Bioactive Compounds and Evaluation of Antioxidant, Cytotoxic and Cytoprotective Effects of Murici Pulp Extracts (Byrsonima crassifolia) Obtained by Supercritical Extraction in HepG2 Cells Treated with H2O2
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sample Preparation
2.2. Sequential Supercritical Extractions: CO2–SFE (Extraction with Supercritical CO2) and CO2+EtOH–SFE (Extraction with Supercritical CO2 and Ethanol)
2.3. Characterization of the Defatted Pulps and Extracts
2.3.1. Lutein Content
2.3.2. Phenolic Compounds
2.3.3. Flavonoid Content
2.3.4. Fatty Acids and Functional Potential
2.3.5. Probable Triglyceride Compositions
2.3.6. Oxygen Radical Absorbance Capacity (ORAC)
2.3.7. Antioxidant Activity (DPPH)
2.4. Cell Culture
2.5. In Vitro Evaluation of Cytotoxicity and Cytoprotection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Global Yield Isotherms
3.2. Lutein Content
3.3. Phenolic Compounds
3.4. Flavonoid Content
3.5. Fatty Acids and Functional Quality
3.6. Probable Triglyceride Composition
3.7. Oxygen Radical Absorbance Capacity (ORAC)
3.8. Antioxidant Capacity (DPPH)
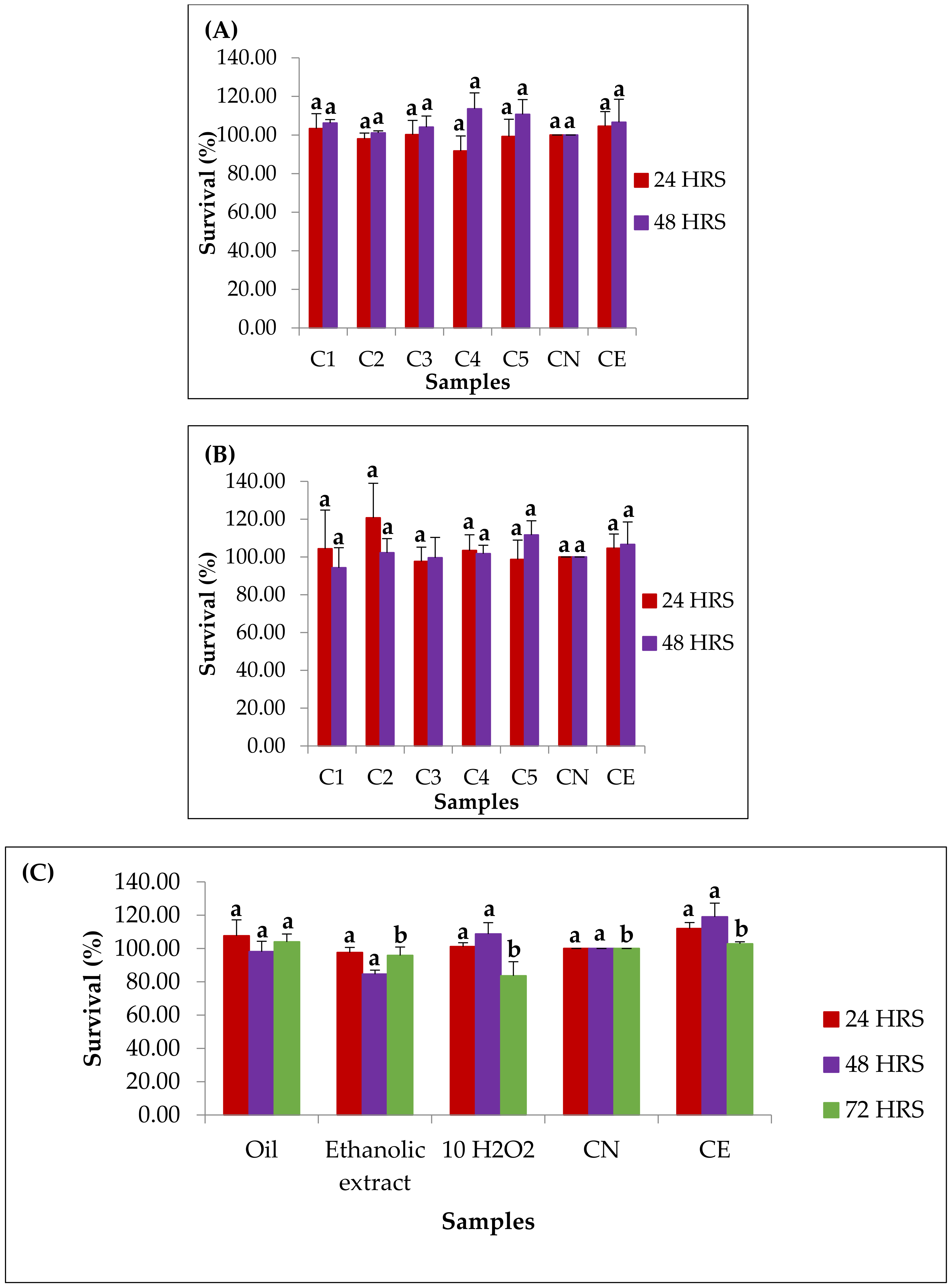
3.9. In Vitro Evaluation of Cytotoxicity and Cytoprotection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Gallic Acid Concentration (mg/L) | Absorbance * |
|---|---|
| White | 0.094 |
| 0.9 | 0.093 |
| 1.8 | 0.188 |
| 2.5 | 0.262 |
| 6.3 | 0.647 |
| 8.3 | 0.828 |
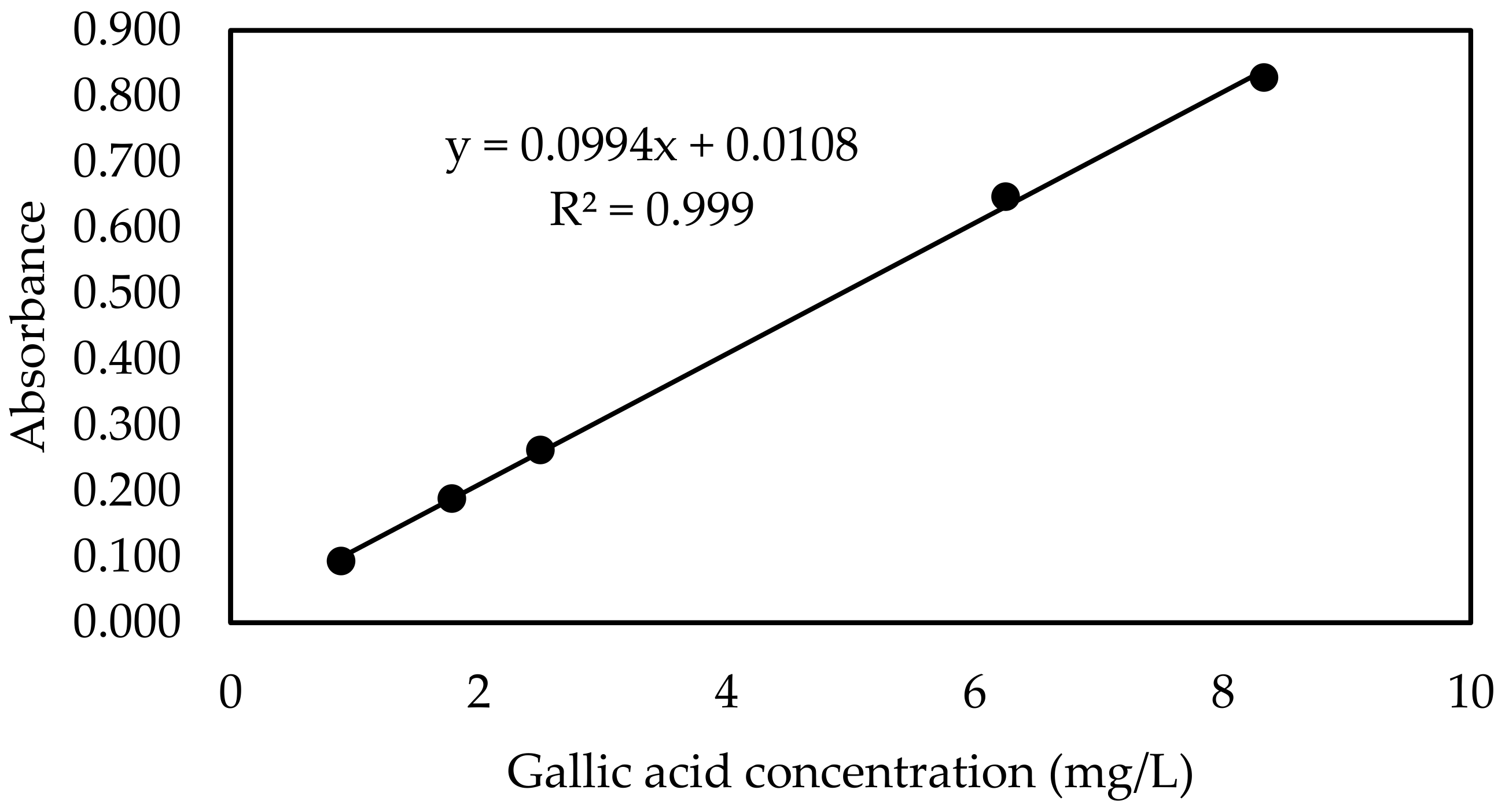
Appendix B
| Quercetin Concentration (mg/L) | Absorbance * |
|---|---|
| White | 0.087 |
| 0.2 | 0.098 |
| 0.5 | 0.233 |
| 2.4 | 0.400 |
| 4.5 | 0.537 |
| 6.5 | 0.669 |
| 8.3 | 0.803 |
| 10.0 | 0.896 |
| 11.5 | 0.973 |
| 13.0 | 0.087 |
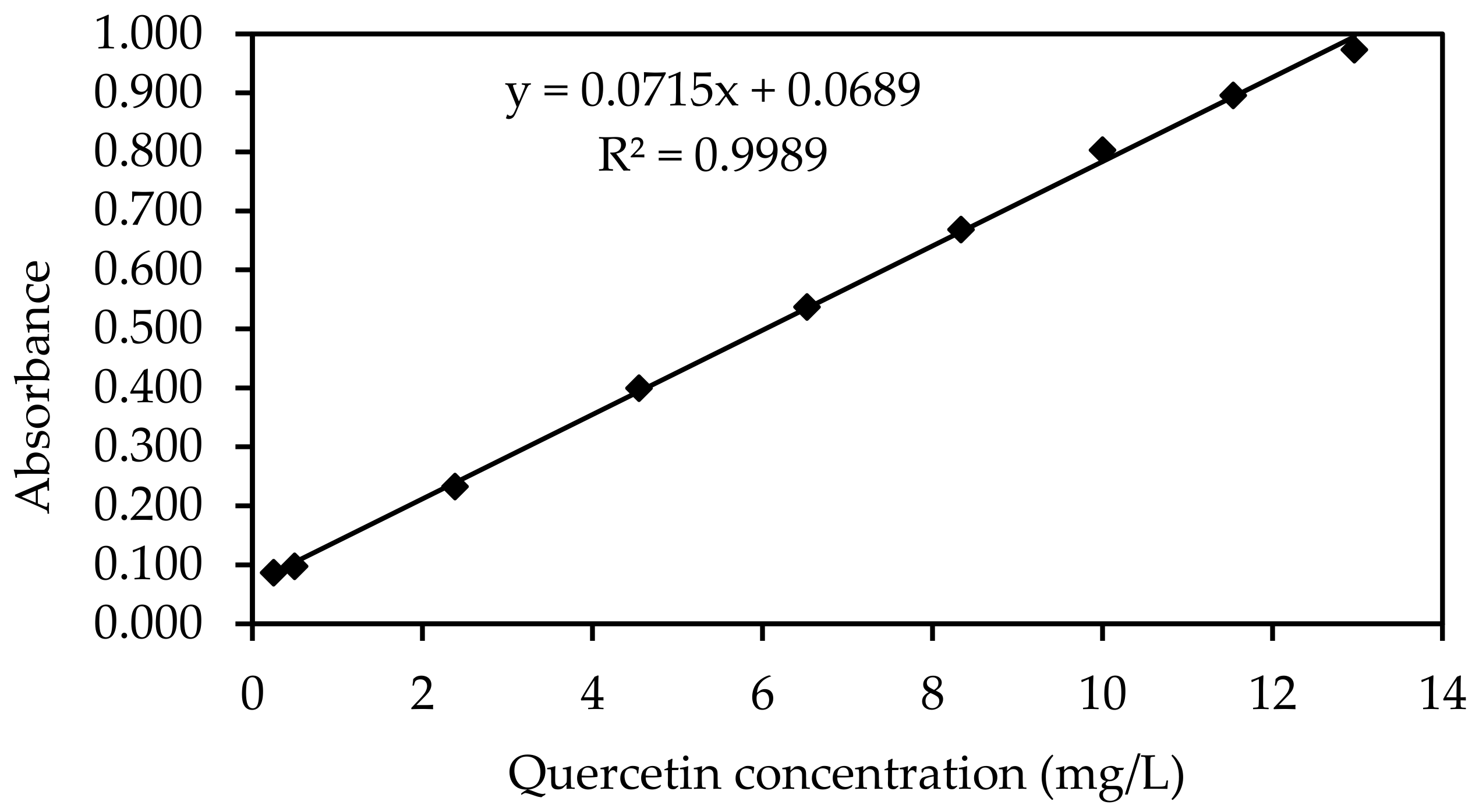
Appendix C
| Trolox Concentration (µmol TE/L) | NET AUC * |
|---|---|
| 0 | 0.921 |
| 0.631 | |
| 0.204 | |
| 0.253 | |
| 1.400 | |
| 1 | 3.774 |
| 3.939 | |
| 3.470 | |
| 3.456 | |
| 2 | 6.449 |
| 6.407 | |
| 5.987 | |
| 5.963 | |
| 6.388 | |
| 5.611 | |
| 4 | 11.491 |
| 11.145 | |
| 11.440 | |
| 11.291 | |
| 8 | 20.761 |
| 19.823 | |
| 21.661 | |
| 20.461 |
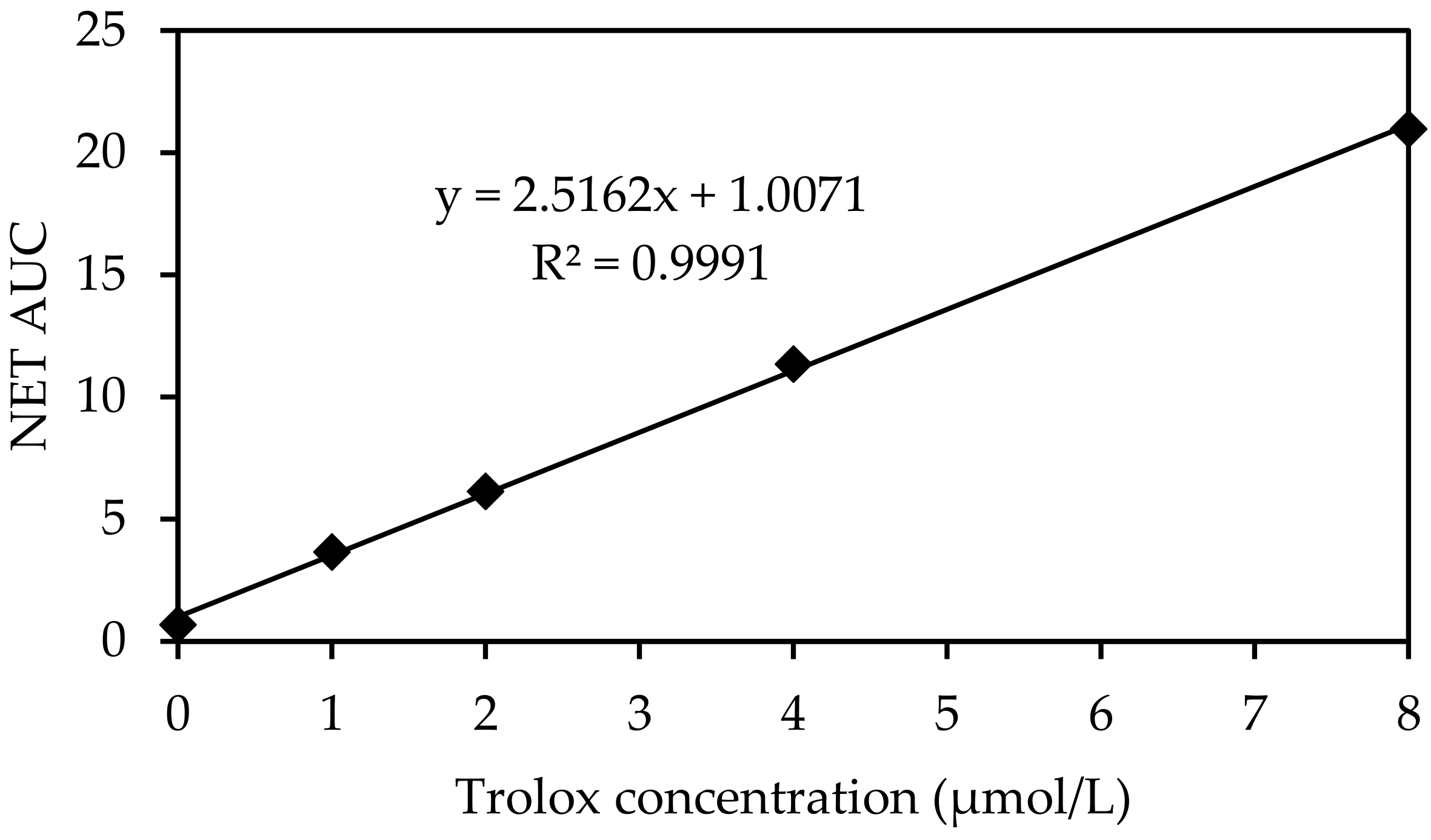
Appendix D
| Trolox Concentration (µmol/L) | Absorbance * |
|---|---|
| 20.00 | 0.460 |
| 12.50 | 0.296 |
| 6.25 | 0.151 |
| 2.50 | 0.056 |
| 0.63 | 0.021 |
| 0 | 0.000 |
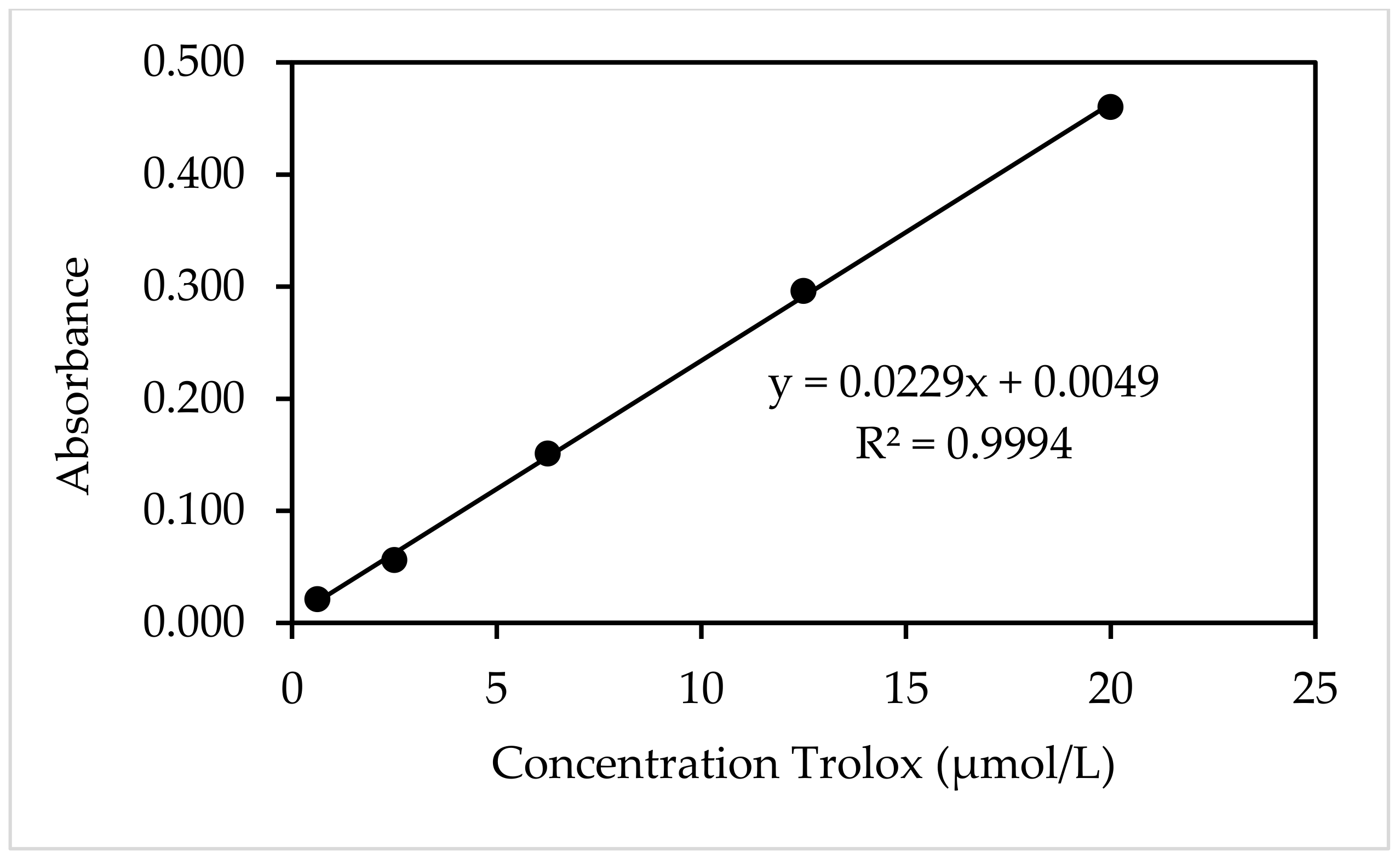
References
- Rezende, C.M.; Fraga, S.R.G. Chemical and aroma determination of the pulp and seeds of murici (Byrsonima crassifolia L.). J. Braz. Chem. Soc. 2003, 14, 425–428. [Google Scholar] [CrossRef]
- Perez-Gutierrez, R.M.; Muñiz-Ramirez, A.; Gomez, Y.G.; Ramírez, E.B. Antihyperglycemic, antihyperlipidemic and antiglycation effects of Byrsonima crassifolia fruit and seed in normal and streptozotocin-induced diabetic rats. Plant Foods Hum. Nutr. 2010, 65, 350–357. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian fruit Byrsonima crassifolia effectively scavenges reactive oxygen and nitrogen species and protects human erythrocytes against oxidative damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An In vitro digestion method adapted for carotenoids and carotenoid esters: Moving forward towards standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Irías-Mata, A.; Jiménez, V.M.; Steingass, C.B.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Carotenoids and xanthophyll esters of yellow and red nance fruits (Byrsonima crassifolia (L.) Kunth) from Costa Rica. Food Res. Int. 2018, 111, 708–714. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Rodrigues, E.; Mercadante, A.Z. Carotenoids from Byrsonima crassifolia: Identification, quantification and in vitro scavenging capacity against peroxyl radicals. J. Food Compos. Anal. 2013, 31, 155–160. [Google Scholar] [CrossRef]
