Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials
Abstract
1. Introduction
2. Coffee By-Products and Their Potential for Use in the Development of Plastics
2.1. The Coffee Industry and Its By-Products
2.1.1. Coffee Pulp and Mucilage
2.1.2. Coffee Husks
2.1.3. Coffee Parchment and Silverskin
2.1.4. Spent Coffee Grounds
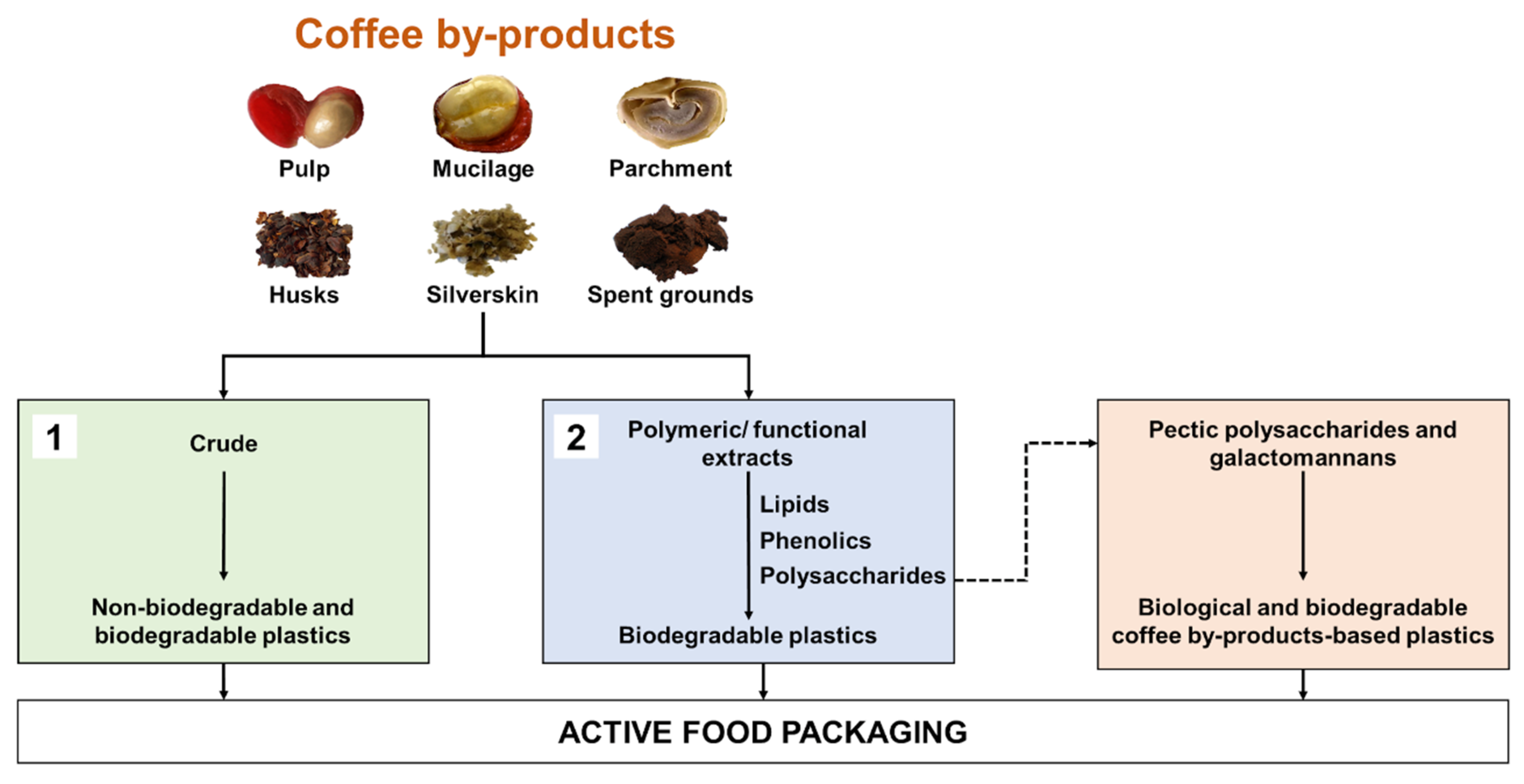
2.2. Coffee By-Products for the Production of Plastics
2.2.1. Crude Coffee By-Products as Functional Additives for Plastics
Nonbiodegradable Formulations
Biodegradable Formulations
2.2.2. Coffee By-Product-Derived Extracts with Film-Forming Ability or Functional Properties
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics—the Facts 2019. Available online: https://www.plasticseurope.org/en/resources/publications/1804-plastics-facts-2019 (accessed on 21 December 2020).
- European Union: European Comission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Commission Work Programme 2018—An Agenda for a More United, Stronger and More Democratic Europe. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52017DC0650&from=en (accessed on 22 December 2020).
- European Bioplastics. What Are Bioplastics? Available online: https://www.european-bioplastics.org/bioplastics (accessed on 4 January 2021).
