Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Raw Material
2.3. Raw Material Chemical Composition
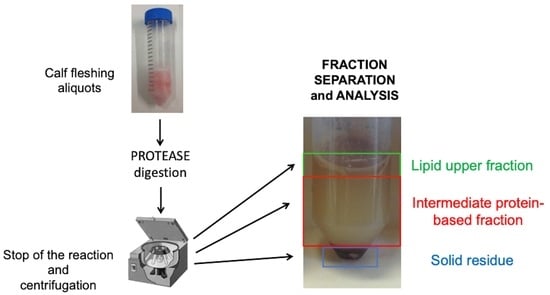
2.4. Proteases Digestion
2.5. Quantification of Free and Total Amino Acids, SDS-PAGE and Degree of Hydrolysis (DH) Analyses
2.6. Peptide Identification by Linear Trap Quadrupole (LTQ)-Orbitrap Analyses
2.7. Fatty Acid Analysis
2.8. Antioxidant and Anti-Tyrosinase Activity Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Calf Fleshing Bulk Characterization
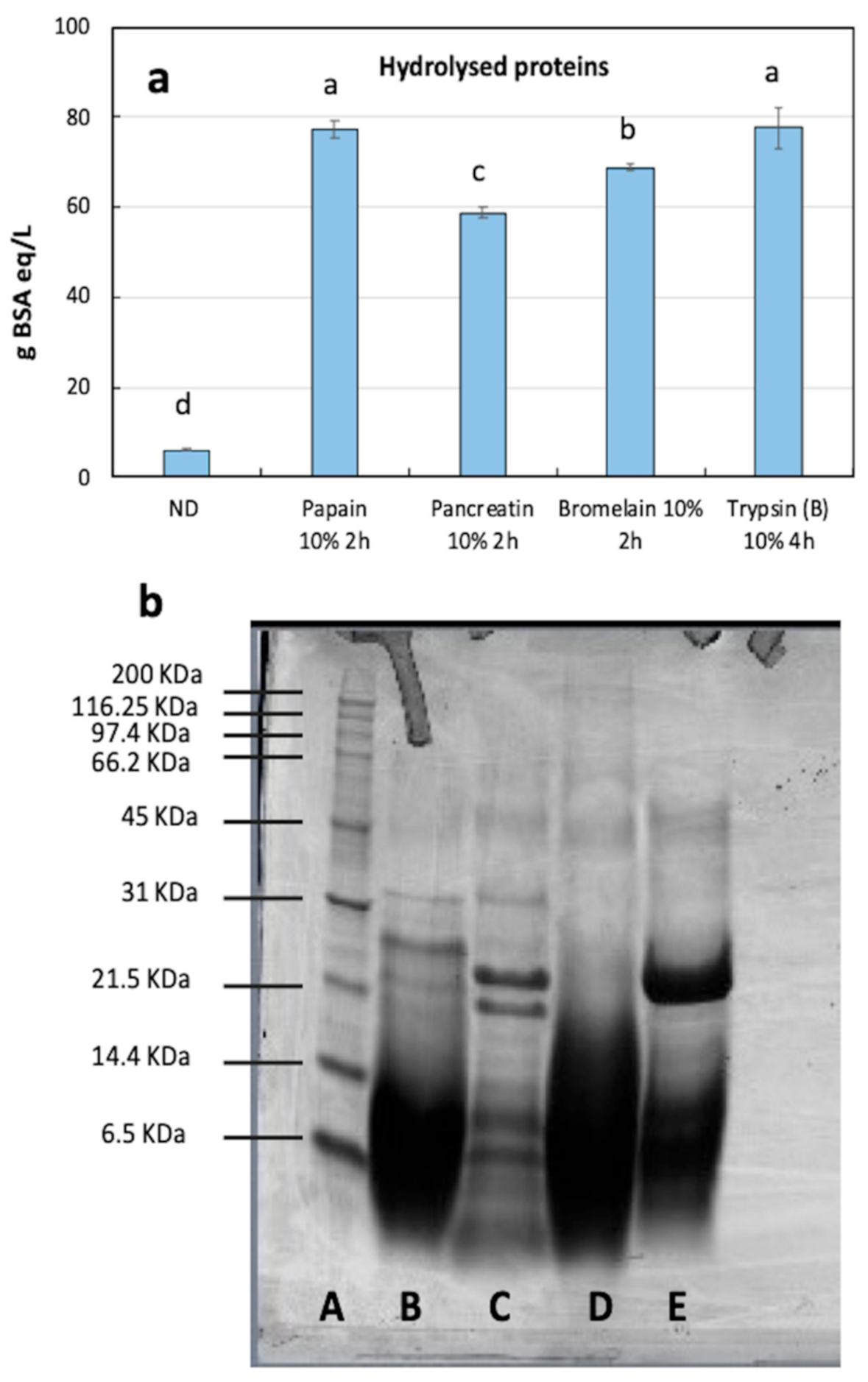
3.2. Optimization of the Enzymatic Reaction
3.3. Protein Fraction Analysis
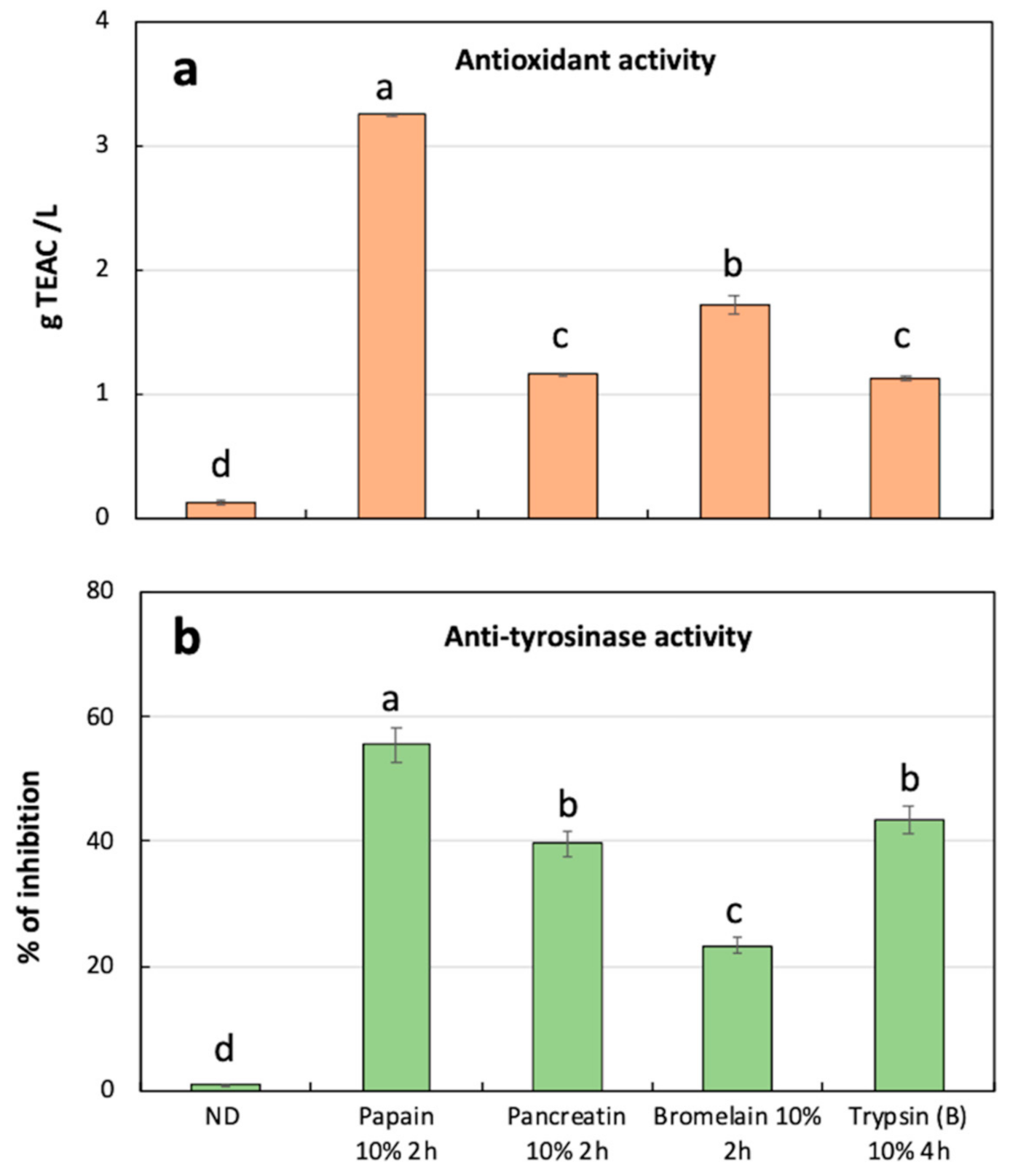
3.4. Antioxidant and Anti-Tyrosinase Activity
3.5. Fatty Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Alternative Pathways to 2050. Available online: http://tools.foodsecurityportal.org/fao-global-perspectives-studies 2019 (accessed on 20 January 2021).