- Gordon, A.; Jungfer, E.; Silva, B.A.; Maia, J.G.S.; Marx, F. Phenolic constituents and antioxidant capacity of four underutilized fruits from the Amazon region. J. Agric. Food Chem. 2011, 59, 7688–7699. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.; Correa, N.C.F.; Carvalho Junior, R.; Costa, C.E.F.; Moraes, J.D.F.C.; da Lannes, S.C.S. Quality parameters and thermogravimetric and oxidative profile of muruci oil (Byrsonima crassifolia L.) obtained by supercritical CO2. Food Sci. Technol. 2018, 38, 172–179. [Google Scholar] [CrossRef]
- Moreira-Araújo, R.S.D.R.; Barros, N.V.D.A.; Porto, R.G.C.L.; de Brandão, A.C.A.S.; de Lima, A.; Fett, R. Bioactive compounds and antioxidant activity three fruit species from the Brazilian Cerrado. Rev. Bras. Frutic. 2019, 41, 1–8. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Machida, H.; Takesue, M.; Smith, R.L. Green chemical processes with supercritical fluids: Properties, materials, separations and energy. J. Supercrit. Fluids 2011, 60, 2–15. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Pires, F.C.S.; de e Silva, A.P.S.; de los Salazar, M.A.R.; da Costa, W.A.; da Costa, H.S.C.; Lopes, A.S.; Rogez, H.; de Carvalho Junior, R.N. Determination of process parameters and bioactive properties of the murici pulp (Byrsonima crassifolia) extracts obtained by supercritical extraction. J. Supercrit. Fluids 2019, 146, 128–135. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Sena, C.; Santos, O.V.; Da Costa, W.A.; Rodrigues, A.M.C.; Carvalho Junior, R.N. Extraction of bacaba (Oenocarpus bacaba) oil with supercritical CO2: Global yield isotherms, fatty acid composition, functional quality, oxidative stability, spectroscopic profile and antioxidant activity. Grasas Aceites 2018, 69, 246. [Google Scholar] [CrossRef]
- Salvador, A.A.; Podestá, R.; Block, J.M.; Ferreira, S.R.S. Increasing the value of pecan nut [Carya illinoinensis (Wangenh) C. Koch] cake by means of oil extraction and antioxidant activity evaluation. J. Supercrit. Fluids 2016, 116, 215–222. [Google Scholar] [CrossRef]
- Carvalho, R.H.R.; Galvão, E.L.; Barros, J.A.C.; Conceição, M.M.; Sousa, E.M.B.D. Extraction, fatty acid profile and antioxidant activity of sesame extract: (Sesamum indicum L.). Braz. J. Chem. Eng. 2012, 29, 409–420. [Google Scholar] [CrossRef]
- Pires, F.C.S.; e de Silva, A.P.S.; Silva, I.Q.; de Oliveira, J.C.; Gama, E.M.O.; Costa, W.A.; de Carvalho Junior, R.N. Application of supercritical technology in the production of dietary supplement based on plant extracts. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, R., Boddula, M.I., Ahamed, A.M., Asiri, Eds.; Solventes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 161–183. [Google Scholar] [CrossRef]
