Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Hens, Housing and Experimental Diets
2.2. Sample Collection
2.3. Color Determination
2.4. Carotenoid Analysis
2.5. Iron-Induced Lipid Oxidation of Egg Yolks
2.6. Sensory Assessment
2.7. Statistical Analysis
3. Results
3.1. Carotenoid Content in Diets and Egg Yolks
3.2. Egg Yolk Color
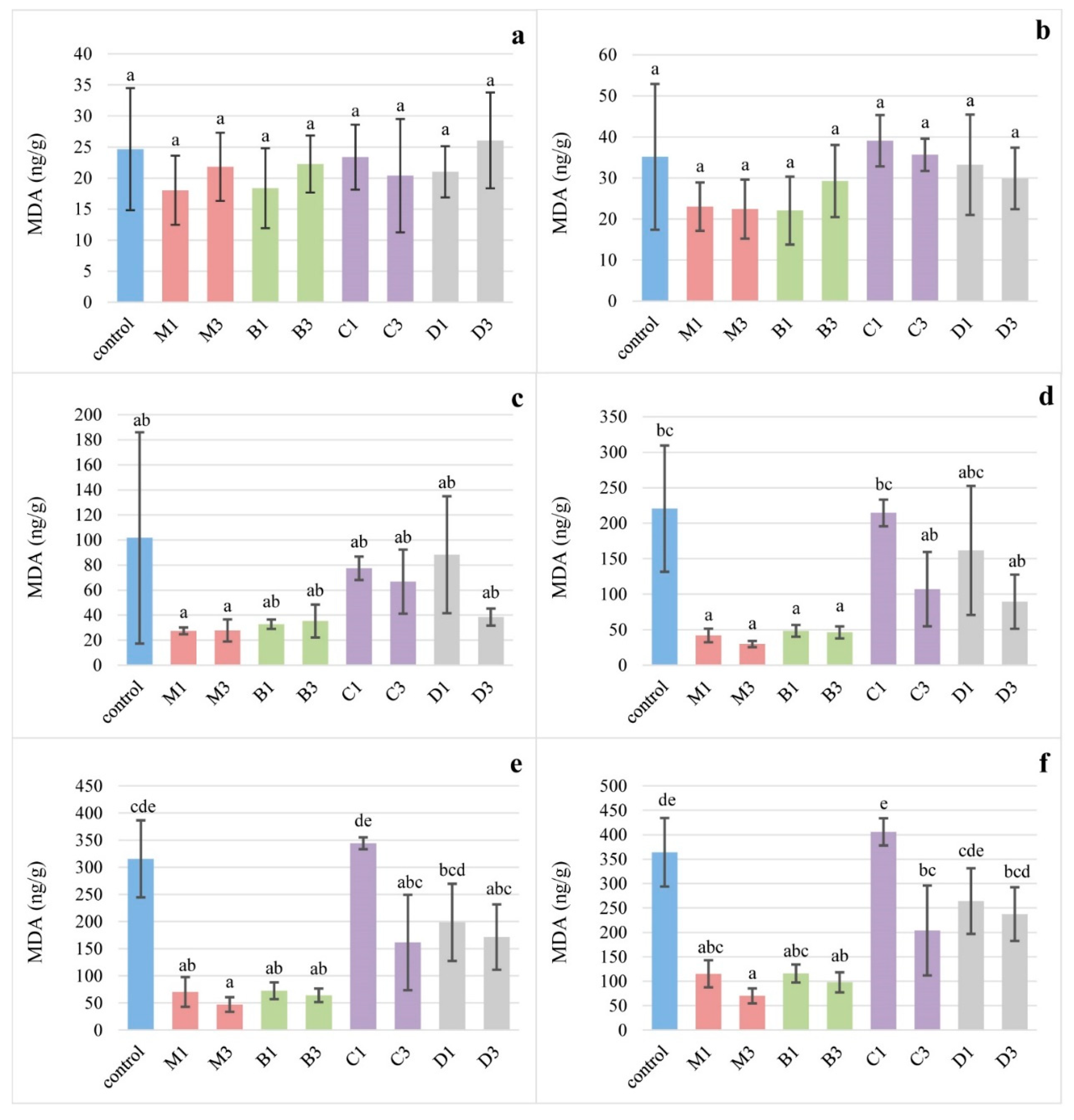
3.3. Iron-Induced Lipid Oxidation of Egg Yolks
3.4. Sensory Traits of Eggs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zaheer, K. An updated review on chicken eggs: Production, consumption, management aspects and nutritional benefits to human health. Food Nutr. Sci. 2015, 6, 1208–1220. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Rezaei, M.; Zakizadeh, S.; Eila, N. Effects of Pigments Extracted from the Marigold Flower on Egg Quality and Oxidative Stability of the Egg Yolk Lipids in Laying Hens. Iran. J. Appl. Anim. Sci. 2019, 9, 541–547. [Google Scholar]
- Karadas, F.; Grammenidis, E.; Surai, P.F.; Acamovic, T.; Sparks, N.H.C. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br. Poult. Sci. 2006, 47, 561–566. [Google Scholar] [CrossRef]
- Santos-Bocanegra, E.; Ospina-Osorio, X.; Oviedo-Rondon, E.O. Evaluation of xanthophylls extracted from Tagetes erectus (Marigold flower) and Capsicum Sp. (Red pepper paprika) as a pigment for egg-yolks compare with Synthetic pigments. Int. J. Poultry Sci. 2004, 3, 685–689. [Google Scholar]
- Rondoni, A.; Asioli, D.; Millan, E. Consumer behaviour, perceptions, and preferences towards eggs: A review of the literature and discussion of industry implications. Trends Food Sci. Tech. 2020, 106, 391–401. [Google Scholar] [CrossRef]
- European Commission. European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003. Annex I: List of additives. Edition 10/2020 (288). 2019. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed-eu-reg-comm_register_feed_additives_1831-03.pdf (accessed on 3 January 2021).
- Grashorn, M. Feed additives for influencing chicken meat and egg yolk color. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 283–302. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific opinion on the safety and efficacy of canthaxanthin as a feed additive for poultry and for ornamental birds and ornamental fish. EFSA J. 2014, 12, 3527. [Google Scholar]