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A Review of Poly(Lactic Acid)-Based Materials for Antimicrobial Packaging. J. Food Sci. 2014, 79, R1477–R1490. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M. On the Use of Plant Cellulose Nanowhiskers to Enhance the Barrier Properties of Polylactic Acid. Cellulose 2010, 17, 987–1004. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Rebocho, A.T.; Pereira, J.R.; Freitas, F.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Reis, M.A.M. Production of Medium-Chain Length Polyhydroxyalkanoates by Pseudomonas Citronellolis Grown in Apple Pulp Waste. Appl. Food Biotechnol. 2019, 6, 71–82. [Google Scholar] [CrossRef]
- Gonçalves, I.; Lopes, J.; Barra, A.; Hernández, D.; Nunes, C.; Kapusniak, K.; Kapusniak, J.; Evtyugin, D.V.; Lopes da Silva, J.A.; Ferreira, P.; et al. Tailoring the Surface Properties and Flexibility of Starch-Based Films Using Oil and Waxes Recovered from Potato Chips Byproducts. Int. J. Biol. Macromol. 2020, 163, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mironescu, M. Investigations on Wastewaters at Potato Processing and Starch Recovery and Characterisation. J. Agroaliment. Process. Technol. 2011, 17, 134–138. [Google Scholar]
- Muangrat, R.; Nuankham, C. Moisture Sorption Isotherm and Changes in Physico-Mechanical Properties of Films Produced from Waste Flour and Their Application on Preservation Quality of Fresh Strawberry. Food Sci. Nutr. 2018, 6, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Gonçalves, I.; Nunes, C.; Ferreira, P.; Coimbra, M.A.; Martin, C.; Bras, J. Feasibility of Chitosan Crosslinked with Genipin as Biocoating for Cellulose-Based Materials. Carbohydr. Polym. 2020, 242, 116429. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, V.; Bhunia, H.; Narula, A.K. Evaluation of Biodegradability of Potato Peel Powder Based Polyolefin Biocomposites. J. Polym. Environ. 2018, 26, 2049–2060. [Google Scholar] [CrossRef]
- Bunmechimma, L.; Leejarkpai, T.; Riyajan, S.A. Fabrication and Physical Properties of a Novel Macroporous Poly(Vinyl Alcohol)/Cellulose Fibre Product. Carbohydr. Polym. 2020, 240, 116215. [Google Scholar] [CrossRef] [PubMed]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; De Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Krishna Mohan, G.V.; Naga Babu, A.; Kalpana, K.; Ravindhranath, K. Removal of Chromium (VI) from Water Using Adsorbent Derived from Spent Coffee Grounds. Int. J. Environ. Sci. Technol. 2019, 16, 101–112. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption Isotherms and Kinetic Modeling of Methylene Blue Dye onto a Carbonaceous Hydrochar Adsorbent Derived from Coffee Husk Waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Wen, X.; Liu, H.; Zhang, L.; Zhang, J.; Fu, C.; Shi, X.; Chen, X.; Mijowska, E.; Chen, M.J.; Wang, D.Y. Large-Scale Converting Waste Coffee Grounds into Functional Carbon Materials as High-Efficient Adsorbent for Organic Dyes. Bioresour. Technol. 2019, 272, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Sério, A.; Ferreira, S.S.; Kukurová, K.; Ciesarová, Z.; Nunes, F.M.; Coimbra, M.A. Microwave Assisted Extraction of Carbohydrate-Rich Fractions from Spent Coffee Grounds: Formulation of Biscuits Enriched in Dietary Fibre. Trends Carbohydr. Res. 2015, 7, 12–17. [Google Scholar]
- Iriondo-DeHond, A.; Aparicio García, N.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of Coffee By-Products as Novel Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- International Coffee Organisation. Monthly Coffee Market Report—November 2020. Available online: http://www.ico.org/Market-Report-20-21-e.asp (accessed on 4 January 2021).
- Nguyen, T.M.T.; Cho, E.J.; Song, Y.; Oh, C.H.; Funada, R.; Bae, H.J. Use of Coffee Flower as a Novel Resource for the Production of Bioactive Compounds, Melanoidins, and Bio-Sugars. Food Chem. 2019, 299, 125120. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. A Review on Coffee Leaves: Phytochemicals, Bioactivities and Applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 1008–1025. [Google Scholar] [CrossRef]
- International Coffee Organization. International Coffee Organization—Field Processing. Available online: https://web.archive.org/web/20150316011430/http://www.ico.org/field_processing.asp (accessed on 8 December 2019).
- del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-DeHond, A.; Mesa, M.D. Coffee By-products. In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 309–334. [Google Scholar]
- Iriondo-DeHond, A.; Iriondo-DeHond, M.; del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- de O. Carvalho, P.L.; Moreira,, I.; Furlan, A.C.; Paiano, D.; Piano, L.M.; Sierra, L.M.P. Sticky Coffee Hull Silage on the Feeding of Growing and Finishing Pigs. Rev. Bras. Zootec. 2011, 40, 343–351. [Google Scholar] [CrossRef][Green Version]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of Ethanol Production from Coffee Husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Wondemagegnehu, E.B.; Gupta, N.K.; Habtu, E. Coffee Parchment as Potential Biofuel for Cement Industries of Ethiopia. Energy Sources Part A Recover Util. Environ. Eff. 2019, 1–12. [Google Scholar] [CrossRef]
- Gurram, R.; Al-Shannag, M.; Knapp, S.; Das, T.; Singsaas, E.; Alkasrawi, M. Technical Possibilities of Bioethanol Production from Coffee Pulp: A Renewable Feedstock. Clean Technol. Environ. Policy 2016, 18, 269–278. [Google Scholar] [CrossRef]
- The World Bank. Agro-Industry Profiles: Coffee. 1986. Available online: http://documents1.worldbank.org/curated/en/311271467993475518/pdf/FAU14.pdf (accessed on 8 December 2019).