- Feilitzsch, M.; Maier, T.; Junginger, J.; Meile, T.; Kueper, M.A.; Koenigsrainer, A.; Glatzle, J. W1834 Enteral Immunonutrition with Long Chain Triglycerides Significantly Reduces the Recruitment of Immune Cells Into the Liver During Experimental Sepsis. Gastroenterology 2008, 134, 911. [Google Scholar] [CrossRef]
- Lira, C.R.G.; Zucco, F.; Negrão, A.N.; Silva, M.A.S.; Murakami, F.S. Nutraceuticals: An overview on safety, quality control and legislation. Rev. Bras. Farmácia 2009, 90, 45–49. [Google Scholar]
- St-Onge, M.P.; Mayrsohn, B.; O’keeffe, M.; Kissileff, H.R.; Choudhury, A.R.; Laferrère, B. Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur. J. Clin. Nutr. 2014, 68, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Daar, J.; Khan, A.; Khan, J.; Khan, A.; Khan, G.M. Studies on self-nanoemulsifying drug delivery system of flurbiprofen employing long, medium and short chain triglycerides. Pak. J. Pharm. Sci. 2017, 30, 601–606. [Google Scholar]
- Cunha, V.M.B.; da Silva, M.P.; de Sousa, S.H.B.; do Bezerra, P.N.; Menezes, E.G.O.; da Silva, N.J.N.; da Banna, D.A.D.S.; Araújo, M.E.; de Carvalho Júnior, R.N. The Journal of Supercritical Fluids Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- de Batista, C.C.R.; de Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; da Souza Filho, A.P.S.; Machado, N.T.; de Carvalho Junior, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2016, 107, 364–369. [Google Scholar] [CrossRef]
- Santos, O.V.; Corrêa, N.C.F.; Carvalho, R.N.; Costa, C.E.F.; França, L.F.F.; Lannes, S.C.S. Comparative parameters of the nutritional contribution and functional claims of Brazil nut kernels, oil and defatted cake. Food Res. Int. 2013, 51, 841–847. [Google Scholar] [CrossRef]
- Pohl, F.; Goua, M.; Bermano, G.; Russell, W.R.; Scobbie, L.; Maciel, P.; Kong Thoo Lin, P. Revalorisation of rapeseed pomace extracts: An in vitro study into its anti-oxidant and DNA protective properties. Food Chem. 2018, 239, 323–332. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Udenigwe, C.C.; Aluko, R.E. Lutein and zeaxanthin: Production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci. Technol. 2016, 49, 74–84. [Google Scholar] [CrossRef]
- Bak, M.J.; Jun, M.; Jeong, W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCL 4-treated mice. Int. J. Mol. Sci. 2012, 13, 2314–2330. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; Maurício, M.M.; Oliveira, L.C.; Luiza, L.H.; Almada-Vilhena, A.O.; Oliveira, R.M.; Pieczarka, J.C.; Davi do Socorro, B.; Carvalho Junior, R.N. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem. 2020, 330, 127173. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Sowndhararajan, K.; Joo, T.; Lim, C.; Cho, H.; Kim, S.; Kim, G.Y.; Jhoo, J.W. Ethanol and supercritical fluid extracts of hemp seed (Cannabis sativa L.) increase gene expression of antioxidant enzymes in HepG2 cells. Asian Pac. J. Reprod. 2015, 4, 147–152. [Google Scholar] [CrossRef]