- Skřivan, M.; Englmaierová, M.; Skřivanová, E.; Bubancová, I. Increase in lutein and zeaxanthin content in the eggs of hens fed marigold flower extract. Czech J. Anim. Sci. 2015, 60, 89–96. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Zhang, H.; Chen, X.; Liang, F.; Qin, H.; Zhang, Z. Carotenoid metabolite and transcriptome dynamics underlying flower color in marigold (Tagetes erecta L.). Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Pintea, A. HPLC analysis of carotenoids in four varieties of Calendula officinalis L. flowers. Acta Biol. Szeged. 2003, 47, 37–40. [Google Scholar]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. HPLC analysis of geometrical isomers of lutein epoxide isolated from dandelion (Taraxacum officinale F. Weber ex Wiggers). Phytochemistry 2006, 67, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and chlorophyll pigments in sweet basil grown in the field and greenhouse. HortScience 2005, 40, 1119D. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Antioxidant, prooxidant, and cytotoxic activities of solvent-fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric. Food Chem. 2003, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sholichah, A.R. Phytochemical screening and antioxidant activity of ethanolic extract and ethyl acetate fraction from basil leaf (Ocimum basilicum L.) by DPPH radical scavenging method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 259, 012008. [Google Scholar]
- Sahingil, D. GC/MS-olfactometric characterization of the volatile compounds, determination antimicrobial and antioxidant activity of essential oil from flowers of calendula (Calendula officinalis L.). J. Essent. Oil-Bear. Plants 2019, 22, 1571–1580. [Google Scholar] [CrossRef]
- Omri, B.; Amraoui, M.; Tarek, A.; Lucarini, M.; Durazzo, A.; Cicero, N.; Santini, A.; Kamoun, M. Arthrospira platensis (Spirulina) supplementation on laying hens’ performance: Eggs physical, chemical, and sensorial qualities. Foods 2019, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, B.; Davood, S.S.; Mohammadi-Sangcheshmeh, A. Dietary supplementation of Gracilariopsis persica is associated with some quality related sera and egg yolk parameters in laying quails. J. Sci. Food Agri. 2015, 95, 643–648. [Google Scholar] [CrossRef]
- Botsoglou, N.; Florou-Paneri, P.; Botsoglou, E.; Dotas, V.; Giannenas, I.; Koidis, A.; Mitrakos, P. The effect of feeding rosemary, oregano, saffron and α-tocopheryl acetate on hen performance and oxidative stability of eggs. S. Afr. J. Anim. Sci. 2005, 35, 143–151. [Google Scholar] [CrossRef]