- Ateş, G.; Elmacı, Y. Coffee Silverskin as Fat Replacer in Cake Formulations and Its Effect on Physical, Chemical and Sensory Attributes of Cakes. LWT 2018, 90, 519–525. [Google Scholar] [CrossRef]
- Borrelli, R.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V.; Borrelli, R.C.; Esposito, F.; Napolitano, A.; Alberto Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Orozco, F.H.; Cegarra, J.; Trujillo, L.M.; Roig, A. Vermicomposting of Coffee Pulp Using the Earthworm Eisenia Fetida: Effects on C and N Contents and the Availability of Nutrients. Biol. Fertil. Soils 1996, 22, 162–166. [Google Scholar] [CrossRef]
- Rodríguez Frómeta, R.A.; Sánchez, J.L.; Ros García, J.M. Evaluation of Coffee Pulp as Substrate for Polygalacturonase Production in Solid State Fermentation. Emirates J. Food Agric. 2020, 32, 117–124. [Google Scholar] [CrossRef]
- Reichembach, L.H.; de Oliveira Petkowicz, C.L. Extraction and Characterization of a Pectin from Coffee (Coffea arabica L.) Pulp with Gelling Properties. Carbohydr. Polym. 2020, 245, 116473. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Silva, A.M.S.; Evtuguin, D.V.; Nunes, F.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. The Hydrophobic Polysaccharides of Apple Pomace. Carbohydr. Polym. 2019, 223, 115132. [Google Scholar] [CrossRef] [PubMed]
- Nawrath, C. The Biopolymers Cutin and Suberin. Arab. B. 2002, 1. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Hernandez, L.; Ruiz, H.A.; Cristina Ramírez, T.; Ascacio, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal Detoxification of Coffee Pulp by Solid-State Fermentation. Biocatal. Agric. Biotechnol. 2020, 23, 101467. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional Properties of Coffee and Coffee By-Products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of Coffee Parchment Waste (Coffea arabica) as a Source of Caffeine and Phenolic Compounds in Antifungal Gellan Gum Films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Shemekite, F.; Gómez-Brandón, M.; Franke-Whittle, I.H.; Praehauser, B.; Insam, H.; Assefa, F. Coffee Husk Composting: An Investigation of the Process Using Molecular and Non-Molecular Tools. Waste Manag. 2014, 34, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee Silverskin as a Source of Dietary Fiber in Bread-Making: Optimization of Chemical Treatment Using Response Surface Methodology. LWT—Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Biobutanol Production from Coffee Silverskin. Microb. Cell Fact. 2018, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee Silverskin: Characterization, Possible Uses, and Safety Aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Passos, C.P.; Coimbra, M.A. Microwave Superheated Water Extraction of Polysaccharides from Spent Coffee Grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef]
- Avallone, S.; Guiraud, J.-P.; Guyot, B.; Olguin, E.; Brillouet, J.-M. Polysaccharide Constituents of Coffee-Bean Mucilage. J. Food Sci. 2000, 65, 1308–1311. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee Parchment as a New Dietary Fiber Ingredient: Functional and Physiological Characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ávila, P.F.; Martins, M.; Goldbeck, R. Enzymatic Production of Xylooligosaccharides from Alkali-Solubilized Arabinoxylan from Sugarcane Straw and Coffee Husk. Bioenergy Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Valdespino-León, M.; Calderón-Domínguez, G.; De La Paz Salgado-Cruz, M.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Morales-Sánchez, E.; Gaona-Sánchez, V.A.; Terrazas-Valencia, F. Biodegradable Electrosprayed Pectin Films: An Alternative to Valorize Coffee Mucilage. Waste Biomass Valorization 2020, 1, 3. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological Potential of Coffee Pulp and Coffee Husk for Bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical Fluid Extraction of Lipids from Spent Coffee Grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Bekalo, S.A.; Reinhardt, H.W. Fibers of Coffee Husk and Hulls for the Production of Particleboard. Mater. Struct. Constr. 