- FAO. SAVE FOOD: Global Initiative on Food Loss and Waste Reduction. 2021. Available online: http://www.fao.org/save-food/resources/en/ (accessed on 20 January 2021).
- Ockerman, H.W.; Hansen, C.L. Introduction and history of processing animal by-products. In Animal By-Products Processing and Utilization; Ockerman, H.W., Hansen, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–22. [Google Scholar]
- Russ, W.; Meyer-Pittroff, R. Utilizing waste products from the food production and processing industries. Crit. Rev. Food Sci. Nutr. 2004, 44, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of by-products and waste materials from meat, poultry and fish processing industries: a review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Alvarez, C. Opportunities and perspectives for utilisation of co-products in the meat industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Bajza, Z.; Vrucek, V. Thermal and enzymatic recovering of proteins from untanned leather waste. Waste Manag. 2001, 21, 79–84. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Olsen, K.; Grossi, A.; Otte, J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013, 141, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Damrongsakkul, S.; Ratanathammapan, K.; Komolpis, K.; Tanthapanichakoon, W. Enzymatic hydrolysis of rawhide using papain and neutrase. J. Ind. Eng. Chem. 2008, 14, 202–206. [Google Scholar] [CrossRef]
- Jian, S.; Wenyi, T.; Wuyong, C. Ultrasound-accelerated enzymatic hydrolysis of solid leather waste. J. Clean Prod. 2008, 16, 591–597. [Google Scholar] [CrossRef]
- Anzani, C.; Prandi, B.; Tedeschi, T.; Baldinelli, C.; Sorlini, G.; Wierenga, P.A.; Dossena, A.; Sforza, S. Degradation of collagen increases nitrogen solubilisation during enzymatic hydrolysis of fleshing meat. Waste Biomass. Valor. 2018, 9, 1113–1119. [Google Scholar] [CrossRef]
- Toldra, F.; Aristoy, M.C.; Mora, L.; Reig, M. Innovations in value-addition of edible meat by-products. Meat Sci. 2012, 92, 290–296. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Arihara, K.; Ohata, M. Functional meat products. In Handbook of Meat Processing; Toldrá, F., Ed.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 423–439. [Google Scholar]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Wang, B.-S.; Chang, L.-W.; Wu, H.-C.; Huang, S.-L.; Chu, H.-L.; Huang, M.-H. Antioxidant and anti-tyrosinase activity of aqueous extracts of green asparagus. Food Chem. 2011, 127, 141–146. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, W. Antioxidant, antibrowning, and cytoprotective activities of Ligustrum robustum (Rxob.) Blume extract. J. Food Sci. Technol. 2013, 78, C1354–1362. [Google Scholar]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, in vitrophotoprotective and skin-aging related enzyme inhibitory activities of Malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Protopappa, A.; Megoulas, N.; Skaltsa, H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorgan. Med. Chem. 2007, 15, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Graen-Heedfeld, J.; Bretz, K.; Guillon, F.; Michelini, E.; Calabretta, M.M.; Lamborghini, M.; Gruarin, N.; Roda, A.; Kraft, A.; et al. Peptide fractions obtained from rice by-products by means of an environment-friendly process show in vitro health-related bioactivities. PLoS ONE 2017, 12, e0170954. [Google Scholar] [CrossRef]