- Jondeau, A.; Dahbi, L.; Bani-Estivals, M.H.; Chagnon, M.C. Evaluation of the sensitivity of three sublethal cytotoxicity assays in human HepG2 cell line using water contaminants. Toxicology 2006, 226, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.G.E.J.; De Roos, J.A.D.M.; Westerink, W.M.A.; Débiton, E. Cytotoxic effects of 110 reference compounds on HepG2 cells and for 60 compounds on HeLa, ECC-1 and CHO cells. II Mechanistic assays on NAD(P)H, ATP and DNA contents. Toxicol. Vitr. 2005, 19, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Darroudi, F.; Natarajan, A.T. Metabolic activation of chemicals to mutagenic carcinogens by human hepatoma microsomal extracts in Chinese hamster ovary cells (in vitro). Mutagenesis 1993, 8, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.T.; Darroudi, F. Use of human hepatoma cells for in vitro metabolic activation of chemical mutagens/carcinogens. Mutagenesis 1991, 6, 399–403. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis, 1st ed.; Breeding Crops for Better Nutrition: Washington, DC, USA, 2004. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Dowd, L.E. Spectrophotometric determination of quercetin. Anal. Chem. 1959, 31, 1184–1187. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society (method Ce 2-66): Preparation of methyl esters of fatty acids. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th ed.; The American Oil Chemists’ Society: Boulder, CO, USA, 2017. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Bessa, R.J.B. Effect of genotype, feeding system and slaughter weight on the quality of light lambs II. Fatty acid composition of meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar]
- Gunstone, F.D.; Norris, F.A. Lipids in Food Chemistry, Biochemistry and Technology, 1st ed.; Elsevier: Pergamon, Turkey, 1983. [Google Scholar]
- Norris, F.A.; Mattil, K.F. A new approach to the glyceride structure of natural fats. J. Am. Oil Chem. Soc. 1947, 24, 274–275. [Google Scholar] [CrossRef]
- Lai, T.N.H.; André, C.; Rogez, H.; Mignolet, E.; Nguyen, T.B.T.; Larondelle, Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem. 2015, 168, 410–416. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Yen, H.W.; Chiang, W.C.; Sun, C.H. Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J. Taiwan Inst. Chem. Eng. 2012, 43, 53–57. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Carle, R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.R.; Brum, M.C.M.; Guimarães, I.D.S.; De Freitas Dos Santos, P.; Do Amaral, T.O.; Abreu, J.P.; Passos, T.; Freitas-Silva, O.; Gimba, E.R.P.; Teodoro, A.J. Amazon fruits inhibit growth and promote pro-apoptotic effects on human ovarian carcinoma cell lines. Biomolecules 2019, 9, 707. [Google Scholar] [CrossRef]
- Cobb, B.F.; Kallenbach, J.; Hall, C.A., III; Pryor, S.W. Optimizing the Supercritical Fluid Extraction of Lutein from Corn Gluten Meal. Food Bioprocess Technol. 2018, 11, 757–764. [Google Scholar] [CrossRef]
- Serra, A.T.; Seabra, I.J.; Braga, M.E.M.; Bronze, M.R.; de Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 1: Recovery of extract fractions rich in bioactive compounds. J. Supercrit. Fluids 2010, 55, 184–191. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; da Silva, M.P.; da Costa, W.A.; de Oliveira, M.S.; Bezerra, F.W.F.; de Melo, A.C.; Pinto, R.H.H.; Machado, N.T.; Araujo, M.E.; de Carvalho Junior, R.N. Carbon Dioxide Use in High-Pressure Extraction Processes. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen: London, UK, 2018; pp. 211–240. [Google Scholar]