2010, 43, 1049–1060. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent Coffee Grounds: A Review on Current Research and Future Prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Bresciani, L.; Calani, L.; Bruni, R.; Brighenti, F.; Del Rio, D. Phenolic Composition, Caffeine Content and Antioxidant Capacity of Coffee Silverskin. Food Res. Int. 2014, 61, 196–201. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving Properties of Thermoplastic Starch Films by Incorporating Active Extracts and Cellulose Fibres Isolated from Rice or Coffee Husk. Food Packag. Shelf Life 2019, 22. [Google Scholar] [CrossRef]
- Garcia, R.; Arriola, D.; De Arriola, M.C.; De Porres, E.; Rolz, C. Characterization of Coffee Pectin. LWT Food Sci. Technol. 1991, 24, 125–129. [Google Scholar]
- Avallon, S.; Guiraud, J.P.; Guyot, B.; Olguin, E.; Brillouet, J.M. Fate of Mucilage Cell Wall Polysaccharides during Coffee Fermentation. J. Agric. Food Chem. 2001, 49, 5556–5559. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.A.; Waldron, K.W.; Selvendran, R.R. Isolation and Characterisation of Cell Wall Polymers from the Heavily Lignified Tissues of Olive (Olea Europaea) Seed Hull. Carbohydr. Polym. 1995, 27, 285–294. [Google Scholar] [CrossRef]
- Passos, C.P.; Moreira, A.S.P.; Domingues, M.R.M.; Evtuguin, D.V.; Coimbra, M.A. Sequential Microwave Superheated Water Extraction of Mannans from Spent Coffee Grounds. Carbohydr. Polym. 2014, 103, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Arabinosyl and Glucosyl Residues as Structural Features of Acetylated Galactomannans from Green and Roasted Coffee Infusions. Carbohydr. Res. 2005, 340, 1689–1698. [Google Scholar] [CrossRef]
- Batista, M.J.P.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Polysaccharide-Rich Fraction of Spent Coffee Grounds as Promising Biomaterial for Films Fabrication. Carbohydr. Polym. 2020, 233, 115851. [Google Scholar] [CrossRef]
- Coelho, G.O.; Batista, M.J.A.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Development and Characterization of Biopolymeric Films of Galactomannans Recovered from Spent Coffee Grounds. J. Food Eng. 2021, 289, 110083. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Galactomannans Use in the Development of Edible Films/Coatings for Food Applications. Trends Food Sci. Technol. 2011, 22, 662–671. [Google Scholar] [CrossRef]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Barbosa, H.M.A.; Passos, C.P.; Silva, C.M. Supercritical Fluid Extraction of Spent Coffee Grounds: Measurement of Extraction Curves, Oil Characterization and Economic Analysis. J. Supercrit. Fluids 2014, 86, 150–159. [Google Scholar] [CrossRef]
- Barbosa, H.M.A.; de Melo, M.M.R.; Coimbra, M.A.; Passos, C.P.; Silva, C.M. Optimization of the Supercritical Fluid Coextraction of Oil and Diterpenes from Spent Coffee Grounds Using Experimental Design and Response Surface Methodology. J. Supercrit. Fluids 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What Kind of Coffee Do You Drink? An Investigation on Effects of Eight Different Extraction Methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, M.; Guna, V.; Hu, C.; Takemura, A.; Leman, Z.; Reddy, N. Dehulled Coffee Husk-Based Biocomposites for Green Building Materials. J. Thermoplast. Compos. Mater. 2019, 089270571987630. [Google Scholar] [CrossRef]
- Leal, H.D.A.; Babetto, A.S.; Bonse, B.C. Properties of Lignocellulosic Composites of Coffee Husk Filled Polypropylene. In Proceedings of the AIP Conference Proceedings; American Institute of Physics Inc.: Melville, NY, USA, 10 January 2020; Volume 2205, p. 020072. [Google Scholar]
- Huang, L.; Mu, B.; Yi, X.; Li, S.; Wang, Q. Sustainable Use of Coffee Husks For Reinforcing Polyethylene Composites. J. Polym. Environ. 2018, 26, 48–58. [Google Scholar] [CrossRef]
- Das, O.; Kim, N.K.; Hedenqvist, M.S.; Lin, R.J.T.; Sarmah, A.K.; Bhattacharyya, D. An Attempt to Find a Suitable Biomass for Biochar-Based Polypropylene Biocomposites. Environ. Manag. 2018, 62, 403–413. [Google Scholar] [CrossRef]