- Horsted, K.; Hammershøj, M.; Allesen-Holm, B.H. Effect of grass–clover forage and whole-wheat feeding on the sensory quality of eggs. J. Sci. Food Agr. 2010, 90, 343–348. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th rev. ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- International Organization for Standardization. ISO 6496:1999 Animal Feeding Stuffs—Determination of Moisture and Other Volatile Matter Content; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- Surai, P.F.; Speake, B.K.; Wood, N.A.; Blount, J.D.; Bortolotti, G.R.; Sparks, N.H. Carotenoid discrimination by the avian embryo: A lesson from wild birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 743–750. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Kornbrust, D.J.; Mavis, R.D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: Correlation with vitamin E content. Lipids 1980, 15, 315–322. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Yannakopoulos, A.L.; Fletouris, D.J.; Tserveni-Goussi, A.S.; Fortomaris, P.D. Effect of dietary thyme on the oxidative stability of egg yolk. J. Agr. Food Chem. 1997, 45, 3711–3716. [Google Scholar] [CrossRef]
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-3. 2015. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 11 June 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 1 February 2020).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Software 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Hencken, H. Chemical and physiological behavior of feed carotenoids and their effects on pigmentation. Poult. Sci. 1992, 71, 711–717. [Google Scholar] [CrossRef]
- Kotrbáček, V.; Skřivan, M.; Kopecký, J.; Pěnkava, O.; Hudečková, P.; Uhríková, I.; Doubek, J. Retention of carotenoids in egg yolks of laying hens supplemented with heterotrophic Chlorella. Czech J. Anim. Sci. 2013, 58, 193–200. [Google Scholar] [CrossRef]
- Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Carotenoids in avian nutrition and embryonic development. 1. Absorption, availability and levels in plasma and egg yolk. J. Poult. Sci. 2001, 38, 1–27. [Google Scholar] [CrossRef]
- Grčević, M.; Kralik, Z.; Kralik, G.; Galović, O. Effects of dietary marigold extract on lutein content, yolk color and fatty acid profile of omega-3 eggs. J. Sci. Food Agr. 2019, 99, 2292–2299. [Google Scholar] [CrossRef]
- Hammershøj, M.; Steenfeldt, S. The effects of kale (Brassica oleracea ssp. acephala), basil (Ocimum basilicum) and thyme (Thymus vulgaris) as forage material in organic egg production on egg quality. Br. Poult. Sci. 2012, 53, 245–256. [Google Scholar] [CrossRef]
