Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds
Abstract
1. Introduction
2. The Bioactive Composition of Soy and Its Effect in Experimental IBD
2.1. Soy Lipid Fraction
2.1.1. Phospholipids
2.1.2. Soyasaponins
2.1.3. Phytosterols
2.2. Soy Protein Fraction
2.2.1. β–Sitosterol
2.2.2. β-Conglycinin and Glycin
2.2.3. Lectin
2.2.4. Lunasin
2.2.5. Bowman–Birk Inhibitor (BBI)
2.3. Soy Carbohydrate Fraction
Soy Oligosaccharides
2.4. Soy-Derived Isoflavones
2.4.1. Genistein
2.4.2. Equol
3. Mechanisms of Action
3.1. Intestinal Mucosa Permeability
3.2. Oxidative Stress
3.3. Myeloperoxidase (MPO) Activity
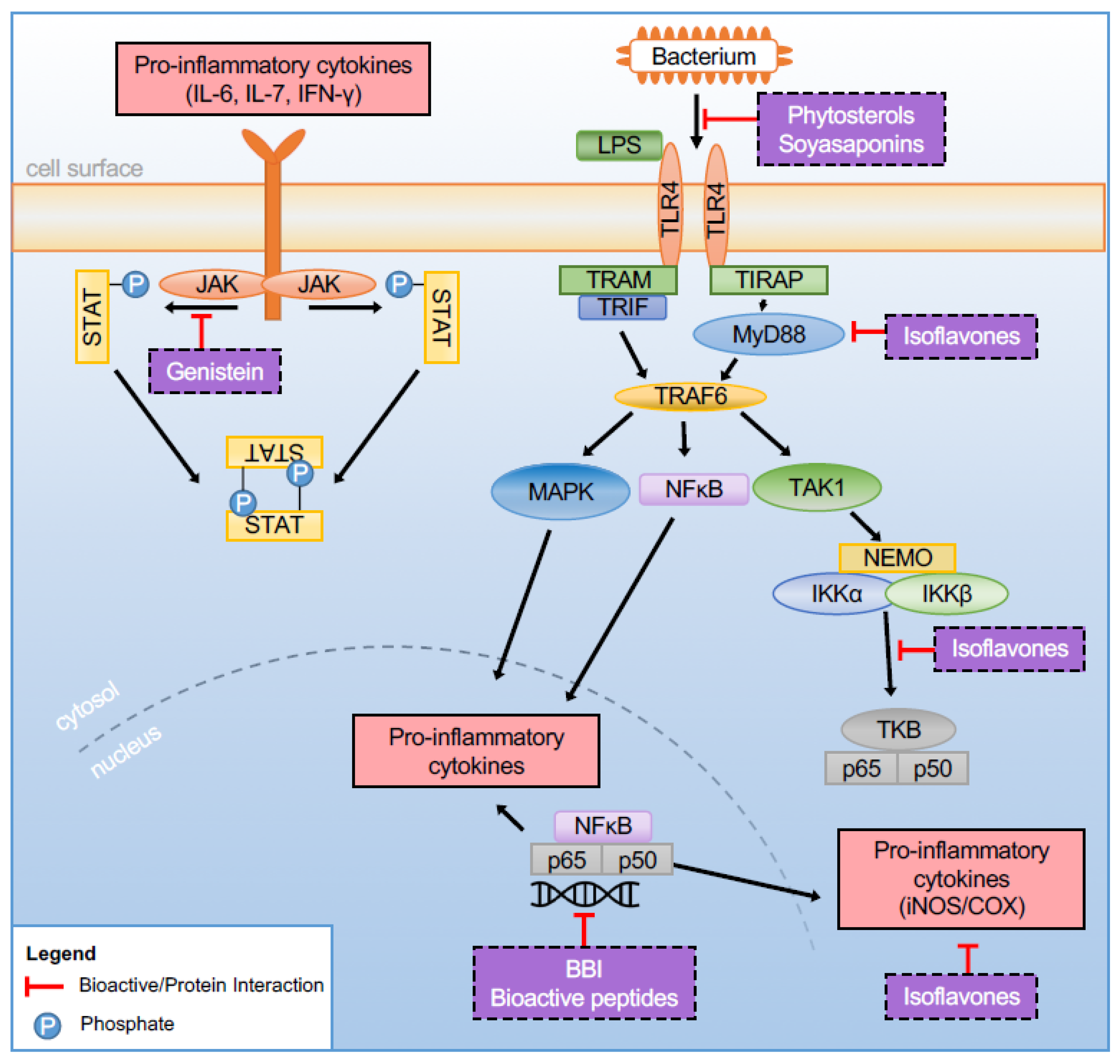
3.4. Pathway Regulation
3.4.1. Cytokines
3.4.2. Cyclooxygenase 2 (COX-2)
3.4.3. Toll-Like Receptors (TLRs)
3.4.4. Peroxisome Proliferator-Activated Receptors (PPARs)
3.5. Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basu, J.; Avila, R.; Ricciardi, R. Hospital Readmission Rates in U.S. States: Are Readmissions Higher Where More Patients with Multiple Chronic Conditions Cluster? Health Serv. Res. 2016, 51, 1135–1151. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hebuterne, X.; Klek, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Ther. Adv. Gastroenterol. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Ishii, H.; Komatsu, M. Recommendation of plant-based diets for inflammatory bowel disease. Transl. Pediatr. 2019, 8, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Metzger, C.E.; Narayanan, S.A.; Zawieja, D.C.; Bloomfield, S.A. A moderately elevated soy protein diet mitigates inflammatory changes in gut and in bone turnover during chronic TNBS-induced inflammatory bowel disease. Appl. Physiol. Nutr. Metab. 2019, 44, 595–605. [Google Scholar] [CrossRef]
- Juritsch, A.F.; Moreau, R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr. Rev. 2018, 76, 618–638. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Wopperer, A.L.; Chrisfield, B.J.; Tao, L.; Cooper, T.K.; Vanamala, J.; Elias, R.J.; Hayes, J.E.; Lambert, J.D. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo. J. Nutr. Biochem. 2017, 40, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; Gomez-Nguyen, A.; LaSalla, A.; Butto, L.; Kulpins, D.; Warner, A.; DiMartino, L.; Ponzani, G.; Osme, A.; Rodriguez-Palacios, A. Replacing Animal Protein with Soy-Pea Protein in an ‘American diet’ Controls Murine Crohn’s Disease-Like Ileitis Regardless of Firmicutes:Bacteroidetes ratio. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Penalvo, J.L.; Gil, J.I.; Medina, S.; Horcajada, M.N.; Lafay, S.; Silberberg, M.; Llorach, R.; Zafrilla, P.; Garcia-Mora, P.; et al. Soy isoflavones and cardiovascular disease epidemiological, clinical and -omics perspectives. Curr. Pharm. Biotechnol. 2012, 13, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Nozue, M.; Shimazu, T.; Charvat, H.; Mori, N.; Mutoh, M.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Inoue, M.; Kokubo, Y.; et al. Fermented soy products intake and risk of cardiovascular disease and total cancer incidence: The Japan Public Health Center-based Prospective study. Eur. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.J.; Persky, V.; Setchell, K.D.; Barnes, S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr. Cancer 1994, 21, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Impact of Soy Foods on the Development of Breast Cancer and the Prognosis of Breast Cancer Patients. Forsch Komplementmed 2016, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Yu, S.; Badger, T.M. Comprehensive phytochemical profile of soy protein isolate. J. Agric. Food Chem. 2004, 52, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Soybean Market Research Report: Market Size, Industry Outlook, Market Forecast, Demand Analysis, Market Share, Market Report 2019–2025. Available online: https://www.industryarc.com/Report/18471/soybean-market-research-report-analysis.html (accessed on 2 January 2021).