- Ángel Hidalgo-Salazar, M.; Pablo Correa-Aguirre, J.; Manuel Montalvo-Navarrete, J.; Fernando Lopez-Rodriguez, D.; Felipe Rojas-González, A. Recycled Polypropylene-Coffee Husk and Coir Coconut Biocomposites: Morphological, Mechanical, Thermal and Environmental Studies. In Thermosoftening Plastics; IntechOpen: London, UK, 2020. [Google Scholar]
- Yiga, V.A.; Pagel, S.; Lubwama, M.; Epple, S.; Olupot, P.W.; Bonten, C. Development of Fiber-Reinforced Polypropylene with NaOH Pretreated Rice and Coffee Husks as Fillers: Mechanical and Thermal Properties. J. Thermoplast. Compos. Mater. 2020, 33, 1269–1291. [Google Scholar] [CrossRef]
- Dominici, F.; García García, D.; Fombuena, V.; Luzi, F.; Puglia, D.; Torre, L.; Balart, R. Bio-Polyethylene-Based Composites Reinforced with Alkali and Palmitoyl Chloride-Treated Coffee Silverskin. Molecules 2019, 24, 3113. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Wang, T.; Rodriguez-Uribe, A.; Misra, M.K. Mohanty, A. Characterization of Wastes and Coproducts from the Coffee Industry for Composite Material Production. BioResources 2016, 11, 7637–7653. [Google Scholar] [CrossRef]
- Wu, H.; Hu, W.; Zhang, Y.; Huang, L.; Zhang, J.; Tan, S.; Cai, X.; Liao, X. Effect of Oil Extraction on Properties of Spent Coffee Ground–Plastic Composites. J. Mater. Sci. 2016, 51, 10205–10214. [Google Scholar] [CrossRef]
- García-García, D.; Carbonell, A.; Samper, M.D.; García-Sanoguera, D.; Balart, R. Green Composites Based on Polypropylene Matrix and Hydrophobized Spend Coffee Ground (SCG) Powder. Compos. Part B Eng. 2015, 78, 256–265. [Google Scholar] [CrossRef]
- Essabir, H.; Raji, M.; Laaziz, S.A.; Rodrique, D.; Bouhfid, R.; Qaiss, A. el kacem Thermo-Mechanical Performances of Polypropylene Biocomposites Based on Untreated, Treated and Compatibilized Spent Coffee Grounds. Compos. Part B Eng. 2018, 149, 1–11. [Google Scholar] [CrossRef]
- Panzella, L.; Cerruti, P.; Ambrogi, V.; Agustin-Salazar, S.; D’Errico, G.; Carfagna, C.; Goya, L.; Ramos, S.; Martín, M.A.; Napolitano, A.; et al. A Superior All-Natural Antioxidant Biomaterial from Spent Coffee Grounds for Polymer Stabilization, Cell Protection, and Food Lipid Preservation. ACS Sustain. Chem. Eng. 2016, 4, 1169–1179. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, H.J.; Park, H.J.; Lee, B.J.; Hwang, T.S. Effect of Compatibilizing Agents on Rice-Husk Flour Reinforced Polypropylene Composites. Compos. Struct. 2007, 77, 45–55. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Cruz, J.; Fangueiro, R. Surface modification of natural fibers in polymer composites. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–41. ISBN 9780081021774. [Google Scholar]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Borghesi, D.C.; Molina, M.F.; Guerra, M.A.; Campos, M.G.N. Biodegradation Study of a Novel Poly-Caprolactone-Coffee Husk Composite Film. Mater. Res. 2016, 19, 752–758. [Google Scholar] [CrossRef]
- Lule, Z.C.; Kim, J. Properties of Economical and Eco-Friendly Polybutylene Adipate Terephthalate Composites Loaded with Surface Treated Coffee Husk. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106154. [Google Scholar] [CrossRef]
- Ortiz-Barajas, D.L.; Arévalo-Prada, J.A.; Fenollar, O.; Rueda-Ordóñez, Y.J.; Torres-Giner, S. Torrefaction of Coffee Husk Flour for the Development of Injection-Molded Green Composite Pieces of Polylactide with High Sustainability. Appl. Sci. 2020, 10, 6468. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, L.; Melo, I.C.N.A.; Marconcini, J.M.; Trugilho, P.F.; Tonoli, G.H.D. Particles of Coffee Wastes as Reinforcement in Polyhydroxybutyrate (PHB) Based Composites. Mater. Res. 2015, 18, 546–552. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Zuorro, A.; Maffei, G.; Lavecchia, R.; Puglia, D.; Dominici, F.; Luzi, F.; Valente, T.; Torre, L. Recycling Coffee Silverskin in Sustainable Composites Based on a Poly(Butylene Adipate-Co-Terephthalate)/Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Matrix. Ind. Crops Prod. 2018, 118, 311–320. [Google Scholar] [CrossRef]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of Different Compatibilizers on Sustainable Composites Based on a PHBV/PBAT Matrix Filled with Coffee Silverskin. Polym. Basel 2018, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Gigante, V.; Seggiani, M.; Cinelli, P.; Signori, F.; Vania, A.; Navarini, L.; Amato, G.; Lazzeri, A. Utilization of Coffee Silverskin in the Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Biopolymer-Based Thermoplastic Biocomposites for Food Contact Applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106172. [Google Scholar] [CrossRef]