- Altuntaş, A.; Aydin, R. Fatty acid composition of egg yolk from chickens fed a diet including Marigold (Tagetes erecta L.). J. Lipids 2014, 2014, 564851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simitzis, P.; Spanou, D.; Glastra, N.; Goliomytis, M. Impact of dietary quercetin on laying hen performance, egg quality and yolk oxidative stability. Anim. Feed Sci. Technol. 2018, 239, 27–32. [Google Scholar] [CrossRef]
- Galobart, J.; Barroeta, A.C.; Baucells, M.D.; Guardiola, F. Lipid oxidation in fresh and spray-dried eggs enriched with ω3 and ω6 polyunsaturated fatty acids during storage as affected by dietary vitamin E and canthaxanthin supplementation. Poult. Sci. 2001, 80, 327–337. [Google Scholar] [CrossRef]
- Botsoglou, E.; Govaris, A.; Fletouris, D.; Iliadis, S. Olive leaves (Olea europea L.) and α-tocopheryl acetate as feed antioxidants for improving the oxidative stability of α-linolenic acid-enriched eggs. J. Anim. Physiol. Anim. Nutr. 2013, 97, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Spada, F.P.; Selani, M.M.; Coelho, A.A.D.; Savino, V.J.M.; Rodella, A.A.; Souza, M.C.; Fischer, F.S.; Lemes, D.E.A.; Canniatti-Brazaca, S.G. Influence of natural and synthetic carotenoids on the color of egg yolk. Sci. Agri. 2016, 73, 234–242. [Google Scholar] [CrossRef][Green Version]
- Berset, C.; Clermont, H.; Cheval, S. Natural red colorant effectiveness as influenced by absorptive supports. J. Food Sci. 1995, 60, 858–861. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Schieber, A.; Wu, J. Effect of domestic cooking methods on egg yolk xanthophylls. J. Agr. Food Chem. 2012, 60, 12547–12552. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Content (g/kg) |
|---|---|
| Maize | 645.2 |
| Soybean meal | 175 |
| Sunflower meal | 80 |
| Calcium carbonate | 80 |
| Monocalcium phosphate | 8 |
| Sodium chloride | 5 |
| DL methionine | 1.8 |
| Premix 1 | 5 |
| Calculated nutrient composition | |
| Crude protein | 149.1 |
| Crude fat | 23 |
| Crude fibre | 26 |
| Crude ash | 101 |
| Calcium | 34.2 |
| Phosphorus | 5 |
| Sodium | 1.9 |
| Metabolic energy (MJ kg−1) | 9.87 |
| Characteristic | Percentage (%) | Characteristic | Percentage (%) |
|---|---|---|---|
| Gender | Household income | ||
| Female | 52.6 | Low | 6.2 |
| Male | 47.4 | Middle | 59.8 |
| Age (years) | Upper Middle | 30.9 | |
| <20 | 9.3 | High | 3.1 |
| 21–30 | 29.9 | Education level | |
| 31–40 | 26.8 | Elementary school | 2.1 |
| 41–50 | 20.6 | High school | 24.7 |
| 51–60 | 6.2 | University degree | 73.2 |
| >61 | 7.2 |
| Carotenoids | Control | M1 | M3 | B1 | B3 | C1 | C3 | D1 | D3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Diets (µg/g DM) | ||||||||||