- Seibel, J.; Molzberger, A.F.; Hertrampf, T.; Laudenbach-Leschowski, U.; Diel, P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 2009, 48, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Dietary fats and coronary heart disease. J. Intern. Med. 2012, 272, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; Chen, C.; Segal, F.; Trotter, A.; Bederman, I.; Gomez-Nguyen, A.; Sundrud, M.; Ilic, S.; Cominelli, F. Regulation of Intestinal Inflammation by Dietary Fats. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Raphael, W.; Sordillo, L.M. Dietary polyunsaturated fatty acids and inflammation: The role of phospholipid biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Turk, H.F.; Fan, Y.Y.; Callaway, E.; Weeks, B.; Yang, P.; McMurray, D.N.; Chapkin, R.S. Antagonizing arachidonic acid-derived eicosanoids reduces inflammatory Th17 and Th1 cell-mediated inflammation and colitis severity. Mediat. Inflamm 2014, 2014, 917149. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture. Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 21; USDA: Washington, DC, USA, 2008. Available online: https://ndb.nal.usda.gov (accessed on 21 December 2020).
- Gibson, P.R.; Muir, J.G. Reinforcing the mucus: A new therapeutic approach for ulcerative colitis? Gut 2005, 54, 900–903. [Google Scholar] [CrossRef] [PubMed]
- More, M.I.; Freitas, U.; Rutenberg, D. Positive effects of soy lecithin-derived phosphatidylserine plus phosphatidic acid on memory, cognition, daily functioning, and mood in elderly patients with Alzheimer’s disease and dementia. Adv. Ther. 2014, 31, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Kullenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Fabia, R.; Ar’Rajab, A.; Willen, R.; Andersson, R.; Ahren, B.; Larsson, K.; Bengmark, S. Effects of phosphatidylcholine and phosphatidylinositol on acetic-acid-induced colitis in the rat. Digestion 1992, 53, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ehehalt, R.; Wagenblast, J.; Erben, G.; Lehmann, W.D.; Hinz, U.; Merle, U.; Stremmel, W. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand. J. Gastroenterol. 2004, 39, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Chen, J.; Sang, S.; Cheng, S. Biological functionality of soyasaponins and soyasapogenols. J. Agric. Food Chem. 2014, 62, 8247–8255. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Reddy, M.B.; Hendrich, S.; Murphy, P.A. Soyasaponin I and sapongenol B have limited absorption by Caco-2 intestinal cells and limited bioavailability in women. J. Nutr. 2004, 134, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Popovich, D.G. Chemical and biological characterization of oleanane triterpenoids from soy. Molecules 2009, 14, 2959–2975. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Chen, J.; Sun, S.; Mao, L.; Chu, X.; Deng, H.; Cai, J.; Li, X.; Liu, Z.; Cao, W. Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-kB pathway. PLoS ONE 2014, 9, e107655. [Google Scholar] [CrossRef]
- Kim, S.L.; Berhow, M.A.; Kim, J.T.; Chi, H.Y.; Lee, S.J.; Chung, I.M. Evaluation of soyasaponin, isoflavone, protein, lipid, and free sugar accumulation in developing soybean seeds. J. Agric. Food Chem. 2006, 54, 10003–10010. [Google Scholar] [CrossRef] [PubMed]
- Westerlund, C.; Ostlund-Lindqvist, A.M.; Sainsbury, M.; Shertzer, H.G.; Sjoquist, P.O. Characterization of novel indenoindoles. Part, I. Structure-activity relationships in different model systems of lipid peroxidation. Biochem. Pharmacol. 1996, 51, 1397–1402. [Google Scholar] [CrossRef]
- Kang, J.; Badger, T.M.; Ronis, M.J.; Wu, X. Non-isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Sung, M.K.; Kawada, T.; Yoo, H.; Kim, Y.K.; Kim, J.S.; Yu, R. Soybean saponins suppress the release of proinflammatory mediators by LPS-stimulated peritoneal macrophages. Cancer Lett. 2005, 230, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Pateras, I.; Giaginis, C.; Tsigris, C.; Patsouris, E.; Theocharis, S. NF-kappaB signaling at the crossroads of inflammation and atherogenesis: Searching for new therapeutic links. Expert Opin. Ther. Targets 2014, 18, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Yadav, S.S.; Tewari, A.K. Recent insights into NF-kappaB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014, 114, 168–176. [Google Scholar] [CrossRef]
- Lee, I.A.; Park, Y.J.; Joh, E.H.; Kim, D.H. Soyasaponin Ab ameliorates colitis by inhibiting the binding of lipopolysaccharide (LPS) to Toll-like receptor (TLR)4 on macrophages. J. Agric. Food Chem. 2011, 59, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.Y.; Mao, L.M.; Lu, X.C.; Deng, H.; Ye, J.F.; Chu, X.W.; Sun, S.X.; Luo, H.J. Anti-inflammatory effect of soyasaponins through suppressing nitric oxide production in LPS-stimulated RAW 264.7 cells by attenuation of NF-kappaB-mediated nitric oxide synthase expression. Bioorg. Med. Chem. Lett. 2011, 21, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Park, Y.J.; Yeo, H.K.; Han, M.J.; Kim, D.H. Soyasaponin I attenuates TNBS-Induced colitis in mice by inhibiting NF-kappaB pathway. J. Agric. Food Chem. 2010, 58, 10929–10934. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002, 41, 457–500. [Google Scholar] [CrossRef]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Meyer, K.A.; Jacobs, D.R., Jr. Cereals, legumes, and chronic disease risk reduction: Evidence from epidemiologic studies. Am. J. Clin. Nutr. 1999, 70, 451S–458S. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and beta-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Kurano, M.; Iso, O.N.; Hara, M.; Noiri, E.; Koike, K.; Kadowaki, T.; Tsukamoto, K. Plant sterols increased IL-6 and TNF-alpha secretion from macrophages, but to a lesser extent than cholesterol. J. Atheroscler. Thromb. 2011, 18, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; Awad, A.B. beta-Sitosterol down-regulates some pro-inflammatory signal transduction pathways by increasing the activity of tyrosine phosphatase SHP-1 in J774A.1 murine macrophages. Int. Immunopharmacol. 2011, 11, 1012–1017. [Google Scholar] [CrossRef]