- Oliveira, G.; Gonçalves, I.; Barra, A.; Nunes, C.; Ferreira, P.; Coimbra, M.A. Coffee Silverskin and Starch-Rich Potato Washing Slurries as Raw Materials for Elastic, Antioxidant, and UV-Protective Biobased Films. Food Res. Int. 2020, 109733. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S. Renewable Resource-Based Green Composites of Surface-Treated Spent Coffee Grounds and Polylactide: Characterisation and Biodegradability. Polym. Degrad. Stab. 2015, 121, 51–59. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of Torrefied Coffee Grounds as Reinforcing Agent to Produce High-Quality Biodegradable PBAT Composites for Food Packaging Applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/Waste Coffee-Grounds Composite: An Efficient and Eco-Friendly Adsorbent for Removal of Pharmaceutical Contaminants from Water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef]
- Gazonato, E.C.; Maia, A.A.D.; Da Silva Moris, V.A.; De Paiva, J.M.F. Thermomechanical Properties of Corn Starch Based Film Reinforced with Coffee Ground Waste as Renewable Resource. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Thiagamani, S.M.K.; Nagarajan, R.; Jawaid, M.; Anumakonda, V.; Siengchin, S. Utilization of Chemically Treated Municipal Solid Waste (Spent Coffee Bean Powder) as Reinforcement in Cellulose Matrix for Packaging Applications. Waste Manag. 2017, 69, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, V.A.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Coffee Grounds as Filler for Pectin: Green Composites with Competitive Performances Dependent on the UV Irradiation. Carbohydr. Polym. 2017, 170, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Sena Neto, A.R.; Pinheiro, A.C.M.; Mattoso, L.H.C.; Martins, M.A. Development and Physical-Chemical Properties of Pectin Film Reinforced with Spent Coffee Grounds by Continuous Casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; del Castillo, M. Coffee Silverskin Extract Protects against Accelerated Aging Caused by Oxidative Agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, Chemical and Antioxidant/pro-Oxidant Profiles of Silverskin, a Coffee Roasting by-Product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Ouarhim, W.; Zari, N.; Bouhfid, R.; Qaiss, A.E.K. Mechanical performance of natural fibers-based thermosetting composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 43–60. ISBN 9780081022924. [Google Scholar]
- Choo, M.Y.; Oi, L.E.; Ling, T.C.; Ng, E.P.; Lee, H.V.; Juan, J.C. Conversion of microalgae biomass to biofuels. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–161. ISBN 9780128175361. [Google Scholar]
- Dyjakon, A.; Noszczyk, T.; Smędzik, M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef]
- Jaisan, C.; Punbusayakul, N. Development of Coffee Pulp Extract-Incorporated Chitosan Film and Its Antimicrobial and Antioxidant Activities. Asia Pac. J. Sci. Technol. 2016, 21, 140–149. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Using Lignocellulosic Fractions of Coffee Husk to Improve Properties of Compatibilised Starch-PLA Blend Films. Food Packag. Shelf Life 2019, 22. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of Polylactic Acid Nanocomposite Films Reinforced with Cellulose Nanocrystals Derived from Coffee Silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and Physicochemical Properties of Carboxymethyl Cellulose Films Enriched with Spent Coffee Grounds Polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chen, Y.; Ning, J.; Hao, C.; Rock, M.; Amer, M.; Feng, S.; Falahati, M.; Wang, L.J.; Chen, R.K.; et al. No Such Thing as Trash: A 3D-Printable Polymer Composite Composed of Oil-Extracted Spent Coffee Grounds and Polylactic Acid with Enhanced Impact Toughness. ACS Sustain. Chem. Eng. 2019, 7, 15304–15310. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-Sustainable Systems Based on Poly(Lactic Acid), Diatomite and Coffee Grounds Extract for Food Packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ounkaew, A.; Kasemsiri, P.; Kamwilaisak, K.; Saengprachatanarug, K.; Mongkolthanaruk, W.; Souvanh, M.; Pongsa, U.; Chindaprasirt, P. Polyvinyl Alcohol (PVA)/Starch Bioactive Packaging Film Enriched with Antioxidants from Spent Coffee Ground and Citric Acid. J. Polym. Environ. 2018, 26, 3762–3772. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive Starch Foam Composite Enriched With Natural Antioxidants from Spent Coffee Ground and Essential Oil. Starch Stärke 2018, 70, 1700238. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Salameh, M.M.; Phetsomphou, S.; Yang, H.; Seo, C.W. Application of Caffeine, 1,3,7-Trimethylxanthine, to Control Escherichia Coli O157:H7. Food Chem. 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Cellulose Fibres, Nanofibrils and Microfibrils: The Morphological Sequence of MFC Components from a Plant Physiology and Fibre Technology Point of View. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–141. ISBN 9780081021774. [Google Scholar]
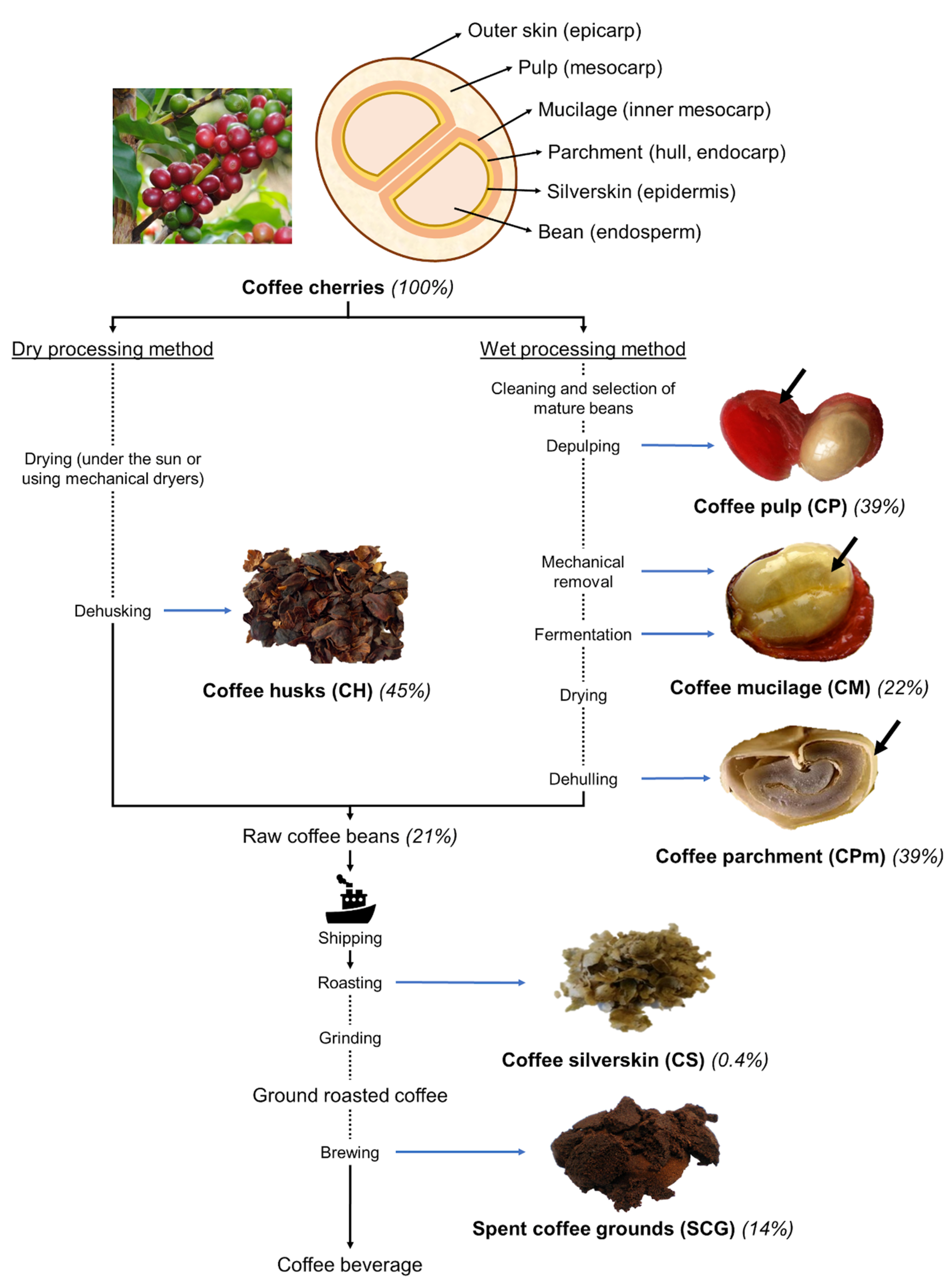
| Composition | Pulp (CP) | Mucilage (CM) | Parchment (CPm) | Husks (CH) | Silverskin (CS) | Spent Coffee Grounds (SCG) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture (% wt) | 78–81 | [31,35] | 84 | [41] | 9 | [42] | 13–15 | [29,43] | 4–7 | [34,44,45,46] | 61 | [47] |
| Component (% dry wt basis) | ||||||||||||
| Free sugars | 5 | [36] | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Cellulose | 36 | 8 | [48] | 12 | [49] | 28 | [50] | 24 | [51] | 16 | [47] | |
| Hemicelluloses | 9 | 18 | 35 | 25 | 16 | 50 33% GM 17% AG | ||||||
| Pectic polysaccharides | 21 | 30 | ND | ND | ND | ND | ND | ND | ND | ND | ||
| GalA (%) | 80 | [37] | 52 | [52] | ||||||||
| DE (%) | 63 | 85 | ||||||||||
| DA (%) | 6 | 6 | ||||||||||
| Mw (g mol−1) | 400,000 | 12,000 | [48] | |||||||||
| Total carbohydrates | 71 * | ND | 56 * | ND | 47 * | ND | 53 * | ND | 40 | [51] | 66 | [47] |
| Lignin | ND | ND | ND | ND | 32 | [49] | 38 | [50] | 29 | ND | ND | |
| Melanoidins | ND | ND | ND | ND | ND | ND | ND | ND | 5 | [34] | 16 | [47] |
| Protein | 9 ** | [36] | 17 | [48] | ND | ND | 8–11 ** | [29,53] | 19 ** | [34,44,45,51] | 5 | |
| Lipids | 0.8 | ND | ND | ND | ND | 1–3 | 2–5 | 13–15 | [47,54] | |||
| Ash | ND | ND | ND | 1 | [55] | 3–7 | [29,55] | 5–7 | 2 | [56] | ||
| Caffeine | 1 | [36] | ND | ND | 0.13 | [42] | 1 | [29,53] | 1 | [57] | 0.01–0.5 | |
| Total phenolics (% w/w GAE) | 0.3 | ND | ND | 0.2 | 1 | [58] | 2 | 1–2 | ||||
| By-Product | Coffee-Based Powder | Polymeric Matrix | Developed Materials and Main Properties | Ref |
|---|---|---|---|---|
| CH | CH powder | PP | Composites with poor interfacial adhesion between CH and the polymeric matrix | [71] |
| PP (plus maleic anhydride grafted PP) | Composites with good interfacial adhesion | [72] | ||
| HDPE (plus maleic anhydride grafted PE) | Composites with good interfacial adhesion | [73] | ||
| PP (plus maleic anhydride grafted PP) | Composites with decreased susceptibility towards fire | [74] | ||
| PP (plus maleic anhydride grafted PP) | Composites with decreased carbon footprint | [75] | ||
| CH powder alkali treated | PP | Composites with improved mechanical and thermal performance | [76] | |
| CS | CS powder | HDPE | Composites with poor interfacial adhesion between CS and the polymeric matrix | [77] |
| CS powder alkali treated and esterified with palmitoyl chloride | HDPE | Composites with decreased water absorption | [77] | |
| SCG | SCG powder | PP | Composites with poor interfacial adhesion between SCG and the polymeric matrix | [78] |
| SCG powder treated by oil removal | PP (plus maleic anhydride grafted PP) | Composites with increase interfacial adhesion, compatibility, and water resistance | [79] | |