| Total carotenes | 1.08 ± 0.13 e | 7.37 ± 0.48 c | 12.73 ± 3.29 b | 2.55 ± 0.26 d,e | 5.15 ± 0.45 d | 8.03 ± 0.42 c | 17.24 ± 2.34 a | 10.67 ± 0.84 b | 25.67 ± 3.87 a | <0.0001 |
| Monohydroxy carotenoids | 6.12 ± 0.19 b | 1.84 ± 0.09 c | 1.85 ± 0.06 c | 1.67 ± 0.26 c | 1.94 ± 0.17 c | 5.83 ± 0.43 b | 12.35 ± 1.75 a | 5.60 ± 1.03 b | 11.67 ± 1.12 a | <0.0001 |
| Dihidroxy carotenoids | 9.44 ± 0.61 f | 106.23 ± 5.35 a,b | 299.49 ± 5.46 a | 10.04 ± 0.97 e,f | 14.14 ± 0.65 d | 11.24 ± 0.87 e | 18.44 ± 0.72 b,c | 8.70 ± 0.27 g | 16.17 ± 1.19 c,d | <0.0001 |
| Polioxy carotenoids | nd | nd | nd | nd | nd | 3.29 ± 0.93 c | 7.69 ± 1.37 b | 1.69 ± 0.18 d | 15.32 ± 0.86 a | <0.0001 |
| Total xanthophylls | 15.40 ± 1.14 f | 108.07 ± 5.37 a,b | 301.34 ± 5.44 a | 12.11 ± 0.55 g | 16.97 ± 0.75 e | 20.36 ± 1.45 d | 38.48 ± 3.13 c,d | 15.99 ± 0.75 e,f | 43.16 ± 1.92 b,c | <0.0001 |
| Total carotenoids | 16.48 ± 1.12 f | 115.44 ± 2.82 a,b | 314.07 ± 4.78 a | 14.66 ± 0.78 f | 22.12 ± 1.11 e | 28.39 ± 1.86 d | 55.72 ± 4.68 c | 26.66 ± 1.26 d,e | 68.83 ± 5.28 b,c | <0.0001 |
| Egg yolks (µg/g) | ||||||||||
| Total carotenes | 1.71 ± 0.25 a,b,c | 1.59 ± 0.42 a,b,c | 1.49 ± 0.47 b,c | 1.31 ± 0.18 b,c | 1.99 ± 0.33 a,b | 1.86 ± 0.21 a,b | 2.60 ± 0.38 a,b | 1.11 ± 0.19 c | 1.62 ± 0.34 a,b,c | <0.0001 |
| Monohydroxy carotenoids | 9.30 ± 1.03 a | 3.30 ± 0.50 b,c | 2.64 ± 0.42 c,d | 2.19 ± 0.40 d | 2.64 ± 0.31c,d | 2.15 ± 0.23 d | 3.56 ± 0.28 a,b,c | 2.19 ± 0.20 d | 3.60 ± 0.39 a,b | <0.0001 |
| Dihidroxy carotenoids | 10.36 ± 1.00 d,e | 29.06 ± 1.32 a,b | 62.85 ± 3.81 a | 10.02 ± 0.60 e | 16.05 ± 0.46 b,c | 10.04 ± 0.66 e | 13.80 ± 0.80 c,d | 9.82 ± 0.29 e | 16.47 ± 0.76 b,c | <0.0001 |
| Polioxy carotenoids | nd | nd | nd | nd | nd | 1.28 ± 0.53 b | 1.95 ± 0.71 a,b | 2.59 ± 0.58 b | 1.96 ± 0.46 a,b | <0.0001 |
| Total xanthophylls | 19.54 ± 1.99 c,d | 32.37 ± 1.10 a,b | 65.46 ± 3.64 a | 12.44 ± 0.95 f | 18.82 ± 0.57 d | 13.41 ± 0.20 e,f | 19.16 ± 1.18 d | 14.25 ± 0.37 e | 21.18 ± 1.39 b,c | <0.0001 |
| Total carotenoids | 21.25 ± 2.10 c,d | 33.96 ± 1.28 a,b | 66.95 ± 3.56 a | 13.75 ± 1.08 e | 20.81 ± 0.88 d | 15.27 ± 0.27 e | 21.76 ± 1.41 c,d | 15.36 ± 0.37 e | 22.80 ± 0.87 b,c | <0.0001 |
| Carotenoids | Control | M1 | M3 | B1 | B3 | C1 | C3 | D1 | D3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| CIE | ||||||||||
| L* | 66.64 ± 0.74 f | 68.33 ± 0.88 e | 66.43 ± 1.05 f | 71.63 ± 1.07 a | 71.18 ± 1.88 a,b | 70.59 ± 0.79 c | 69.42 ± 1.72 d | 71.66 ± 1.74 a | 71.00 ± 1.28 b,c | <0.0001 |
| a* | 18.76 ± 0.92 a | 7.28 ± 1.34 c | 12.18 ± 1.88 b | 2.03 ± 1.51 f | 2.99 ± 1.06 e | 4.79 ± 1.68 d | 7.00 ± 2.90 c | 2.55 ± 1.19 e,f | 3.13 ± 1.85 e | <0.0001 |