- Plat, J.; Hendrikx, T.; Bieghs, V.; Jeurissen, M.L.; Walenbergh, S.M.; Van Gorp, P.J.; De Smet, E.; Konings, M.; Vreugdenhil, A.C.; Guichot, Y.D.; et al. Protective role of plant sterol and stanol esters in liver inflammation: Insights from mice and humans. PLoS ONE 2014, 9, e110758. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhuo, Z.; Fang, S.; Zhang, Y.; Feng, J. Phytosterols improve immunity and exert anti-inflammatory activity in weaned piglets. J. Sci. Food Agric. 2017, 97, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Bouic, P.J. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Hasegawa, K.; Kunimi, M.; Hara, M.; Yatomi, Y.; Teramoto, T.; Tsukamoto, K. Sitosterol prevents obesity-related chronic inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Kim, E.J.; Kim, D.H. Inhibitory effect of beta-sitosterol on TNBS-induced colitis in mice. Planta Med. 2012, 78, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.; Wang, H.; Chen, J.; Luo, Z.; Yang, C.S. beta-Sitosterol and stigmasterol ameliorate dextran sulfate sodium-induced colitis in mice fed a high fat Western-style diet. Food Funct. 2017, 8, 4179–4186. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yang, J.; He, J.; Chen, X.; Zhang, H.; Jia, M.; Liu, K.; Jia, C.; Pan, Y.; Wei, J. Stigmasterol alleviates cerebral ischemia/reperfusion injury by attenuating inflammation and improving antioxidant defenses in rats. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.A.; Taylor, O.A.; Prendergast, D.R.; Zimmerman, T.L.; Von Furstenberg, R.; Moore, D.D.; Karpen, S.J. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr. Res. 2007, 62, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.H.; Kim, J.S.; Kim, J.M.; Kim, N.; Jung, H.C.; Song, I.S. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm. Bowel. Dis. 2006, 12, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef]
- Lee, W.T.; Weisell, R.; Albert, J.; Tome, D.; Kurpad, A.V.; Uauy, R. Research Approaches and Methods for Evaluating the Protein Quality of Human Foods Proposed by an FAO Expert Working Group in 2014. J. Nutr. 2016, 146, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Lee, I.A.; Gu, W.; Hyam, S.R.; Kim, D.H. beta-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-kappaB pathway. Mol. Nutr. Food Res. 2014, 58, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Awaad, A.S.; El-Meligy, R.M.; Al-Jaber, N.A.; Al-Muteeri, H.S.; Zain, M.E.; Alqasoumi, S.I.; Alafeefy, A.M.; Donia Ael, R. Anti-ulcerative colitis activity of compounds from Euphorbia granuleta Forssk. Phytother. Res. 2013, 27, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Torre-Villalvazo, I.; Tovar, A.R. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J. Nutr. Biochem. 2006, 17, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Beaubernard, L.; Lamothe, V.; Bennetau-Pelissero, C. New Evaluation of Isoflavone Exposure in the French Population. Nutrients 2019, 11, 2308. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef]
- Ren, J.; Yang, B.; Lv, Y.; Guo, S. Protective and reparative effects of peptides from soybean beta-conglycinin on mice intestinal mucosa injury. Int. J. Food Sci. Nutr. 2014, 65, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2012, 142, 363–368. [Google Scholar] [CrossRef]
- Shen, C.L.; Chen, W.H.; Zou, S.X. In vitro and in vivo effects of hydrolysates from conglycinin on intestinal microbial community of mice after Escherichia coli infection. J. Appl. Microbiol. 2007, 102, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, X.; Bao, X.; Lv, Y.; Zhang, J.; Guo, S. Glycopeptide derived from soybean β-conglycinin inhibits the adhesion of Escherichia coli and Salmonella to human intestinal cells. Food Res. Int. 2008, 41. [Google Scholar] [CrossRef]
- Chatterjee, C.; Liu, J.; Wood, C.; Gagnon, C.; Cober, E.R.; Fregeau-Reid, J.A.; Gleddie, S.; Xiao, C.W. The alpha’ subunit of beta-conglycinin and various glycinin subunits of soy are not required to modulate hepatic lipid metabolism in rats. Eur. J. Nutr. 2018, 57, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Kohno, S.; Yama, T.; Ochi, A.; Suto, T.; Hirasaka, K.; Ohno, A.; Teshima-Kondo, S.; Okumura, Y.; Oarada, M.; et al. Soy Glycinin Contains a Functional Inhibitory Sequence against Muscle-Atrophy-Associated Ubiquitin Ligase Cbl-b. Int. J. Endocrinol. 2013, 2013, 907565. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta 2012, 1820, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A. The oligopeptide transporter (Pept-1) in human intestine: Biology and function. Gastroenterology 1997, 113, 332–340. [Google Scholar] [CrossRef]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Maebuchi, M.; Samoto, M.; Kohno, M.; Ito, R.; Koikeda, T.; Hirotsuka, M.; Nakabou, Y. Improvement in the intestinal absorption of soy protein by enzymatic digestion to oligopeptide in healthy adult men. Food Sci. Technol. Res. 2007, 13, 45–53. [Google Scholar] [CrossRef]
- King, T.P.; Begbie, R.; Cadenhead, A. Nutritional toxicity of raw kidney beans in pigs. Immunocytochemical and cytopathological studies on the gut and the pancreas. J. Sci. Food Agric. 1983, 34, 1404–1412. [Google Scholar] [CrossRef]
- Pan, L.; Farouk, M.H.; Qin, G.; Zhao, Y.; Bao, N. The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals. Int. J. Mol. Sci. 2018, 19, 554. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Behera, B.; Bhol, C.S.; Dey, S.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K.; et al. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Terashima, S.; Takano, Y.; Ohori, T.; Kanno, T.; Kimura, T.; Motoki, R.; Kawaguchi, T. Soybean agglutinin binding as a useful prognostic indicator in stomach cancer. Surg. Today 1997, 27, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ye, X.; Ng, T. Purification of melibiose-binding lectins from two cultivars of Chinese black soybeans. Acta Biochim. Biophys. Sin. 2008, 40, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, Y.; Yuan, Z.; Farouk, M.H.; Zhang, S.; Bao, N.; Qin, G. The Integrins Involved in Soybean Agglutinin-Induced Cell Cycle Alterations in IPEC-J2. Mol. Cells 2017, 40, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Qin, G.; Zhao, Y.; Wang, J.; Liu, F.; Che, D. Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets. Int. J. Mol. Sci. 2013, 14, 21689–21704. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.; Pusztai, A. Toxicity of kidney bean (Phaseolus vulgaris) in rats: Changes in intestinal permeability. Digestion 1985, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Reisner, Y.; Sharon, N. Fractionation of subpopulations of mouse and human lymphocytes by peanut agglutinin or soybean agglutinin. Methods Enzymol. 1984, 108, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A.; Grant, G.; Spencer, R.J.; Duguid, T.J.; Brown, D.S.; Ewen, S.W.; Peumans, W.J.; Van Damme, E.J.; Bardocz, S. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. J. Appl. Bacteriol. 1993, 75, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, G.; Sun, Z.; Che, D.; Bao, N.; Zhang, X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011, 12, 8502–8512. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.F.; Figueiredo, R.C.; Henriques, M.G.; Barja-Fidalgo, C. Inflammatory and anti-inflammatory effects of soybean agglutinin. Braz. J. Med. Biol. Res. 1997, 30, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.; van Drtessche, E. Legume lectins. Physicochemlcal and nutritional properties. In Recent Advances of Research in Ant-nutritional Factors in Legume Seeds; van der Poel, A., Huisman, J., Sam, H., Eds.; Wagenmgen Pers: Wagenmgen, The Netherlands, 1993; pp. 219–234. [Google Scholar]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 2018, 107, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Lopez, O.; Harry, G. Changes in Selected Chemical and Antinutritional Components during Tempeh Preparation Using Fresh and Hardened Common Beans. J. Food Sci. 1989, 54. [Google Scholar] [CrossRef]