- Schurink, M.; van Berkel, W.; Wichers, H.; Boeriu, C. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef]
- Mapiye, C.; Dugan, M.E.R.; Juarez, M.; Basarab, J.A.; Baron, V.S.; Turner, T.; Yang, X.; Aldai, N.; Aalhus, J.L. Influence of alpha-tocopherol supplementation on trans-18:1 and conjugated linoleic acid profiles in beef from steers fed a barley-based diet. Animal 2012, 6, 1888–1896. [Google Scholar] [CrossRef]
- Marzocchi, S.; Pasini, F.; Baldinelli, C.; Caboni, M.F. Value-addition of beef meat by-products: Lipid characterization by chromatographic techniques. J. Oleo Sci. 2018, 67, 143–150. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis. 2002. Available online: http://www.aoac.org/ (accessed on 14 December 2020).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Verardo, V.; Gomez-Caravaca, A.M.; Gori, A.; Losi, G.; Caboni, M.F. Bioactive lipids in the butter production chain from Parmigiano Reggiano cheese area. J. Sci. Food Agr. 2013, 93, 3625–3633. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Rai, A.K.; Nived, C.; Sakhare, P.Z.; Suresh, P.V.; Bhaskar, N.; Mahendrakar, N.S. Optimization of acid hydrolysis conditions of delimed tannery fleshings by response surface method. J. Sci. Ind. Res. India 2009, 68, 967–974. [Google Scholar]
- Bhaskar, N.; Sakhare, P.Z.; Suresh, P.V.; Gowda, L.R.; Mahendrakar, N.S. Biostabilization and preparation of protein hydrolysates from delimed leather fleshings. J. Sci. Ind. Res. India 2007, 66, 1054–1063. [Google Scholar]
- USDA-United States Department of Agriculture. Agricultural Research Service, National Nutrient Database for Standard Reference Legacy. Available online: https://fdc.nal.usda.gov (accessed on 14 December 2020).
- White, J.S.; White, D.C. Source Book of Enzymes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Olsen, J.V.; Ong, S.E.; Mann, M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef]
- Haslaniza, H.; Maskat, M.Y.; Aida, W.M.W.; Mamot, S.; Saadiah, I. Optimization of enzymatic hydrolysis of cockle (Anadara Granosa) meat wash water precipitate for the development of seafood flavor. Int. Food Res. J. 2013, 20, 3053–3059. [Google Scholar]
- Ryder, K.; Bekhit, A.E.D.; McConnell, M.; Carne, A. Towards generation of bioactive peptides from meat industry waste proteins: Generation of peptides using commercial microbial proteases. Food Chem. 2016, 208, 42–50. [Google Scholar] [CrossRef]
