Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Plant Extracts
2.3. Preparation of Fish Burgers
2.4. Total Phenols and Antioxidant Activity of S. nigra Flower Extract (SNE) and Essential Oils of Clove and Oregano (OEO and CLEO)
2.5. Fish Burger Analyses
2.5.1. pH and Water Activity (aw)
2.5.2. Color Determination
2.5.3. Microbial Analysis of Burgers
2.5.4. Inhibition of Lipid Peroxidation. Thiobarbituric Acid Reactive Substances (TBARS)
2.5.5. Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Total Phenols and Antioxidant Activity in S. nigra Flower Extract (SNE) and Essential Oils of Clove and Oregano (OEO and CLEO)
3.2. Fish Burger Analysis
3.2.1. pH, Water Activity (aw) and Color
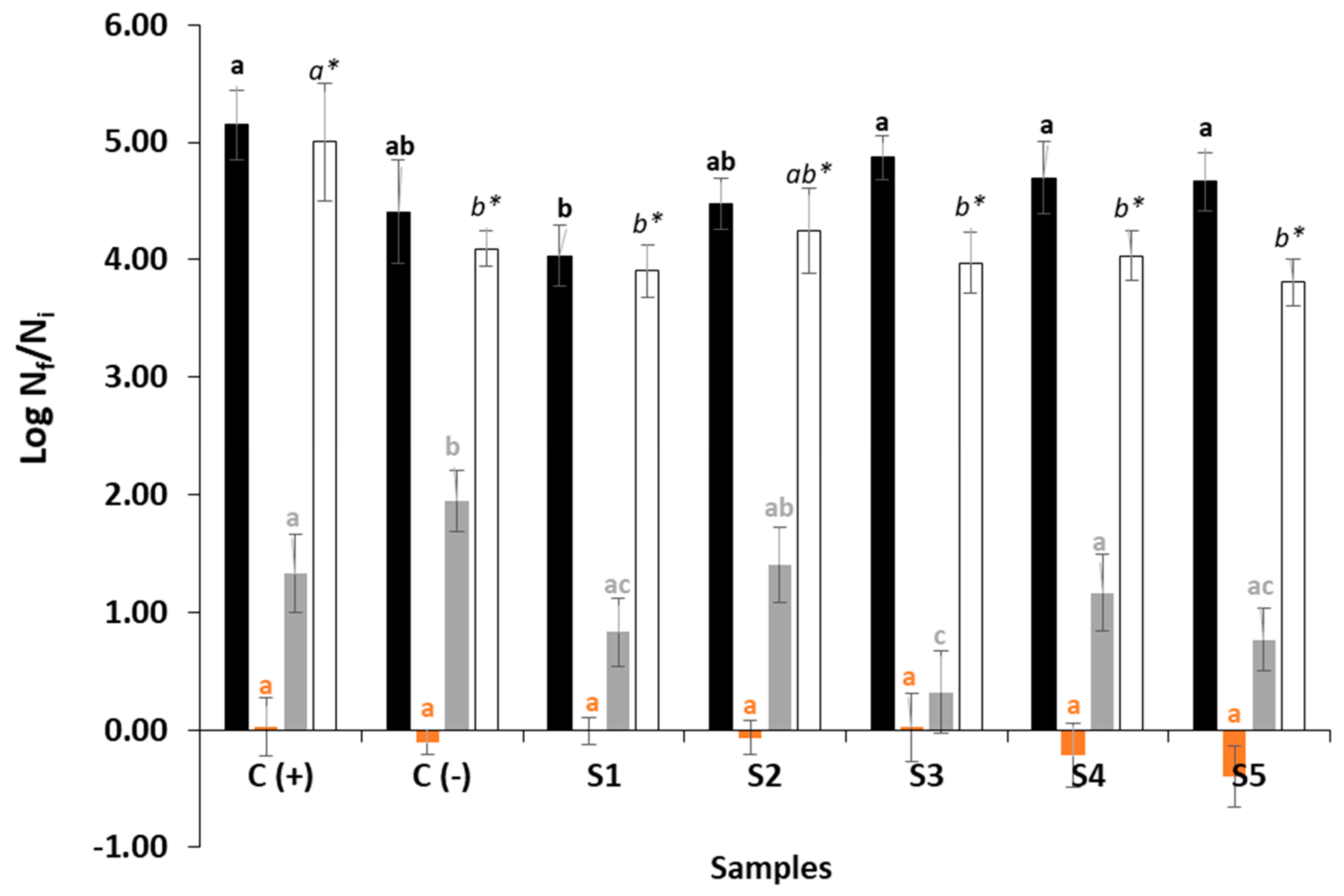
