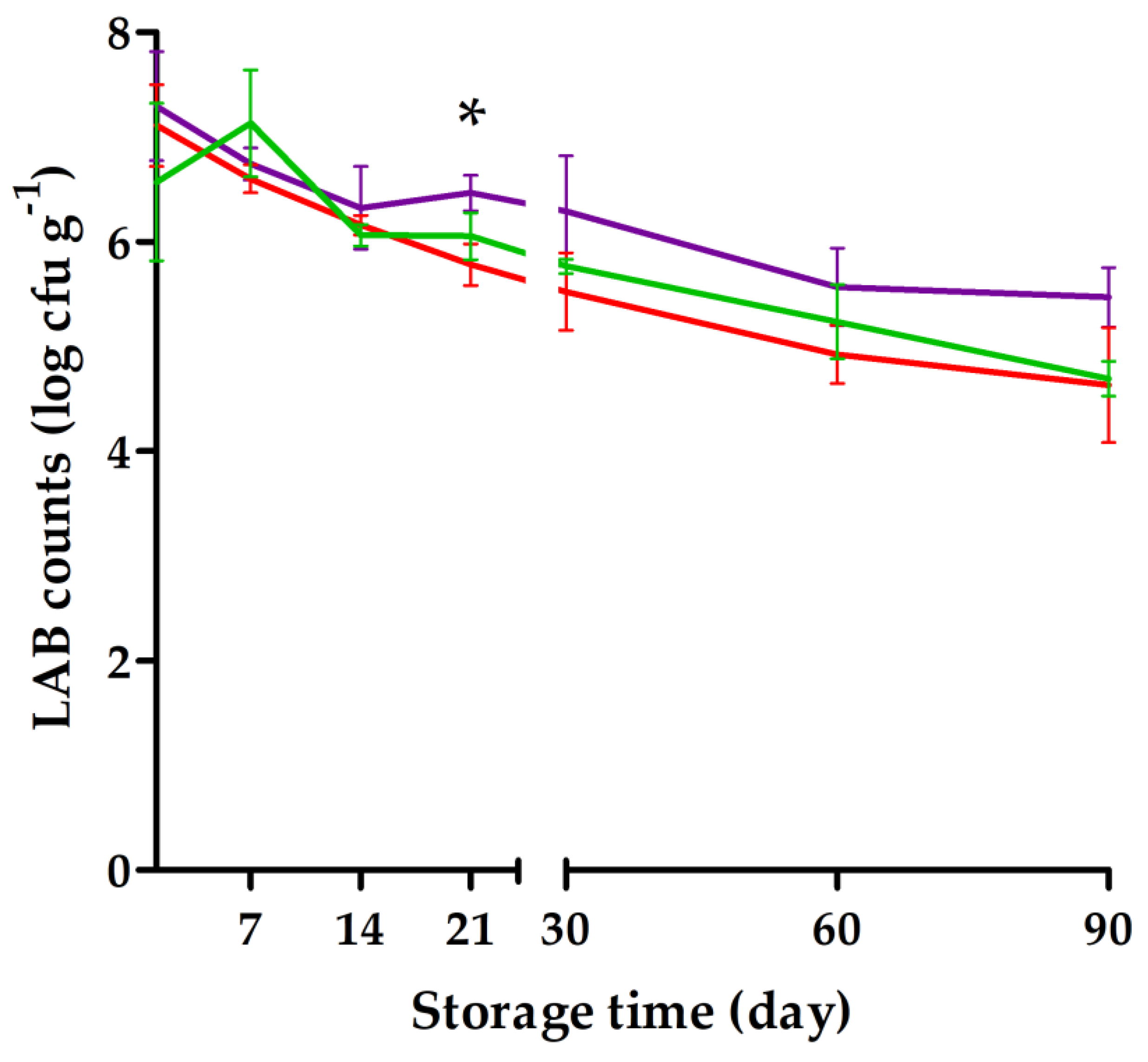
 ), 14 (
), 14 ( ), and 21 (
), and 21 ( ) days of storage at −20 °C. The viable counts with different letters are significantly different according to Tukey’s test (p < 0.05). Results are means of triplicates.
) days of storage at −20 °C. The viable counts with different letters are significantly different according to Tukey’s test (p < 0.05). Results are means of triplicates.