- Choi, J.S.; Moon, H.E.; Roh, M.K.; Ha, V.M.; Lee, B.B.; Cho, K.K.; Choi, I.S. Physiological properties of Scomber japonicus meat hydrolysate prepared by subcritical water hydrolysis. J. Environ. Biol. 2016, 37, 57–63. [Google Scholar]
- Baggio, S.R.; Bragagnolo, N. The effect of heat treatment on the cholesterol oxides, cholesterol, total lipid and fatty acid contents of processed meat products. Food Chem. 2006, 95, 611–619. [Google Scholar] [CrossRef]
- Muchenje, V.; Hugo, A.; Dzama, K.; Chimonyo, M.; Strydom, P.E.; Raats, J.G. Cholesterol levels and fatty acid profiles of beef from three cattle breeds raised on natural pasture. J. Food Compos. Anal. 2009, 22, 354–358. [Google Scholar] [CrossRef]
- Brugiapaglia, A.; Lussiana, C.; Destefanis, G. Fatty acid profile and cholesterol content of beef at retail of Piemontese, Limousin and Friesian breeds. Meat Sci. 2014, 96, 568–573. [Google Scholar] [CrossRef] [PubMed]
| Amino Acids | % on Total Amino Acids | Reference (%) |
|---|---|---|
| Hyp | 12.1 ± 0.6 | 1.4 |
| Asp | 7.0 ± 0.5 | 9.4 |
| Ser | 3.2 ± 0.1 | 4.2 |
| Glu | 13.2 ± 0.7 | 15.6 |
| Gly | 8.2 ± 0.6 | 7.1 |
| His | 2.2 ± 0.3 | 3.4 |
| Arg | 6.3 ± 0.5 | 6.8 |
| Thr | 3.4 ± 0.3 | 4.0 |
| Ala | 5.3 ± 0.8 | 6.5 |
| Pro | 10.7 ± 1.2 | 5.3 |
| Tyr | 2.7 ± 0.4 | 3.2 |
| Val | 3.3 ± 0.2 | 5.1 |
| Met | 3.4 ± 0.3 | 2.7 |
| Lys | 6.6 ± 0.4 | 8.6 |
| Ile | 3.3 ± 0.3 | 4.6 |
| Leu | 5.8 ± 0.5 | 8.1 |
| Phe | 3.4 ± 0.3 | 4.1 |
| Amino Acids | Papain | Pancreatin | Bromelain | Trypsin (B) |
|---|---|---|---|---|
| Hyp | 3.46 ± 0.33 b | 3.54 ± 0.07 b | 2.79 ± 0.60 b | 5.75 ± 0.30 a |
| Asp | 0.14 ± 0.03 c | 1.81 ± 0.01 a | 0.81 ± 0.01 b | 1.78 ± 0.36 a |
| Ser | 3.55 ± 0.06 a | 4.17 ± 0.66 a | 3.73 ± 0.20 a | 1.75 ± 0.01 b |
| Glu | 4.66 ± 0.04 b | 6.86 ± 0.25 a | 2.44 ± 0.37 c | 2.77 ± 0.05 c |
| Gly | 9.00 ± 0.02 a | 1.67 ± 0.15 c | 3.27 ± 0.13 b | 1.57 ± 0.04 c |
| His | 2.62 ± 0.06 c | 5.80 ± 0.08 a | 4.65 ± 0.16 b | 2.47 ± 0.02 c |
| Arg | 14.58 ± 0.29 a | 16.63 ± 1.69 a | 3.74 ± 0.34 b | 14.27 ± 1.04 a |
| Thr | 2.93 ± 0.08 b | 3.79 ± 0.07 b | 8.37 ± 0.24 a | 3.48 ± 0.05 b |
| Ala | 3.44 ± 0.03 b | 3.70 ± 0.67 a | 2.29 ± 0.09 b | 3.28 ± 0.80 b |
| Pro | 7.67 ± 0.04 b | 1.32 ± 0.23 c | 11.89 ± 0.62 a | 1.36 ± 0.21 c |
| Tyr | 7.89 ± 0.21 a | 8.93 ± 0.86 a | 4.96 ± 0.47 b | 7.52 ± 0.73 a |
| Val | 1.85 ± 0.09 c | 4.43 ± 0.15 a | 3.08 ± 0.01 b | 3.46 ± 0.69 b |
| Met | 3.93 ± 0.13 b | 4.20 ± 0.41 b | 4.55 ± 0.22 b | 5.69 ± 0.03 a |
| Lys | 15.36 ± 0.13 b | 14.89 ± 1.84 b | 10.55 ± 0.06 c | 22.51 ± 1.89 a |
| Ile | 3.96 ± 0.12 b | 4.05 ± 0.20 b | 19.26 ± 0.23 a | 4.47 ± 0.69 b |
| Leu | 11.20 ± 0.47 a | 7.82 ± 0.33 c | 9.04 ± 0.16 b | 9.45 ± 0.38 b |
| Phe | 3.75 ± 0.33 c | 6.40 ± 0.58 b | 4.59 ± 0.09 c | 8.40 ± 0.45 a |
| Enzyme | Identified Peptides | Parent Protein | Average Peptide Length (N°AA) |
|---|---|---|---|
| Papain | 78 | Collagen alpha-1(I) chain | 14 |
| 71 | Actin, alpha skeletal muscle | 11 | |
| 56 | Collagen alpha-2(I) chain | 13 | |
| 54 | Collagen alpha-1(II) chain | 12 | |