- Terefe, N.S.; Kleintschek, T.; Gamage, T.; Fanning, K.J.; Netzel, G.; Versteeg, C.; Netzel, M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov. Food Sci. Emerg. Technol. 2013, 19, 57–65. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Villalobos, M.C.; Serradilla, M.J.; Queirós, R.P.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. High pressure extraction of phenolic compounds from citrus peels†. High Press. Res. 2014, 34, 447–451. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Barros, L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Saraiva, J.A.; Ferreira, I.C.F.R. Cold extraction of phenolic compounds from watercress by high hydrostatic pressure: Process modelling and optimization. Sep. Purif. Technol. 2018, 192, 501–512. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of Flavonoids in Human: A Comprehensive Review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.N.S.; Silva, E.M.; Loir, A.; ßois Rees, J.-F.; Rogez, H.; Larondelle, Y. Antioxidant capacity of four polyphenol-rich Amazonian plant extracts: A correlation study using chemical and biological in vitro assays. Food Chem. 2008, 106, 331–339. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Raju, P.S.; Ravi, N.; Roopa, N.; Bawa, A.S. Studies on retention of antioxidant activity, phenolics and flavonoids in high pressure processed black grape juice and their modelling. Int. J. Food Sci. Technol. 2011, 46, 2562–2568. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. Two-step cleanup procedure for the identification of carotenoid esters by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J. Chromatogr. A 2016, 1457, 116–124. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Menezes, E.G.O.; Freitas, L.C.; de Andrade, E.H.A.; Ribeiro-Costa, R.M.; Silva Júnior, J.O.C.; Carvalho Junior, R.N. Supercritical CO2 extraction of uxi (Endopleura uchi) oil: Global yield isotherms, fatty acid profile, functional quality and thermal stability. J. Supercrit. Fluids 2020, 165, 104932. [Google Scholar] [CrossRef]
- Silva, M.P.; Cunha, V.M.B.; Sousa, S.H.B.; Menezes, E.G.O.; Bezerra, P.D.N.; De Farias Neto, J.T.; Filho, G.N.R.; Araújo, M.E.; De Carvalho, R.N. Supercritical CO2 extraction of lyophilized Açaí (Euterpe oleracea Mart.) pulp oil from three municipalities in the state of Pará, Brazil. J. CO2 Util. 2019, 31, 226–234. [Google Scholar] [CrossRef]
- de Almeida Rabello Oliveira, M.; da Rocha Ataíde, T.; de Oliveira, S.L.; de Melo Lucena, A.L.; de Lira, C.E.P.R.; Soares, A.A.; de Almeida, C.B.S.; Ximenes-da-Silva, A. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci. Lett. 2008, 434, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Aburahma, M.H.; El-Laithy, H.M.; Hamza, Y.E.-S. Oral bioavailability enhancement of vinpocetine using self-microemulsifying drug delivery system containing long chain triglycerides: Preparation and in vitro/in vivo evaluation. Clin. Res. Regul. Aff. 2010, 27, 97–107. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; Vargas, M.L.V.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Rosa, A.; Maxia, A.; Putzu, D.; Atzeri, A.; Era, B.; Fais, A.; Sanna, C.; Piras, A. Chemical composition of Lycium europaeum fruit oil obtained by supercritical CO2 extraction and evaluation of its antioxidant activity, cytotoxicity and cell absorption. Food Chem. 2017, 230, 82–90. [Google Scholar] [CrossRef]
 ) (A) and CO2+EtOH-SFE (
) (A) and CO2+EtOH-SFE ( ) (B), at 343.15 K (Standard deviations ≤ 0.4%).