- Odani, S.; Koide, T.; Ono, T. Amino acid sequence of a soybean (Glycine max) seed polypeptide having a poly(L-aspartic acid) structure. J. Biol. Chem. 1987, 262, 10502–10505. [Google Scholar] [CrossRef]
- Liu, J.; Jia, S.H.; Kirberger, M.; Chen, N. Lunasin as a promising health-beneficial peptide. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2070–2075. [Google Scholar] [PubMed]
- Dia, V.P.; Gonzalez de Mejia, E. Lunasin potentiates the effect of oxaliplatin preventing outgrowth of colon cancer metastasis, binds to alpha5beta1 integrin and suppresses FAK/ERK/NF-kappaB signaling. Cancer Lett. 2011, 313, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Lule, V.K.; Garg, S.; Pophaly, S.D.; Hitesh; Tomar, S.K. “Potential health benefits of lunasin: A multifaceted soy-derived bioactive peptide”. J. Food Sci. 2015, 80, R485–R494. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Ben, O.; Hsieh, C.-C. 1997-2012: Fifteen years of research on peptide lunasin. In Bioactive Food Peptides in Health and Disease; InTech Open: London, UK, 2013; pp. 3–22. [Google Scholar]
- Dia, V.; Wang, W.; Oh, V.; De Lumen, B.; De Mejia, E. Isolation, purification and characterisation of lunasin from defatted soybean flour and in vitro evaluation of its anti-inflammatory activity. Food Chem. 2009, 114, 108–115. [Google Scholar] [CrossRef]
- Hernandez-Ledesma, B.; Hsieh, C.C.; De Lumen, B.O. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Dia, V.P. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-kappaB pathway in the macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Kusmardi, K.; Nessa, N.; Estuningtyas, A.; Tedjo, A. The effect of lunasin from Indonesian soybean extract on histopatologic examination and cox-2 expression in dextran sodium sulfate-induced mice colon. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 154–162. [Google Scholar] [PubMed]
- Suzuki, R.; Kohno, H.; Sugie, S.; Nakagama, H.; Tanaka, T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis 2006, 27, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Torres, S.; De Lumen, B.O.; Erdman, J.W., Jr.; De Mejia, E.G. Presence of lunasin in plasma of men after soy protein consumption. J. Agric. Food Chem. 2009, 57, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Sonnante, G.; Domoney, C. Bowman-Birk inhibitors from legumes and human gastrointestinal health: Current status and perspectives. Curr. Protein Pept. Sci. 2011, 12, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Jiménez, E.; Marín-Manzan, M.; Rubio, L. Active Bowman-Birk inhibitors survive gastrointestinal digestion at the terminal ileum of pigs fed chickpea-based diets. J. Sci. Food Agric. 2008, 88, 523–531. [Google Scholar] [CrossRef]
- Wan, X.S.; Lu, L.J.; Anderson, K.E.; Ware, J.H.; Kennedy, A.R. Urinary excretion of Bowman-Birk inhibitor in humans after soy consumption as determined by a monoclonal antibody-based immunoassay. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 741–747. [Google Scholar] [PubMed]
- Fang, E.F.; Wong, J.H.; Ng, T.B. Thermostable Kunitz trypsin inhibitor with cytokine inducing, antitumor and HIV-1 reverse transcriptase inhibitory activities from Korean large black soybeans. J. Biosci. Bioeng. 2010, 109, 211–217. [Google Scholar] [CrossRef]
- Muzard, J.; Fields, C.; O’Mahony, J.J.; Lee, G.U. Probing the soybean Bowman-Birk inhibitor using recombinant antibody fragments. J. Agric. Food Chem. 2012, 60, 6164–6172. [Google Scholar] [CrossRef]
- Hackler, L.; Van Buren, J.; Steinkraus, K.; el Rawi, I.; Hand, D. Effect of Heat Treatment on Nutritive Value of Soymilk Protein Fed to Weanling Rats. J. Food Sci. 1964, 30, 723–728. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Szuhaj, B.F.; Newberne, P.M.; Billings, P.C. Preparation and production of a cancer chemopreventive agent, Bowman-Birk inhibitor concentrate. Nutr. Cancer 1993, 19, 281–302. [Google Scholar] [CrossRef]
- Yavelow, J.; Collins, M.; Birk, Y.; Troll, W.; Kennedy, A.R. Nanomolar concentrations of Bowman-Birk soybean protease inhibitor suppress x-ray-induced transformation in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 5395–5399. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R. Chemopreventive agents: Protease inhibitors. Pharmacol. Ther. 1998, 78, 167–209. [Google Scholar] [CrossRef]
- Safavi, F.; Rostami, A. Role of serine proteases in inflammation: Bowman-Birk protease inhibitor (BBI) as a potential therapy for autoimmune diseases. Exp. Mol. Pathol. 2012, 93, 428–433. [Google Scholar] [CrossRef]
- Ware, J.H.; Wan, X.S.; Newberne, P.; Kennedy, A.R. Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig. Dis. Sci. 1999, 44, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.C.; Zhou, R.H.; Wang, X.; Li, J.L.; Sang, M.; Zhou, L.; Zhuang, K.; Hou, W.; Guo, D.Y.; Ho, W.Z. Soybean-derived Bowman-Birk Inhibitor (BBI) Inhibits HIV Replication in Macrophages. Sci. Rep. 2016, 6, 34752. [Google Scholar] [CrossRef] [PubMed]
- Moussa, L.; Bezirard, V.; Salvador-Cartier, C.; Bacquie, V.; Lencina, C.; Leveque, M.; Braniste, V.; Menard, S.; Theodorou, V.; Houdeau, E. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS ONE 2012, 7, e49547. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, L.; Cook, D.R.; Wang, X.; Liu, J.; Kolson, D.L.; Persidsky, Y.; Ho, W.Z. Soybean-derived Bowman-Birk inhibitor inhibits neurotoxicity of LPS-activated macrophages. J. Neuroinflamm. 2011, 8, 15. [Google Scholar] [CrossRef]
- Dai, H.; Ciric, B.; Zhang, G.X.; Rostami, A. Interleukin-10 plays a crucial role in suppression of experimental autoimmune encephalomyelitis by Bowman-Birk inhibitor. J. Neuroimmunol. 2012, 245, 1–7. [Google Scholar] [CrossRef]
- Safavi, F.; Rasouli, J.; Mari, E.; Zhang, G.; Rostami, A. Bowman-Birk protease inhibitor (BBI) induces IL10 production in human T cells and suppresses effector phase of experimental autoimmune encephalomyelitis (EAE) by Tr1 induction. Mult. Scler. J. 2013, 19, 233. [Google Scholar]