 ), 14 (
), 14 ( ), and 21 (
), and 21 ( ) days of storage at −20 °C. The viable counts with different letters are significantly different according to Tukey’s test (p < 0.05). Results are means of triplicates.
) days of storage at −20 °C. The viable counts with different letters are significantly different according to Tukey’s test (p < 0.05). Results are means of triplicates.
| Product | SBP | VBP | VLBP |
|---|---|---|---|
| pH | 3.09 ± 0.01 a | 3.07 ± 0.01 ab | 3.03 ± 0.03 b |
| TTA (mL of NaOH) | 25.5 ± 0.87 a | 25.6 ± 0.21 ab | 27.0 ± 0.21 b |
| Viscosity (Pas) | 79.9 ± 3.59 a | 75.4 ± 5.43 a | 74.2 ± 3.45 a |
| Overrun (%) | 116.9 ± 1.54 a | 137.9 ± 6.4 b | 118.4 ± 0.75 a |
| Moisture | 61.9 ± 0.04 a | 61.6 ± 0.03 c | 61.0 ± 0.01 b |
| Ash | 0.35 ± 0.05 a | 0.37 ± 0.02 a | 0.35 ± 0.02 a |
| Protein | 0.78 ± 0.04 a | 0.74 ± 0.00 a | 0.75 ± 0.07 a |
| Fat 1 | 6.8 a | 6.8 a | 6.8 a |
| Carbohydrates 2 | 30.1 ± 0.06 a | 30.4 ± 0.03 c | 31.1 ± 0.05 b |
| Total fiber 3 | 7.2 | 7.2 | 7.2 |
| SBP | VBP | VLBP | |
|---|---|---|---|
| Overall liking | 7.2 ± 1.45 | 7.6 ± 1.19 | 7.6 ± 1.11 |
| Sweetness | 7.3 ± 1.61 | 7.4 ± 1.58 | 7.7 ± 1.17 |
| Sourness | 6.9 ± 1.83 | 7.2 ± 1.63 | 7.4 ± 1.48 |
| Berryness | 7.7 ± 1.46 | 7.5 ± 1.56 | 7.8 ± 1.23 |
| Texture | 7.2 ± 1.79 | 7.3 ± 1.63 | 7.3 ± 1.56 |
| Component | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Explained variance % | 40.5 | 24.0 | 23.3 |
| Carbohydrates | −0.998 | ||
| Moisture | 0.998 | ||
| pH | 0.994 | ||
| TTA | −0.978 | 0.200 | |
| Viability | −0.853 | 0.520 | |
| Viscosity | 0.848 | 0.527 | |
| Overrun | −0.984 | ||
| Ash | −0.982 | ||
| Protein | 0.438 | 0.898 | |
| Liking | 0.884 | ||
| Berryness | 0.821 | ||
| Sourness | 0.817 | ||
| Sweetness | 0.777 | ||
| Texture | 0.721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Väkeväinen, K.; Rinkinen, N.; Willman, R.-M.; Lappi, J.; Raninen, K.; Kårlund, A.; Mikkonen, S.; Plumed-Ferrer, C.; Kolehmainen, M. Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability. Foods 2021, 10, 792. https://doi.org/10.3390/foods10040792
Väkeväinen K, Rinkinen N, Willman R-M, Lappi J, Raninen K, Kårlund A, Mikkonen S, Plumed-Ferrer C, Kolehmainen M. Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability. Foods. 2021; 10(4):792. https://doi.org/10.3390/foods10040792
Chicago/Turabian StyleVäkeväinen, Kati, Noora Rinkinen, Roosa-Maria Willman, Jenni Lappi, Kaisa Raninen, Anna Kårlund, Santtu Mikkonen, Carme Plumed-Ferrer, and Marjukka Kolehmainen. 2021. "Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability" Foods 10, no. 4: 792. https://doi.org/10.3390/foods10040792
APA StyleVäkeväinen, K., Rinkinen, N., Willman, R.-M., Lappi, J., Raninen, K., Kårlund, A., Mikkonen, S., Plumed-Ferrer, C., & Kolehmainen, M. (2021). Potential of Probiotic Frozen Blackcurrant Products: Consumer Preference, Physicochemical Characterization, and Cell Viability. Foods, 10(4), 792. https://doi.org/10.3390/foods10040792