| 30 | Myosin-1 | 11 | |
| Pancreatin | 70 | Titin | 12 |
| 57 | Collagen alpha-1(I) chain | 11 | |
| 53 | Collagen alpha-2(I) chain | 10 | |
| 29 | Collagen, type III, alpha 1 | 14 | |
| 24 | IgG heavy chain | 18 | |
| Bromelain | 24 | Collagen alpha-1(I) | 9 |
| 17 | Hemoglobin subunit beta | 23 | |
| 15 | Hemoglobin subunit alpha | 25 | |
| 15 | Collagen alpha-2(I) chain | 9 | |
| 13 | Myosin light chain 1/3, skeletal muscle isoform | 13 | |
| Trypsin (B) | 59 | Titin | 11 |
| 46 | Myosin-2 | 11 | |
| 28 | Myosin-1 | 10 | |
| 23 | Collagen alpha-1(I) chain | 13 | |
| 10 | Myosin-7 | 12 |
| FA | Papain | Pancreatin | Bromelain | Trypsin (B) |
|---|---|---|---|---|
| C12:0 | 0.69 ± 0.00 | n.d. | n.d | 0.51 ± 0.02 |
| C14:0 | 5.82 ± 0.09 | 5.88 ± 0.04 | 5.66 ± 0.30 | 5.41 ± 0.33 |
| C14:1c | 2.30 ± 0.19 | 2.67 ± 0.56 | 3.79 ± 0.15 | 2.14 ± 0.01 |
| C15:0 | 0.31 ± 0.01 | n.d | n.d | 0.28 ± 0.00 |
| C16:0 | 24.66 ± 0.03 | 23.99 ± 1.95 | 26.55 ± 0.38 | 24.72 ± 0.19 |
| C16:1 t | 0.37 ± 0.02 | n.d | n.d | 0.27 ± 0.07 |
| C16:1 c | 5.80 ± 0.02 | 5.89 ± 0.60 | 5.88 ± 0.96 | 5.92 ± 0.28 |
| C17:0 | 0.58 ± 0.01 | n.d | n.d | 0.59 ± 0.07 |
| C17:1 | 0,61 ± 0.04 | n.d | n.d | 0.64 ± 0.10 |
| C18:0 | 7.77 ± 0.03 | 9.91 ± 0.66 | 8.21 ± 0.47 | 7.59 ± 0.40 |
| C18:1 t9 | 1.56 ± 0.11 | n.d | n.d | n.d |
| C18:1 c9 | 40.52 ± 0.02 | 39.59 ± 4.69 | 42.80 ± 1.97 | 41.61 ± 1.37 |
| C18:1 c11 | 1.99 ± 0.14 | 2.06 ± 0.42 | 2.12 ± 0.32 | 2.13 ± 0.10 |
| C18:2 n6 | 4.91 ± 0.15 | 4.78 ± 0.38 | 4.99 ± 0.95 | 4.38 ± 0.60 |
| C18:3 n3 | 0.71 ± 0.08 | n.d | n.d | 0.90 ± 0.08 |
| C20:0 | 0.64 ± 0.06 a | n.d | n.d | 0.42 ± 0.01 b |
| C20:3 n6 | 0.76 ± 0.66 | 5.24 ± 0.77 | n.d | 0.85 ± 0.10 |
| SFA | 40.46 ± 0.08 | 39.77 ± 0.75 | 40.41 ± 1.16 | 39.52 ± 0.98 |
| MUFA | 53.16 ± 0.51 | 50.20 ± 3.15 | 54.60 ± 2.11 | 54.36 ± 1.40 |
| PUFA | 6.39 ± 0.59 b | 10.03 ± 2.39 a | 4.99 ± 0.95 c | 6.12 ± 0.42 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, T.; Anzani, C.; Ferri, M.; Marzocchi, S.; Caboni, M.F.; Monari, S.; Tassoni, A. Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids. Foods 2021, 10, 755. https://doi.org/10.3390/foods10040755
Tedeschi T, Anzani C, Ferri M, Marzocchi S, Caboni MF, Monari S, Tassoni A. Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids. Foods. 2021; 10(4):755. https://doi.org/10.3390/foods10040755
Chicago/Turabian StyleTedeschi, Tullia, Cecilia Anzani, Maura Ferri, Silvia Marzocchi, Maria Fiorenza Caboni, Stefania Monari, and Annalisa Tassoni. 2021. "Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids" Foods 10, no. 4: 755. https://doi.org/10.3390/foods10040755
APA StyleTedeschi, T., Anzani, C., Ferri, M., Marzocchi, S., Caboni, M. F., Monari, S., & Tassoni, A. (2021). Enzymatic Digestion of Calf Fleshing Meat By-Products: Antioxidant and Anti-Tyrosinase Activity of Protein Hydrolysates, and Identification of Fatty Acids. Foods, 10(4), 755. https://doi.org/10.3390/foods10040755