- Zhou, X.L.; Kong, X.F.; Yang, X.J.; Yin, Y.L. Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J. Anim. Sci. 2012, 90 (Suppl. 4), 37–39. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Giovanni, V.; Meng, X. Effects of soybean oligosaccharides on intestinal microbial communities and immune modulation in mice. Saudi J. Biol. Sci. 2017, 24, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, J.A. Prebiotics promote good health: The basis, the potential, and the emerging evidence. J. Clin. Gastroenterol. 2004, 38, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Stahl, B.; Jelinek, J.; Knol, J.; Miniello, V.; Moro, G.E. Prebiotic carbohydrates in human milk and formulas. Acta Paediatr. Suppl. 2005, 94, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chao, Y.; Wan, Q. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar]
- Xu, C.; Liang, A.; Zhu, Y.; Tao, S.Y. Immunopotentiating effect of soybean oligosaccharides. Pharm. J. Chin. People’s Lib. Army 2005, 21, 37–39. [Google Scholar]
- Celiberto, L.S.; Bedani, R.; Dejani, N.N.; Ivo de Medeiros, A.; Sampaio Zuanon, J.A.; Spolidorio, L.C.; Tallarico Adorno, M.A.; Amancio Varesche, M.B.; Carrilho Galvao, F.; Valentini, S.R.; et al. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. PLoS ONE 2017, 12, e0175935. [Google Scholar] [CrossRef]
- Tamura, K.; Sasaki, H.; Shiga, K.; Miyakawa, H.; Shibata, S. The Timing Effects of Soy Protein Intake on Mice Gut Microbiota. Nutrients 2019, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Murphy, P.A.; Barua, K.; Hauck, C.C. Solvent extraction selection in the determination of isoflavones in soy foods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 129–138. [Google Scholar] [CrossRef]
- Nielsen, I.L.; Williamson, G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr. Cancer 2007, 57, 1–10. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Sang, L.X.; Chang, B. Isoflavones and inflammatory bowel disease. World J. Clin. Cases 2020, 8, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Mueller, M.; Jungbauer, A. Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutrients 2010, 2, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131, 1362S–1375S. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharm. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 423S–430S. [Google Scholar] [CrossRef]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wang, H.W.; Su, H.Y.; Hao, N.J. The structure-activity relationships of flavonoids as inhibitors of cytochrome P-450 enzymes in rat liver microsomes and the mutagenicity of 2-amino-3-methyl-imidazo[4,5-f]quinoline. Mutagenesis 1994, 9, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Chemler, J.A.; Lim, C.G.; Daiss, J.L.; Koffas, M.A. A versatile microbial system for biosynthesis of novel polyphenols with altered estrogen receptor binding activity. Chem. Biol. 2010, 17, 392–401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aso, T. Equol improves menopausal symptoms in Japanese women. J. Nutr. 2010, 140, 1386S–1389S. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp. Ther. Med. 2017, 14, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Moussa, L.; Bezirard, V.; Salvador-Cartier, C.; Bacquie, V.; Houdeau, E.; Theodorou, V. A new soy germ fermented ingredient displays estrogenic and protease inhibitor activities able to prevent irritable bowel syndrome-like symptoms in stressed female rats. Clin. Nutr. 2013, 32, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Echizen, K.; Hirose, O.; Maeda, Y.; Oshima, M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016, 107, 391–397. [Google Scholar] [CrossRef]
- Inui, K.; Fukuta, Y.; Ikeda, A.; Kameda, H.; Kokuba, Y.; Sato, M. The effect of alpha-linolenic acid-rich emulsion on fatty acid metabolism and leukotriene generation of the colon in a rat model with inflammatory bowel disease. Ann. Nutr. Metab. 1996, 40, 175–182. [Google Scholar] [CrossRef]
- Liu, J.; Chang, S.K.; Wiesenborn, D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J. Agric. Food Chem. 2005, 53, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wei, H. Effect of dietary genistein on antioxidant enzyme activities in SENCAR mice. Nutr. Cancer 1996, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 2009, 587, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Peterson, T.G.; Barnes, S. Mechanisms of action of the soy isoflavone genistein: Emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 1998, 68, 1418S–1425S. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Zafar, S.F.; El-Rayes, B.F. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr. Rev. 2013, 71, 562–572. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Montani, C.; Romani, A.; Vignolini, P.; Pampaloni, B.; Tanini, A.; Brandi, M.L.; Alonso-Magdalena, P.; Nadal, A.; Ottobrini, L.; et al. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 2006, 147, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Babu, P.V.; Si, H.; Nallasamy, P.; Zhu, H.; Zhen, W.; Misra, H.P.; Li, Y.; Liu, D. Genistein inhibits TNF-alpha-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. Int. J. Cardiol. 2013, 168, 2637–2645. [Google Scholar] [CrossRef]
- Khan, A.Q.; Khan, R.; Rehman, M.U.; Lateef, A.; Tahir, M.; Ali, F.; Sultana, S. Soy isoflavones (daidzein & genistein) inhibit 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced cutaneous inflammation via modulation of COX-2 and NF-kappaB in Swiss albino mice. Toxicology 2012, 302, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Kannappan, S.; Anuradha, C.V. Genistein modulates NF-kappaB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur. J. Pharmacol. 2011, 667, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, J.; Zhao, J.; Chen, Y. Genistein improves inflammatory response and colonic function through NF-kappaB signal in DSS-induced colonic injury. Oncotarget 2017, 8, 61385–61392. [Google Scholar] [CrossRef] [PubMed]
- Abron, J.D.; Singh, N.P.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS ONE 2018, 13, e0199631. [Google Scholar] [CrossRef]
- Chiriac, M.; Buchen, B.; Wandersee, A.; Hundorfean, G.; Günther, C.; Bourjau, Y.; Doyle, S.E.; Frey, B.; Ekici, A.B.; Büttner, C.; et al. Activation of Epithelial Signal Transducer and Activator of Transcription 1 by Interleukin 28 Controls Mucosal Healing in Mice With Colitis and Is Increased in Mucosa of Patients With Inflammatory Bowel Disease. Gastroenterology 2017, 153, 123–138.e128. [Google Scholar] [CrossRef] [PubMed]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine 2009, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 45673. [Google Scholar] [CrossRef]