) (B), at 343.15 K (Standard deviations ≤ 0.4%).
 ) (A) and CO2+EtOH-SFE (
) (A) and CO2+EtOH-SFE ( ) (B), at 343.15 K (Standard deviations ≤ 0.4%).
) (B), at 343.15 K (Standard deviations ≤ 0.4%).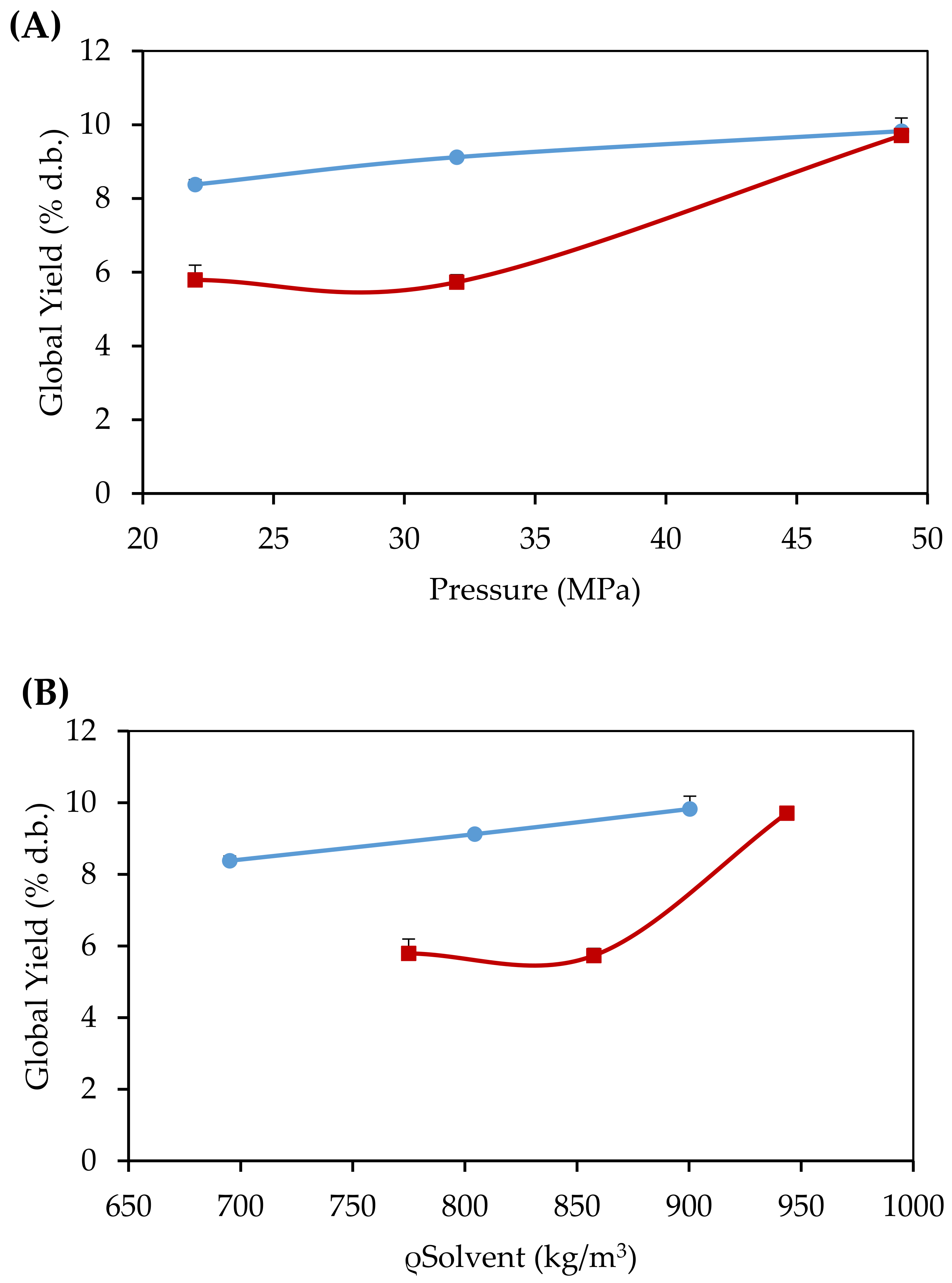
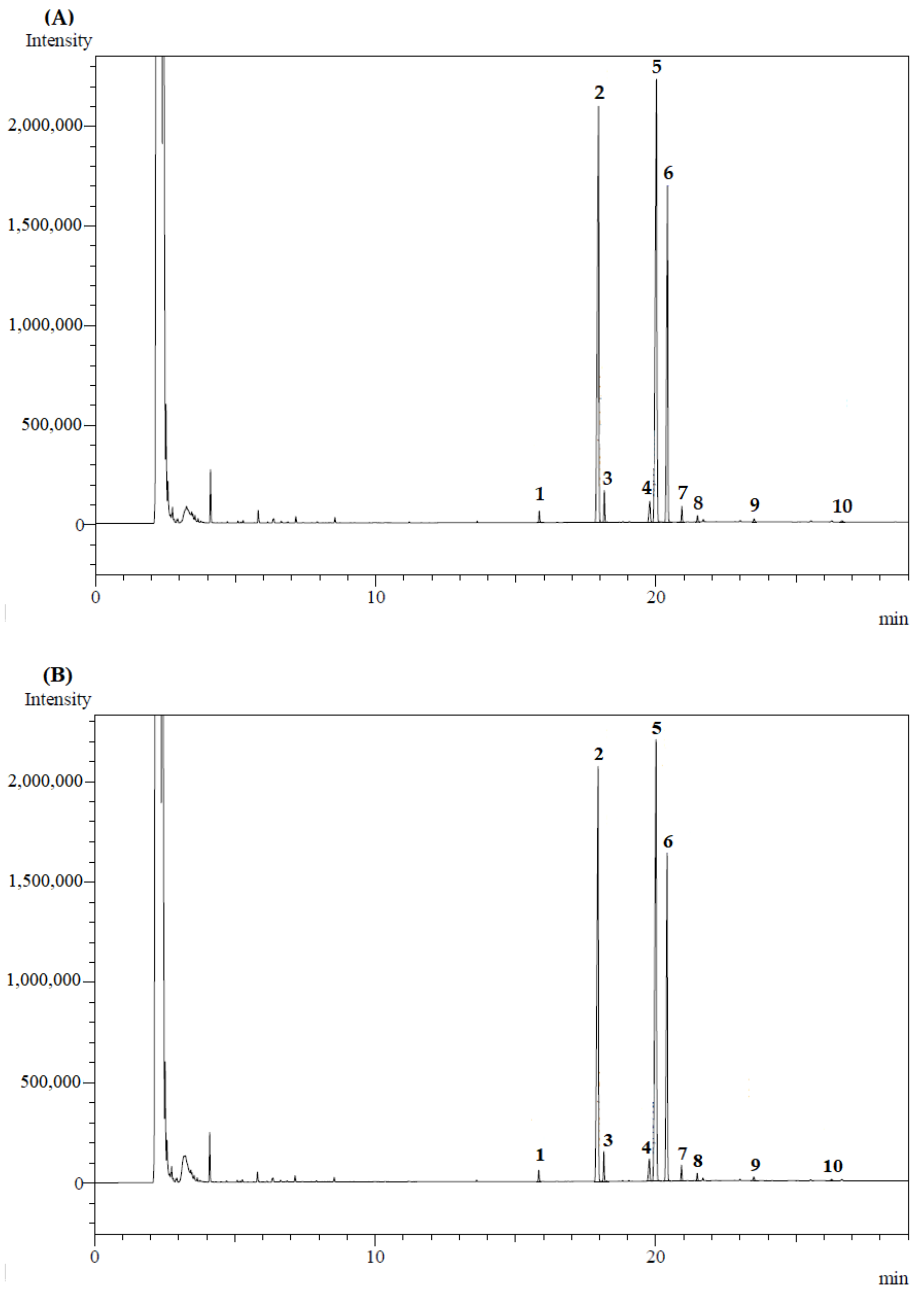
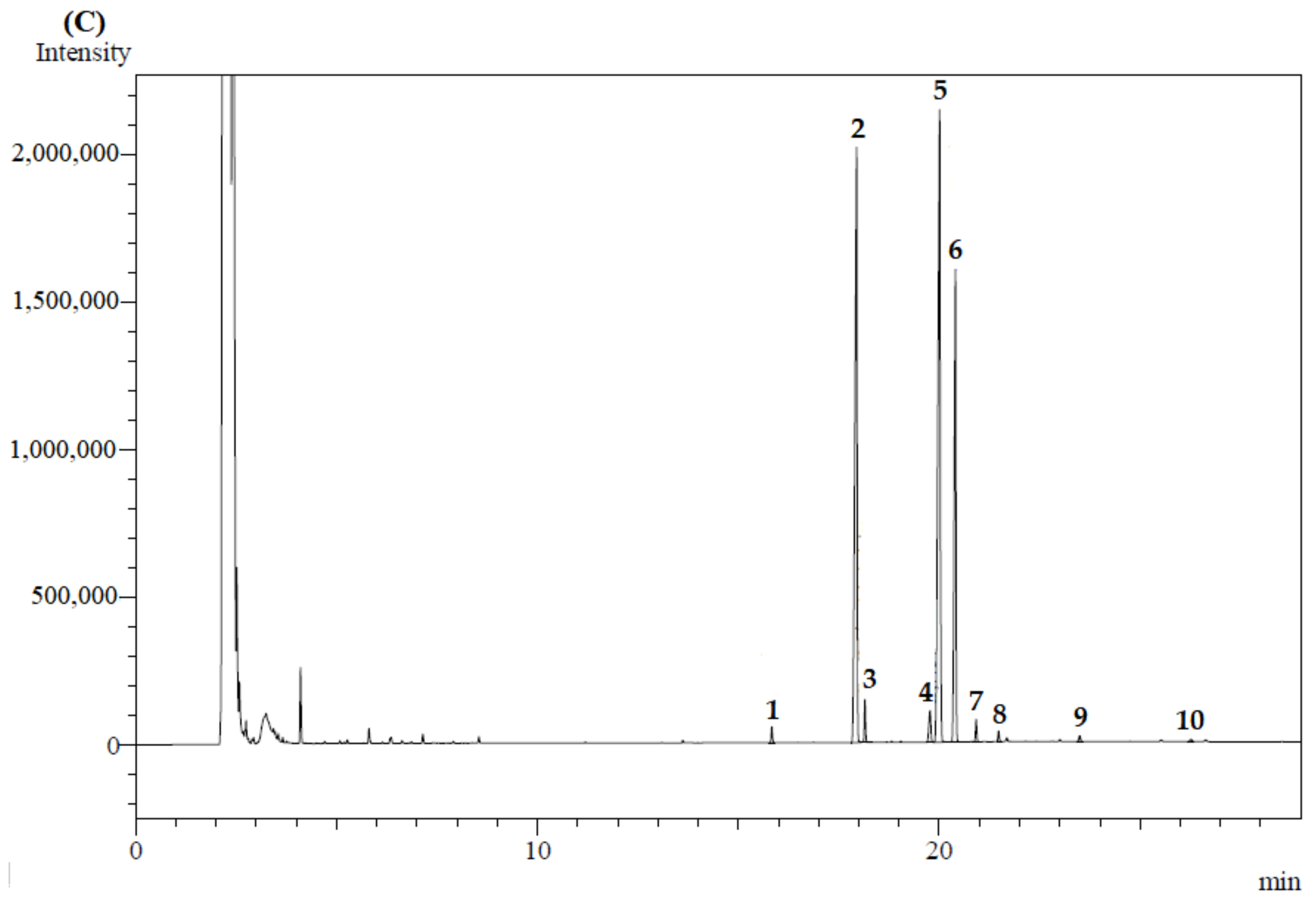
| Samples | Pressure (MPa) | ρSolvent (kg/m3) | Luteín Content (µg/g d.b.) * | Phenolic Compounds (mg GAE/g d.b.) * | Flavonoids Content (mg QE/g d.b.) * | ORAC (µmol TE/g d.b.) * | DPPH (µmol TE/g d.b.) * |
|---|---|---|---|---|---|---|---|
| Oils (CO2 –SFE) | 22 | 695 | 62.38 ± 0.67 c | n.d. | n.d. | 34.44 ± 0.21 b | 6.01 ± 0.31 a |
| 32 | 804 | 196.18 ± 0.90 b | n.d. | n.d. | 43.48 ± 0.88 a | 6.04 ± 0.19 a | |
| 49 | 900 | 224.77 ± 0.67 a | n.d. | n.d. | 32.83 ± 0.27 c | 6.01 ± 0.19 a | |
| Defatted pulps | 22 | 695 | 30.31 ± 0.06 a | 24.58 ± 0.86 a | 0.43 ± 0.01 b | 1.90 ± 0.06 a | 2.47 ± 0.10 a |
| 32 | 804 | 21.93 ± 0.10 c | 19.67 ± 0.27 b | 0.52 ± 0.01 a | 1.45 ± 0.03 c | 1.69 ± 0.15 b | |
| 49 | 900 | 22.58 ± 0.10 b | 12.02 ± 0.42 c | 0.34 ± 0.01 c | 1.58 ± 0.04 b | 1.37 ± 0.13 c | |
| Ethanolic extracts (CO2+EtOH–SFE) | 22 | 775 | 242.16 ± 0.55 a | 6.73 ± 0.15 c | 0.65 ± 0.07 a | 100.88 ± 1.41 b | 12.87 ± 0.38 c |
| 32 | 858 | 163.76 ± 0.94 b | 7.93 ± 0.27 b | 0.59 ± 0.02 a | 117.45 ± 2.40 a | 15.01 ± 0.19 b | |
| 49 | 944 | 88.46 ± 0.58 c | 20.63 ± 0.76 a | 0.64 ± 0.01 a | 122.61 ± 3.79 a | 17.14 ± 0.45 a |
| Peak Number | Relative Area (% d.b.) * | |||
|---|---|---|---|---|
| Fatty Acids | 22 MPa- 695 kg/m3 | 32 MPa- 804 kg/m3 | 49 MPa- 900 kg/m3 | |
| 1 | C14:0 | 0.51 a | 0.51 a | 0.52 a |
| 2 | C16:0 | 34.18 a | 34.38 a | 34.39 a |