3.2.2. Microbial Counts
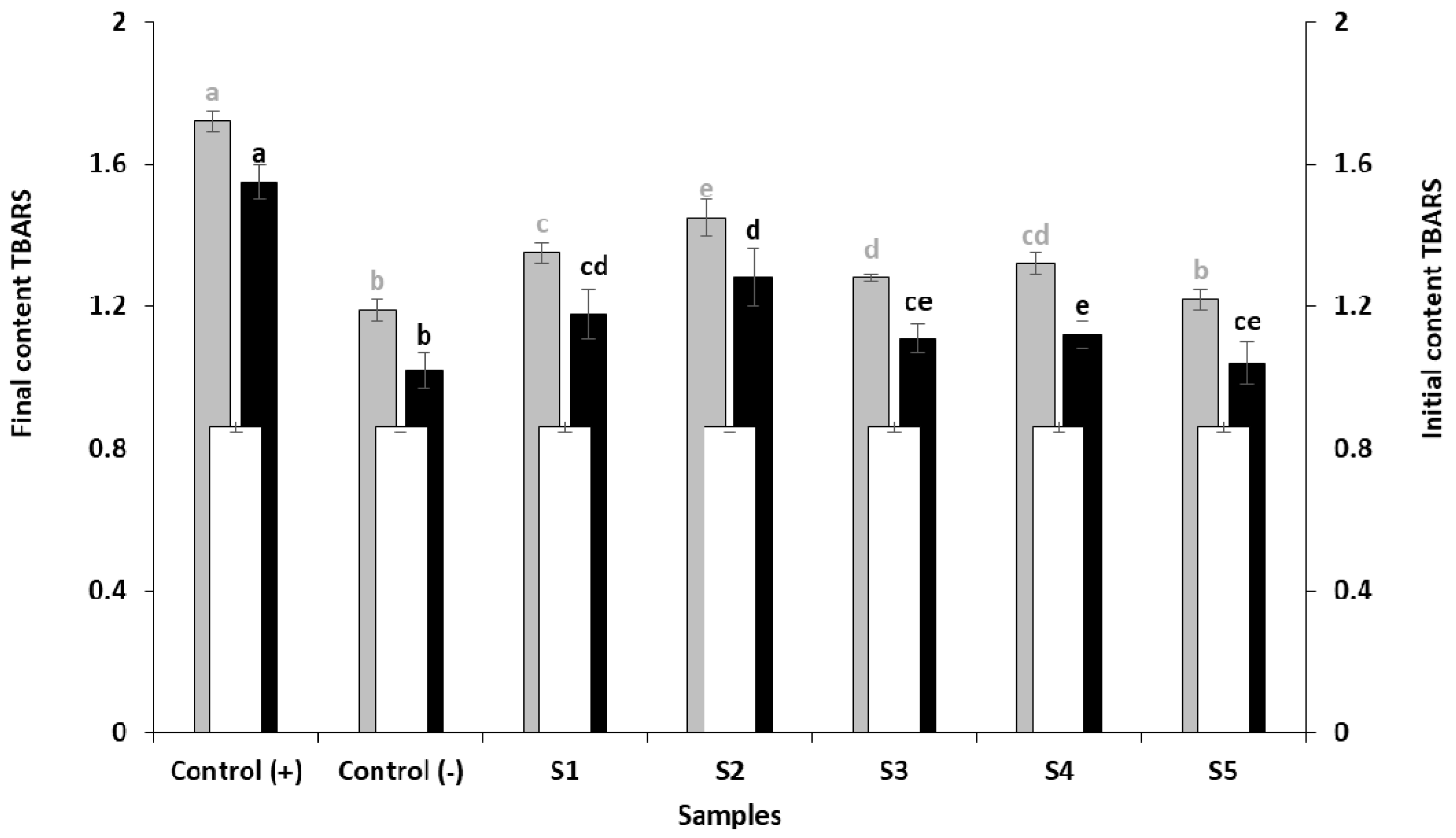
3.2.3. Inhibition of Lipid Peroxidation. Thiobarbituric Acid Reactive Substances (TBARS)
3.2.4. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gray, J.I.; Gomaa, E.A.; Buckley, D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996, 43, 111–123. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Dominguez, R.; Sant’Ana, A.S.; Khaneghah, A.M.; Gavahian, M.; Gomez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Race, S. Antioxidants. The Truth about BHA, BHT, TBHQ and Other Antioxidants Used as Food Additives, 1st ed.; Tigmor Books: Canterbury, UK, 2009; ISBN 9781907119002. [Google Scholar]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union 2008, L354, 16-33.