- Paradkar, P.N.; Blum, P.S.; Berhow, M.A.; Baumann, H.; Kuo, S.-M. Dietary isoflavones suppress endotoxin-induced inflammatory reaction in liver and intestine. Cancer Lett. 2004, 215. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Seo, S.W.; Park, S.J.; Ka, S.O.; Na, L.; Kim, K.A.; Ryu, D.G.; So, H.S.; et al. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol. Cell Endocrinol. 2007, 278, 18–28. [Google Scholar] [CrossRef]
- Gao, F.; Wei, D.; Bian, T.; Xie, P.; Zou, J.; Mu, H.; Zhang, B.; Zhou, X. Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology 2012, 89, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xu, Y.; Xie, J.; Sun, C.; Zheng, X.; Chen, W. Systematic evaluation of phenolic compounds and protective capacity of a new mulberry cultivar J33 against palmitic acid-induced lipotoxicity using a simulated digestion method. Food Chem. 2018, 258, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Bhargava, A.; Bhanot, V.; Gupta, R.; Jain, N.; Panwar, J. Formation and Characterization of Protein Corona Around Nanoparticles: A Review. J. Nanosci. Nanotechnol. 2018, 18, 6653–6670. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C.; Young, E.R.; Tam, B.; Kosobud, A.; Belknap, J.K.; Laursen, S.E. Genetic differences in anticonvulsant sensitivity in mouse lines selectively bred for ethanol withdrawal severity. J. Pharmacol. Exp. Ther. 1986, 239, 154–159. [Google Scholar] [PubMed]
- Vanden Braber, N.L.; Novotny Nunez, I.; Bohl, L.; Porporatto, C.; Nazar, F.N.; Montenegro, M.A.; Correa, S.G. Soy genistein administered in soluble chitosan microcapsules maintains antioxidant activity and limits intestinal inflammation. J. Nutr. Biochem. 2018, 62, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef]
- Shor, D.; Sathyapalan, T.; Atkin, S.L.; Thatcher, N.J. Does equol production determine soy endocrine effects? Eur. J. Nutr. 2012, 51, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Potter, J.; Kroon, P.A.; Wilson, P.; Dhatariya, K.; Sampson, M.; Cassidy, A. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; McGrath, B.P.; DeSilva, L.; Cehun, M.; Fassoulakis, A.; Nestel, P.J. Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arter. Thromb. Vasc. Biol. 2003, 23, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Hazim, S.; Curtis, P.J.; Schar, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.; Fujii, A.; Zhang, L. An isoflavone metabolite reduces arterial stiffness and blood pressure in overweight men and postmenopausal women. Atherosclerosis 2007, 192, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef]
- Calvello, R.; Aresta, A.; Trapani, A.; Zambonin, C.; Cianciulli, A.; Salvatore, R.; Clodoveo, M.L.; Corbo, F.; Franchini, C.; Panaro, M.A. Bovine and soybean milk bioactive compounds: Effects on inflammatory response of human intestinal Caco-2 cells. Food Chem. 2016, 210, 276–285. [Google Scholar] [CrossRef]
- Sakai, T.; Furoku, S.; Nakamoto, M.; Shuto, E.; Hosaka, T.; Nishioka, Y.; Sone, S. Soy isoflavone equol perpetuates dextran sulfate sodium-induced acute colitis in mice. Biosci. Biotechnol. Biochem. 2011, 75, 593–595. [Google Scholar] [CrossRef]
- Lampe, J.W.; Karr, S.C.; Hutchins, A.M.; Slavin, J.L. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc. Soc. Exp. Biol. Med. 1998, 217, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Plaut, A.G. Trefoil peptides in the defense of the gastrointestinal tract. N. Engl. J. Med. 1997, 336, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Ota, H.; Akamatsu, T.; Sugiyama, A.; Katsuyama, T. Histochemistry of the surface mucous gel layer of the human colon. Gut 1997, 40, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Gerova, V.A.; Stoynov, S.G.; Katsarov, D.S.; Svinarov, D.A. Increased intestinal permeability in inflammatory bowel diseases assessed by iohexol test. World J. Gastroenterol. 2011, 17, 2211–2215. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Bruewer, M.; Nusrat, A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr. Opin. Gastroenterol. 2006, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef]
- Woo, J.K.; Choi, S.; Kang, J.H.; Kim, D.E.; Hurh, B.S.; Jeon, J.E.; Kim, S.Y.; Oh, S.H. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement. Altern Med. 2016, 16, 498. [Google Scholar] [CrossRef]
- Soderholm, J.D.; Streutker, C.; Yang, P.C.; Paterson, C.; Singh, P.K.; McKay, D.M.; Sherman, P.M.; Croitoru, K.; Perdue, M.H. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumour necrosis factor alpha. Gut 2004, 53, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Garcia-Villar, R.; Ferrier, L.; Larauche, M.; Vergnolle, N.; Bunnett, N.W.; Coelho, A.M.; Fioramonti, J.; Bueno, L. Proteinase-activated receptor-2-induced colonic inflammation in mice: Possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J. Immunol. 2003, 170, 4296–4300. [Google Scholar] [CrossRef]
- Cenac, N.; Andrews, C.N.; Holzhausen, M.; Chapman, K.; Cottrell, G.; Andrade-Gordon, P.; Steinhoff, M.; Barbara, G.; Beck, P.; Bunnett, N.W.; et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Investig. 2007, 117, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Annahazi, A.; Ferrier, L.; Bezirard, V.; Leveque, M.; Eutamene, H.; Ait-Belgnaoui, A.; Coeffier, M.; Ducrotte, P.; Roka, R.; Inczefi, O.; et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am. J. Gastroenterol. 2013, 108, 1322–1331. [Google Scholar] [CrossRef]
- Annahazi, A.; Gecse, K.; Dabek, M.; Ait-Belgnaoui, A.; Rosztoczy, A.; Roka, R.; Molnar, T.; Theodorou, V.; Wittmann, T.; Bueno, L.; et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 2009, 144, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.; Roka, R.; Ferrier, L.; Leveque, M.; Eutamene, H.; Cartier, C.; Ait-Belgnaoui, A.; Rosztoczy, A.; Izbeki, F.; Fioramonti, J.; et al. Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008, 57, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Roka, R.; Rosztoczy, A.; Leveque, M.; Izbeki, F.; Nagy, F.; Molnar, T.; Lonovics, J.; Garcia-Villar, R.; Fioramonti, J.; Wittmann, T.; et al. A pilot study of fecal serine-protease activity: A pathophysiologic factor in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2007, 5, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.J.; Eakin, M.N.; Czyzewski, D.I.; Jarrett, M.; Ou, C.N. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J. Pediatr. 2008, 153, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Negri, G.; De Paula, J.A.; Di Carlo, M.; Yapur, V.; Facente, A.; De Paula, A. Colonic proteinases: Increased activity in patients with ulcerative colitis. Medicina 1998, 58, 262–264. [Google Scholar] [PubMed]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef]