| b* | 63.08 ± 0.64 f | 68.50 ± 1.06 d | 66.80 ± 1.44 e | 69.14 ± 1.11 c,d | 69.45 ± 2.28 b,c | 68.54 ± 0.45 c,d | 68.22 ± 1.96 d | 70.26 ± 2.08 a,b | 70.44 ± 2.02 a | <0.0001 |
| YCF | 13.47 ± 0.52 a | 10.67 ± 0.72 b,c | 11.47 ± 0.83 b | 7.67 ± 0.82 d | 8.13 ± 0.52 d | 9.73 ± 1.33 c | 9.73 ± 1.10 c | 7.73 ± 0.59 d | 7.87 ± 0.52 d | <0.0001 |
| Carotenoids | Control | M1 | M3 | B1 | B3 | C1 | C3 | D1 | D3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh yolk | ||||||||||
| Sensory color | 7.17 ± 2.15 a | 5.63 ± 2.09 b | 7.28 ± 1.77 a | 4.94 ± 2.16 b,c | 5.18 ± 1.96 b,c | 5.51 ± 1.86 b,c | 5.19 ± 1.92 b,c | 4.65 ± 2.07 c | 5.72 ± 1.79 b | <0.0001 |
| Hard-boiled egg yolk | ||||||||||
| Sensory color | 7.13 ± 2.08 a | 6.58 ± 1.93 a,b | 7.28 ± 2.14 a | 5.38 ± 2.19 b,c | 5.33 ± 1.91 c | 6.02 ± 2.16 b,c | 6.14 ± 1.88 b,c | 5.94 ± 2.21 b,c | 6.10 ± 2.01 b,c | <0.0001 |
| Aroma | 6.08 ± 2.43 | 6.09 ± 2.08 | 5.79 ± 2.56 | 5.44 ± 2.29 | 5.37 ± 2.05 | 5.92 ± 2.18 | 5.82 ± 2.30 | 5.56 ± 2.26 | 5.60 ± 2.22 | 0.338 |
| Flavor | 7.21 ± 1.81 a | 6.62 ± 2.06 a,b | 6.11 ± 2.33 b | 6.12 ± 2.16 b | 6.30 ± 2.16 a,b | 6.31 ± 1.94 a,b | 6.39 ± 1.86 a,b | 6.18 ± 1.99 b | 6.21 ± 1.96 b | 0.035 |
| Texture | 7.10 ± 1.76 | 6.77 ± 1.67 | 6.46 ± 2.18 | 6.38 ± 1.92 | 6.16 ± 1.86 | 6.62 ± 1.95 | 6.29 ± 1.91 | 6.41 ± 1.92 | 6.37 ± 2.04 | 0.140 |
| Overall acceptability | 6.86 ± 1.74 | 6.89 ± 1.48 | 6.40 ± 2.08 | 6.08 ± 1.83 | 6.16 ± 1.56 | 6.31 ± 1.84 | 6.40 ± 1.75 | 6.32 ± 1.68 | 6.18 ± 1.93 | 0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kljak, K.; Carović-Stanko, K.; Kos, I.; Janječić, Z.; Kiš, G.; Duvnjak, M.; Safner, T.; Bedeković, D. Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs. Foods 2021, 10, 721. https://doi.org/10.3390/foods10040721
Kljak K, Carović-Stanko K, Kos I, Janječić Z, Kiš G, Duvnjak M, Safner T, Bedeković D. Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs. Foods. 2021; 10(4):721. https://doi.org/10.3390/foods10040721
Chicago/Turabian StyleKljak, Kristina, Klaudija Carović-Stanko, Ivica Kos, Zlatko Janječić, Goran Kiš, Marija Duvnjak, Toni Safner, and Dalibor Bedeković. 2021. "Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs" Foods 10, no. 4: 721. https://doi.org/10.3390/foods10040721
APA StyleKljak, K., Carović-Stanko, K., Kos, I., Janječić, Z., Kiš, G., Duvnjak, M., Safner, T., & Bedeković, D. (2021). Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs. Foods, 10(4), 721. https://doi.org/10.3390/foods10040721