- Pokorný, J. Are natural antioxidants better and safer than synthetic antioxidants? Eur. J. Lipid Sci. Tech. 2007, 109, 629–664. [Google Scholar] [CrossRef]
- Sasse, A.; Colindres, P.; Brewer, M.S. Effect of natural and synthetic antioxidants on the oxidative stability of cooked, frozen pork patties. J. Food Sci. 2009, 74, 30–35. [Google Scholar] [CrossRef]
- FDA. Chapter I—Food and Drug Administration Department Of Health And Human Services. Title 21—Food and Drugs. Part 172—Food Additives Permitted For Direct Addition to Food for Human Consumption. Subpart B—Food Preservatives. In Code of Federal Regulations; Sec. 172.110 BHA; Office of the Federal Register: College Park, MD, USA, 2019. [Google Scholar]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Patel, S. Plant essential oils and allied volatile fractions as multifunctional additives in meat and fish-based food products: A review. Food Addit. Contam. A 2015, 32, 1049–1064. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Potential application of essential oils as natural antioxidants in meat and meat products: A review. Food Rev. Int. 2014, 30, 71–90. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Racanicci, A.M.C.; Danielsen, B.; Skibsted, L.H. Mate (Ilex paraguariensis) as a source of water extractable antioxidant for use in chicken meat. Eur. Food Res. Tech. 2008, 27, 255–260. [Google Scholar] [CrossRef]
- Alghazeer, R.; Saeed, S.; Howell, N.K. Aldehyde formation in frozen mackerel (Scomber scombrus) in the presence and absence of instant green tea. Food Chem. 2008, 108, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Ayas, D.; Yazgan, H.; Özogul, F.; Boga, E.K.; Ozyurt, G. The capability of rosemary extract in preventing oxidation of fish lipid. Int. J. Food Sci. Technol. 2010, 45, 1717–1723. [Google Scholar] [CrossRef]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Zielinska—Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). Lebensm. Wiss. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluid. 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Jonušaitė, K. High pressure biorefinery of essential oil yielding plants into valuable ingredients. Acta Hortic. 2016, 1125, 399–406. [Google Scholar] [CrossRef]
- Eberhardt, M.; Lee, C.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Part II: Antioxidants in foods and beverages. Chapter 14. Assessing the activity of natural food antioxidants. In Oxidation in Foods and Beverages and Antioxidant Applications. Understanding Mechanisms of Oxidation and Antioxidant Activity; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Cambridge, UK, 2010; pp. 345–346. [Google Scholar]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Hunter, R.S.; Harold, R.W. The Measurement of Appearance, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
- ISO—International Organization for Standardization. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms; ISO: Geneva, Switzerland, 2019; 7410:2019(en). [Google Scholar]
- ISO—International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli; ISO: Geneva, Switzerland, 2001; 16649-2:2001(en). [Google Scholar]
- ISO—International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; ISO: Geneva, Switzerland, 2008; 21527-1:2008(en). [Google Scholar]
- ISO—International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C; ISO: Geneva, Switzerland, 1998; 15214:1998. [Google Scholar]