- Jiang, H.; Przybyszewski, J.; Mitra, D.; Becker, C.; Brehm-Stecher, B.; Tentinger, A.; MacDonald, R.S. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-alpha in colon of dextran sodium sulfate-treated C57BL/6 mice. J. Nutr. 2011, 141, 1239–1246. [Google Scholar] [CrossRef]
- Zeng, B.; Wang, D.; Wang, H.; Chen, T.; Luo, J.; Xi, Q.; Sun, J.; Zhang, Y. Dietary Soy Protein Isolate Attenuates Intestinal Immunoglobulin and Mucin Expression in Young Mice Compared with Casein. Nutrients 2020, 12, 2739. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B.; et al. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef] [PubMed]
- Utama, Z.; Okazaki, Y.; Tomotake, H.; Kato, N. Tempe consumption modulates fecal secondary bile acids, mucins, immunoglobulin A, enzyme activities, and cecal microflora and organic acids in rats. Plant. Foods Hum. Nutr. 2013, 68, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; de Sousa Moraes, L.F.; Lebow, N.; Zhu, M.J. Dietary Green Pea Protects against DSS-Induced Colitis in Mice Challenged with High-Fat Diet. Nutrients 2017, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hanyou, N.; Sonaka, I.; Minami, H. An elemental diet controls inflammation in indomethacin-induced small bowel disease in rats: The role of low dietary fat and the elimination of dietary proteins. Dig. Dis. Sci. 2005, 50, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kwon, S.J.; Ju, J.; Bose, M.; Lee, M.J.; Hong, J.; Hao, X.; Yang, C.S. Effect of genistein on the bioavailability and intestinal cancer chemopreventive activity of (-)-epigallocatechin-3-gallate. Carcinogenesis 2008, 29, 2019–2024. [Google Scholar] [CrossRef]
- Lazarevic-Pasti, T.; Leskovac, A.; Vasic, V. Myeloperoxidase Inhibitors as Potential Drugs. Curr. Drug. Metab. 2015, 16, 168–190. [Google Scholar] [CrossRef]
- Koratkar, R.; Rao, A.V. Effect of soya bean saponins on azoxymethane-induced preneoplastic lesions in the colon of mice. Nutr. Cancer 1997, 27, 206–209. [Google Scholar] [CrossRef]
- Lopez, P.; Sanchez, M.; Perez-Cruz, C.; Velazquez-Villegas, L.A.; Syeda, T.; Aguilar-Lopez, M.; Rocha-Viggiano, A.K.; Del Carmen Silva-Lucero, M.; Torre-Villalvazo, I.; Noriega, L.G.; et al. Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxemia, and Cognitive Function in Mice Fed a High-Fat Diet. Mol. Nutr. Food Res. 2018, 62, e1800313. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Choi, J.H.; Seo, J.H.; Lim, Y.I.; Park, K.Y. Anti-colitic effects of kanjangs (fermented soy sauce and sesame sauce) in dextran sulfate sodium-induced colitis in mice. J. Med. Food 2014, 17, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bryant, C.E.; Doyle, S.L. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol. Rev. 2009, 61, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lin, Q.; Han, L.; Wang, Z.; Luo, M.; Kang, W.; Liu, J.; Wang, J.; Ma, T.; Liu, H. Soy hull dietary fiber alleviates inflammation in BALB/C mice by modulating the gut microbiota and suppressing the TLR-4/NF-kappaB signaling pathway. Food Funct. 2020, 11, 5965–5975. [Google Scholar] [CrossRef] [PubMed]
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef]
- Mezei, O.; Banz, W.J.; Steger, R.W.; Peluso, M.R.; Winters, T.A.; Shay, N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003, 133, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, M.L.; Moore, D.D.; Banz, W.J.; Mezei, O.; Shay, N.F. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J. Nutr. Biochem. 2005, 16, 321–330. [Google Scholar] [CrossRef]
- Ronis, M.J.; Chen, Y.; Badeaux, J.; Badger, T.M. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 2009, 139, 1431–1438. [Google Scholar] [CrossRef]
- Tovar, A.R.; Torre-Villalvazo, I.; Ochoa, M.; Elias, A.L.; Ortiz, V.; Aguilar-Salinas, C.A.; Torres, N. Soy protein reduces hepatic lipotoxicity in hyperinsulinemic obese Zucker fa/fa rats. J. Lipid Res. 2005, 46, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Lopez, L.; Tovar, A.R.; Gonzalez-Granillo, M.; Hernandez-Pando, R.; Escalante, B.; Santillan-Doherty, P.; Torres, N. Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J. Biol. Chem. 2007, 282, 20657–20666. [Google Scholar] [CrossRef] [PubMed]
- Larcher, J.C.; Vayssiere, J.L.; Le Marquer, F.J.; Cordeau, L.R.; Keane, P.E.; Bachy, A.; Gros, F.; Croizat, B.P. Effects of peripheral benzodiazepines upon the O2 consumption of neuroblastoma cells. Eur. J. Pharmacol. 1989, 161, 197–202. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Lewis, J.D.; Wu, G.D. Food and the gut microbiota in inflammatory bowel diseases: A critical connection. Curr. Opin. Gastroenterol. 2012, 28, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Varankovich, N.; Grigoryan, A.; Brown, K.; Inglis, G.D.; Uwiera, R.R.E.; Nickerson, M.T.; Korber, D.R. Pea-protein alginate encapsulation adversely affects development of clinical signs of Citrobacter rodentium-induced colitis in mice treated with probiotics. Can. J. Microbiol. 2018, 64, 744–760. [Google Scholar] [CrossRef] [PubMed]
- Butteiger, D.N.; Hibberd, A.A.; McGraw, N.J.; Napawan, N.; Hall-Porter, J.M.; Krul, E.S. Soy Protein Compared with Milk Protein in a Western Diet Increases Gut Microbial Diversity and Reduces Serum Lipids in Golden Syrian Hamsters. J. Nutr. 2016, 146, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Panasevich, M.R.; Schuster, C.M.; Phillips, K.E.; Meers, G.M.; Chintapalli, S.V.; Wankhade, U.D.; Shankar, K.; Butteiger, D.N.; Krul, E.S.; Thyfault, J.P.; et al. Soy compared with milk protein in a Western diet changes fecal microbiota and decreases hepatic steatosis in obese OLETF rats. J. Nutr. Biochem. 2017, 46, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef]
- Murakami, Y.; Tanabe, S.; Suzuki, T. High-fat Diet-induced Intestinal Hyperpermeability is Associated with Increased Bile Acids in the Large Intestine of Mice. J. Food Sci. 2016, 81, H216–H222. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Palau-Rodriguez, M.; Tulipani, S.; Isabel Queipo-Ortuno, M.; Urpi-Sarda, M.; Tinahones, F.J.; Andres-Lacueva, C. Metabolomic insights into the intricate gut microbial-host interaction in the development of obesity and type 2 diabetes. Front. Microbiol. 2015, 6, 1151. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Santoro, P.; Barone, M.V.; Pappacoda, S.; Barretta, M.L.; Nanayakkara, M.; Apicella, C.; Capasso, L.; Paludetto, R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G906–G913. [Google Scholar] [CrossRef] [PubMed]