| 3 | C16:1 | 1.43 a | 1.39 a | 1.39 a |
| 4 | C18:0 | 1.57 a | 1.66 a | 1.67 a |
| 5 | C18:1 | 39.21 a | 39.04 a | 39.04 a |
| 6 | C18:2 | 21.78 a | 21.58 a | 21.53 a |
| 7 | C18:3 | 0.72 a | 0.72 a | 0.74 a |
| 8 | C20:0 | 0.30 a | 0.36 a | 0.37 a |
| 9 | C22:0 | 0.18 a | 0.23 a | 0.24 a |
| 10 | C24:0 | 0.11 a | 0.12 a | 0.13 a |
| - | SFA | 36.85 a | 37.26 a | 37.32 a |
| - | UFA | 63.14 a | 62.73 a | 62.70 a |
| - | MUFA | 40.64 a | 40.43 a | 40.43 a |
| - | PUFA | 22.50 a | 22.30 a | 22.27 a |
| - | S/U | 0.58 a | 0.59 a | 0.60 a |
| - | AI | 0.57 a | 0.58 a | 0.58 a |
| - | IT | 1.09 a | 1.10 a | 1.10 a |
| - | HH | 1.82 a | 1.80 a | 1.80 a |
| Triglycerides | X:Y * | MM (g/mol) | Fração Molar (% d.b.) | ||
|---|---|---|---|---|---|
| 22 MPa- 695 kg/m3 | 32 MPa- 804 kg/m3 | 49 MPa- 900 kg/m3 | |||
| PPP | 48:0 | 806 | 3.63 | 4.05 | 4.07 |
| OOO | 54:3 | 884 | 6.45 | 5.98 | 5.99 |
| LiLiLi | 54:6 | 878 | 1.14 | 1.02 | 1.01 |
| PPO | 50:1 | 832 | 13.18 | 13.83 | 13.88 |
| POO | 52:2 | 858 | 15.97 | 15.76 | 15.79 |
| LiPP | 50:2 | 830 | 7.40 | 7.68 | 7.68 |
| PLiLi | 52:4 | 854 | 5.03 | 4.86 | 4.83 |
| LiOO | 54:4 | 882 | 10.87 | 9.97 | 9.93 |
| OLiLi | 54:5 | 880 | 6.10 | 5.54 | 5.49 |
| POLi | 52:3 | 856 | 17.93 | 17.50 | 17.46 |
| OPaP | 50:2 | 830 | 1.17 | 1.14 | 1.13 |
| PSO | 52:1 | 860 | 1.05 | 1.32 | 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, F.C.S.; Oliveira, J.C.d.; Menezes, E.G.O.; Silva, A.P.d.S.e.; Ferreira, M.C.R.; Siqueira, L.M.M.; Almada-Vilhena, A.O.; Pieczarka, J.C.; Nagamachi, C.Y.; Carvalho Junior, R.N.d. Bioactive Compounds and Evaluation of Antioxidant, Cytotoxic and Cytoprotective Effects of Murici Pulp Extracts (Byrsonima crassifolia) Obtained by Supercritical Extraction in HepG2 Cells Treated with H2O2. Foods 2021, 10, 737. https://doi.org/10.3390/foods10040737