- Silbande, A.; Adenet, S.; Smith-Ravin, J.; Joffraud, J.J.; Rochefort, K.; Leroi, F. Quality assessment of ice-stored tropical yellowfin tuna (Thunnus albacares) and influence of vacuum and modified atmosphere packaging. Food Microbiol. 2016, 60, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press Inc.: Boca Ratón, FL, USA, 2006. [Google Scholar]
- Kingchaiyaphum, W.; Rachtanapun, C. Antimicrobial and antioxidative activities of essential oils in Chinese sausage (Kun-Chiang). As. J. Food Ag-Ind. 2012, 5, 156–162. [Google Scholar]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Virgata, G.; Barcellona, M.L.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mastelic, J.; Jerkovic, I.; Blaževic, I.; Poljak-Blaži, M.; Borovic, S.; Ivancic-Bace, I.; Smrecki, V.; Žarkovic, N.; Brcic-Kostic, K.; Vikic-Topic, D.; et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, I.; Wilker, M.; Stoyanova, A.; Krastanov, A.; Stanchev, V. Antioxidant activity of extract from elder flower (Sambucus nigra L.). Herba Polonica 2007, 53, 45–54. [Google Scholar]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Dehpour, A.A.; Ebrahimzadeh, M.A.; Nabavi, S.F.; Nabavi, S.M. Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites 2009, 60, 405–412. [Google Scholar]
- Wong, P.Y.; Tan, S.T. Comparison of total phenolic content and antioxidant activities in selected coloured plants. Br. Food J. 2020, 122, 3193–3201. [Google Scholar] [CrossRef]
- Mørkøre, T.; Rødbotten, M.; Vogt, G.; Fjæra, S.O.; Kristiansen, I.; Manseth, E. Relevance of season and nucleotide catabolism on changes in fillet quality during chilled storage of raw Atlantic salmon (Salmo salar L.). Food Chem. 2010, 119, 1417–1425. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Motallebi, A.A.; Hosseini, H.; Haratian, P.; Ahmadi, H.; Mohammadi, M.; Khaksar, R. Quality assessment of fish burgers from deep flounder (Pseudorhombus elevatus) and brushtooth lizardfish (Saurida undosquamis) during storage at −18 ºC. Iran. J. Fish. Sci. 2010, 91, 111–126. [Google Scholar]
- Rostamzad, H.; Shabanpour, B.; Shabani, A.; Shahiri, H. Enhancement of the storage quality of frozen Persian sturgeon fillets by using of ascorbic acid. Int. Food Res. J. 2011, 18, 109–116. [Google Scholar]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT—Food Sci. Technol. 2014, 59, 122–129. [Google Scholar] [CrossRef]
- Jezek, F.; Buchtova, H. Effect of modified atmosphere packaging on the course of physical and chemical changes in chilled muscle tissue of silver carp (Hypophthalmichthys molitrix V.). Polish J. Vet. Sci. 2012, 15, 439–445. [Google Scholar] [CrossRef]
- Pakawatchai, C.; Siripongvutikorn, S.; Usawakesmanee, W. Effect of herb and spice pastes on the quality changes in minced salmon flesh waste during chilled storage. As. J. Food Ag-Ind. 2009, 2, 481–492. [Google Scholar]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Marshall, M.R. Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and mahi mahi (Coryphaena hippurus). J. Food Sci. 2007, 72, C509–C515. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Ponce-Alquicira, E.; Jaramillo-Flores, M.E.; Guerrero-Legarreta, I. Antioxidant effect rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) extracts on TBARS and color of model raw pork batters. Meat Sci. 2009, 81, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Valipour, S. Effect of carboxymethyl cellulose edible coating containing Zataria multiflora essential oil and grape seed extract on chemical attributes of rainbow trout meat. Vet. Res. Forum 2014, 5, 89–93. [Google Scholar]