- Lajczak-McGinley, N.K.; Porru, E.; Fallon, C.M.; Smyth, J.; Curley, C.; McCarron, P.A.; Tambuwala, M.M.; Roda, A.; Keely, S.J. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol. Rep. 2020, 8, e14456. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Ni Gabhann, J.; Franco, P.; Tambuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Florez, A.B.; Guadamuro, L.; Mayo, B. Effect of Soy Isoflavones on Growth of Representative Bacterial Species from the Human Gut. Nutrients 2017, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Liu, X.Z.; Pan, W.; Zou, D.J. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 2017, 15, 2765–2787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yufeng, Z.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Nat. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- O’Malley, D.; Julio-Pieper, M.; Gibney, S.M.; Dinan, T.G.; Cryan, J.F. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress-induced anxiety and depression-like behaviour. Stress 2010, 13, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef]
- Tsinberg, M.B.; Deriabin, D.G.; Denisova, I.V.; Nikiian, A.N. Growth and morphological characteristics of industrial strains of Bifidobacterium and Lactobacillus cultivated in hydrolysate-milk and hydrolysate-soybean media. Antibiot. Khimioter. 2003, 48, 9–13. [Google Scholar] [PubMed]
- Pham, T.T.; Shah, N.P. Fermentation of reconstituted skim milk supplemented with soy protein isolate by probiotic organisms. J. Food Sci. 2008, 73, M62–M66. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Nemoto, M.; Nakata, T.; Kondo, S.; Takahashi, H.; Kimura, B.; Kuda, T. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int. Immunopharmacol. 2015, 26, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Yazaki, T.; Ono, M.; Takahashi, H.; Kimura, B. In vitro cholesterol-lowering properties of Lactobacillus plantarum AN6 isolated from aji-narezushi. Lett. Appl. Microbiol. 2013, 57, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ye, L.; Du, G.; Huang, Y.; Wu, Y.; Ge, S.; Yang, Z.; Zhu, G. Effects of curcumin plus Soy oligosaccharides on intestinal flora of rats with ulcerative colitis. Cell Mol. Biol. 2017, 63, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kuda, T.; An, C.; Kanno, T.; Takahashi, H.; Kimura, B. Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe 2012, 18, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Kawahara, M.; Nemoto, M.; Takahashi, H.; Kimura, B. In vitro antioxidant and anti-inflammation properties of lactic acid bacteria isolated from fish intestines and fermented fish from the Sanriku Satoumi region in Japan. Food Res. Int. 2014, 64, 248–255. [Google Scholar] [CrossRef]
- Levit, R.; de Giori, G.S.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Evaluation of the effect of soymilk fermented by a riboflavin-producing Lactobacillus plantarum strain in a murine model of colitis. Benef. Microbes 2017, 8, 65–72. [Google Scholar] [CrossRef]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Protective effect of the riboflavin-overproducing strain Lactobacillus plantarum CRL2130 on intestinal mucositis in mice. Nutrition 2018, 54, 165–172. [Google Scholar] [CrossRef]
- Jeong, J.K.; Chang, H.K.; Park, K.Y. Doenjang prepared with mixed starter cultures attenuates azoxymethane and dextran sulfate sodium-induced colitis-associated colon carcinogenesis in mice. J. Carcinog. 2014, 13, 9. [Google Scholar] [CrossRef]
- Jeong, J.K.; Chang, H.K.; Park, K.Y. Inhibitory effects of meju prepared with mixed starter cultures on azoxymethane and dextran sulfate sodium-induced colon carcinogenesis in mice. J. Carcinog. 2012, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.; LaSalla, A.; Lam, G.; Kulpins, D.; Moen, E.; Sundrud, M.; Miyoshi, J.; Ilic, S.; Theriault, B.; Cominelli, F.; et al. Artificial microbiome heterogeneity spurs six practical action themes and examples to increase study power-driven reproducibility. Sci. Rep. 2019, 10, 5039. [Google Scholar] [CrossRef] [PubMed]
| Bioactive | Effects | Mechanisms |
|---|---|---|
| Phosphatidylcholine | Blocks hydrophobic bacteria and hydrophilic antigens from entering the intestine; improves mucus layer integrity and mucus secretion; ↓ oxidative stress | Mechanisms not described in detail |
| Soyasaponins | Antioxidant, anti-inflammatory, and immunomodulatory activity | Inhibit LPS binding to TLR4, NF-κB, and iNOS inhibition |
| Phytosterols | Anti-inflammatory and anti-oxidative effects; FXR antagonist (stigmasterol) | NF-κB inhibition and COX-2 downregulation |
| β-conglycinin and Glycin | Maintain intestinal mucosa integrity; improve epithelial cell growth; inhibit enteropathogen adhesion (E. coli, S. typhimurium and S. enteritidis); ↓ MPO | NF-kB/p65 inhibition |
| Lectin | Antibacterial, antifungal, and antiviral activities; disrupt gut barrier function; induce local inflammatory responses; ↓ immunological response; interfere with the balance of the intestinal microbiota | By binding to small bowel epithelial cells; serving as a nutrient source of bacteria; altering the gut mucosal system |
| Lunasin | Suppresses LPS-induced inflammatory reactions in macrophage, decrease pro-inflammatory cytokine production | Suppress PGE2 via COX-2, and NF-κB inhibition |
| Equol | ↓ NO production; antioxidant and estrogenic activity | Inhibition of iNOS mRNA expression, ↓ NF-kB activation |
| Bowman-Birk Inhibitor (BBI) | Anti-inflammatory activity in the gut; suppress oxidative stress; decrease pro- IL-1β, TNF-α, IL-6, and increase IL-10 in macrophages | Inhibition of serine proteases released from inflammation-mediating cells |
| Soy Oligosaccharides | Benefit immune function by promoting the metabolism of beneficial commensal gut bacteria; increase levels of SOD and IgG; promote splenocyte proliferation; increase abundance in SCFA-producing bacterial taxa | Enhanced T-lymphocyte and lymphocyte proliferation |
| Genistein | Inhibit TNF-ɑ-induced endothelial and vascular inflammation; improve cell viability and cellular permeability; convert M1 macrophages toward the M2 phenotype | Mediation of protein kinase pathway; NF-κB inhibition; activation of the JAK signal transduction and transcription (STAT) pathway |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods 2021, 10, 774. https://doi.org/10.3390/foods10040774
Basson AR, Ahmed S, Almutairi R, Seo B, Cominelli F. Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods. 2021; 10(4):774. https://doi.org/10.3390/foods10040774
Chicago/Turabian StyleBasson, Abigail Raffner, Saleh Ahmed, Rawan Almutairi, Brian Seo, and Fabio Cominelli. 2021. "Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds" Foods 10, no. 4: 774. https://doi.org/10.3390/foods10040774
APA StyleBasson, A. R., Ahmed, S., Almutairi, R., Seo, B., & Cominelli, F. (2021). Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods, 10(4), 774. https://doi.org/10.3390/foods10040774