Pires FCS, Oliveira JCd, Menezes EGO, Silva APdSe, Ferreira MCR, Siqueira LMM, Almada-Vilhena AO, Pieczarka JC, Nagamachi CY, Carvalho Junior RNd. Bioactive Compounds and Evaluation of Antioxidant, Cytotoxic and Cytoprotective Effects of Murici Pulp Extracts (Byrsonima crassifolia) Obtained by Supercritical Extraction in HepG2 Cells Treated with H2O2. Foods. 2021; 10(4):737. https://doi.org/10.3390/foods10040737
Chicago/Turabian StylePires, Flávia Cristina Seabra, Joicy Corrêa de Oliveira, Eduardo Gama Ortiz Menezes, Ana Paula de Souza e Silva, Maria Caroline Rodrigues Ferreira, Leticia Maria Martins Siqueira, Andryo Orfi Almada-Vilhena, Julio Cesar Pieczarka, Cleusa Yoshiko Nagamachi, and Raul Nunes de Carvalho Junior. 2021. "Bioactive Compounds and Evaluation of Antioxidant, Cytotoxic and Cytoprotective Effects of Murici Pulp Extracts (Byrsonima crassifolia) Obtained by Supercritical Extraction in HepG2 Cells Treated with H2O2" Foods 10, no. 4: 737. https://doi.org/10.3390/foods10040737
APA StylePires, F. C. S., Oliveira, J. C. d., Menezes, E. G. O., Silva, A. P. d. S. e., Ferreira, M. C. R., Siqueira, L. M. M., Almada-Vilhena, A. O., Pieczarka, J. C., Nagamachi, C. Y., & Carvalho Junior, R. N. d. (2021). Bioactive Compounds and Evaluation of Antioxidant, Cytotoxic and Cytoprotective Effects of Murici Pulp Extracts (Byrsonima crassifolia) Obtained by Supercritical Extraction in HepG2 Cells Treated with H2O2. Foods, 10(4), 737. https://doi.org/10.3390/foods10040737