- Estévez, M.; Ramírez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT—Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Romero, M.M.; Doval, M.M.; Romero, M.A.; Sturla, M.A.; Judis, M.A. Antioxidant properties of soya sprout hydrophilic extracts. Application to cooked chicken patties. Elec. J. Env. Agricult. Food Chem. 2008, 7, 3196–3206. [Google Scholar]
- Abou-Arab, E.A.; Abu-Salem, F.M. Effect of natural antioxidants on the stability of ostrich meat during storage. Grasas Aceites 2010, 61, 102–108. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]

| Samples | E-W (%) | BHT (%) | SNE (%) | CLEO (%) | OEO (%) |
|---|---|---|---|---|---|
| Control (+) | 0.01 | - | - | - | - |
| Control (−) | - | 0.01 | - | - | - |
| S1 | - | - | 0.005 | 0.005 | - |
| S2 | - | - | - | 0.01 | - |
| S3 | - | - | 0.01 | - | - |
| S4 | - | - | - | - | 0.01 |
| S5 | - | - | 0.005 | - | 0.005 |
| Samples | aw | pH | COLOR | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| C (+) | 0.948 ± 0.002 a | 6.10 ± 0.03 a | 40.39 ± 10.78 b | 7.69 ± 3.54 b | 15.94 ± 2.42 b |
| C (−) | 0.949 ± 0.003 a | 5.81 ± 0.03 b | 34.96 ± 7.32 b | 10.78 ± 3.55 b | 13.09 ± 4.47 b |
| S1 | 0.950 ± 0.003 a | 5.84 ± 0.03 b | 35.55 ± 6.25 b | 10.98 ± 2.59 b | 12.75 ± 4.37 b |
| S2 | 0.951 ± 0.002 a | 5.86 ± 0.02 b | 34.78 ± 6.75 b | 10.55 ± 4.57 b | 13.57 ± 3.47 b |
| S3 | 0.949 ± 0.003 a | 5.82 ± 0.03 b | 34.45 ± 8.11 b | 10.57 ± 3.55 b | 13.72 ± 5.47 b |
| S4 | 0.951 ± 0.002 a | 5.84 ± 0.03 b | 34.87 ± 9.21 b | 11.03 ± 5.52 b | 13.25 ± 6.53 b |
| S5 | 0.950 ± 0.002 a | 5.83 ± 0.02 b | 34.29 ± 9.18 b | 10.58 ± 4.54 b | 13.73 ± 2.57 b |
| Samples | Color | Odor | Flavor | Overall Acceptability |
|---|---|---|---|---|
| C (+) | 6.8 ± 0.79 a | 6,02 ± 1.03 a | 6.7 ± 0.82 a | 6.7 ± 0.82 a |
| C (−) | 7.5 ± 0.71 a | 7.5 ± 1.08 a | 7.6 ± 0.97 a | 7.7 ± 0.82 a |
| S1 | 7.2 ± 0.92 a | 7.3 ± 0.67 a | 7.5 ± 0.71 a | 7.3 ± 0.82 a |
| S2 | 7.1 ± 1.1 a | 6.8 ± 0.79 a | 7.2 ± 0.79 a | 7.0 ± 1.05 a |
| S3 | 7.1 ± 0.99 a | 7.1 ± 0.57 a | 7.3 ± 0.67 a | 7.2 ± 0.79 a |
| S4 | 7.2 ± 0.92 a | 6.9 ± 0.99 a | 7.3 ± 0.95 a | 7.1 ± 0.74 a |
| S5 | 7.3 ± 1.16 a | 7.0 ± 1.05 a | 7.0 ± 0.67 a | 7.1 ± 0.88 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonušaite, K.; Venskutonis, P.R.; Martínez-Hernández, G.B.; Taboada-Rodríguez, A.; Nieto, G.; López-Gómez, A.; Marín-Iniesta, F. Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers. Foods 2021, 10, 776. https://doi.org/10.3390/foods10040776
Jonušaite K, Venskutonis PR, Martínez-Hernández GB, Taboada-Rodríguez A, Nieto G, López-Gómez A, Marín-Iniesta F. Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers. Foods. 2021; 10(4):776. https://doi.org/10.3390/foods10040776
Chicago/Turabian StyleJonušaite, Kristina, Petras Rimantas Venskutonis, Gines Benito Martínez-Hernández, Amaury Taboada-Rodríguez, Gema Nieto, Antonio López-Gómez, and Fulgencio Marín-Iniesta. 2021. "Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers" Foods 10, no. 4: 776. https://doi.org/10.3390/foods10040776
APA StyleJonušaite, K., Venskutonis, P. R., Martínez-Hernández, G. B., Taboada-Rodríguez, A., Nieto, G., López-Gómez, A., & Marín-Iniesta, F. (2021). Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers. Foods, 10(4), 776. https://doi.org/10.3390/foods10040776