| SCG powder esterified with palmitoyl chloride | PP | Composites with better particle dispersion and decreased water uptake | [80] | |
| SCG powder alkali treated and bleached | PP (plus silane and styrene-ethylene-butene-styrene-graft-maleic anhydride) | Composites with improved interfacial adhesion and mechanical properties | [81] | |
| SCG powder treated by acid hydrolysis | PE | Antioxidant films with improved biocompatibility | [82] |
| By-Product | Coffee-Based Powder | Polymeric Matrix | Developed Materials and Main Properties | Ref |
|---|---|---|---|---|
| CH | CH powder | PCL | Films with increased biodegradation rate | [86] |
| CH powder treated with (3-glycidoxypropyl)trimethoxysilane | PBAT | Composites with increased hydrophobicity, stiffness, and reduced production cost | [87] | |
| Torrefied CH powder | PLA | Injection specimens with increased mechanical resistance and thermal stability | [88] | |
| CH and CPm | CH and CPm powder | PHB | Composites with increased thermal stability and water absorption | [89] |
| CS | CS powder | PBAT and P(3HB-co-3HV) | Composites with antioxidant activity | [90] |
| CS powder treated with (3-aminopropyl)triethoxysilane | PBAT and P(3HB-co-3HV) | Composites with antioxidant activity and increased interfacial adhesion | [91] | |
| CS powder | P(3HB-co-3HV) | Composites possessing an overall migration below the limit required for food packaging materials | [92] | |
| CS powder | Potato starch | Antioxidant and UV-protective films with increased elasticity, stretchability, and water resistance | [93] | |
| SCG | SCG powder treated with tetraethyl orthosilicate | Maleic-anhydride-grafted PLA formulation | Homogeneous composites with increased water resistance and biodegradability | [94] |
| Torrefied SCG powder | PBAT | Composites with increased hydrophobicity | [95] | |
| SCG powder | PVA (plus chitosan) | Homogeneous composites suitable for the adsorption of pharmaceuticals contaminants from water | [96] | |
| Corn starch | Films with increased tensile strength | [97] | ||
| Cellulose | Photosensitive films | [98] | ||
| Pectin | Films with increased water resistance | [99,100] |
| By-Product | Coffee-Based Molecules | Polymeric Matrix | Developed Materials and Main Properties | Ref |
|---|---|---|---|---|
| CP | Phenolic-rich extract | Chitosan | Films with increased water resistance, antioxidant, and antimicrobial properties | [108] |
| CM | Pectic polysaccharides | Pectic polysaccharides | Biodegradable films with rigidity and water insolubility | [52] |
| CPm | Phenolic-rich extract | Gellan gum | Films with improved antifungal properties | [42] |
| CH | Antioxidant and antibacterial aqueous extract | Corn starch | Antioxidant and antibacterial films with increased tensile strength and decreased water vapor and oxygen permeability | [58] |
| Cellulose fibers | Corn starch | Films with increased stiffness | [58] | |
| Antioxidant and antibacterial aqueous extract | Corn starch/PLA | Antioxidant films with decreased oxygen permeability | [111] | |
| Cellulose nanocrystals | Corn starch/PLA | Films with increased tensile strength and decreased gas permeabilities | [111] | |
| CS | Cellulose nanocrystals | PLA | Films with increased tensile strength and decreased gas permeability | [117] |
| SCG | Polysaccharide-rich extract | Carboxymethyl cellulose | Active brown films with increased light barrier, hydrophobicity, and tensile resistance | [118] |
| Oil | PLA | Composites with increased toughness suitable for 3D-printing applications | [106] | |
| Fatty acids-rich extract | PLA (plus diatomite) | Films with increased interfacial adhesion and decreased oxygen permeability | [107] | |
| Phenolic-rich extract | PVA/cassava starch | Antioxidant, antimicrobial, and antibacterial films | [112] | |
| Cassava starch | Antioxidant, antimicrobial, and antibacterial films | [113] | ||
| Polysaccharide-rich extract | Galactomannans | Heterogeneous films with light-brownish coloration | [64] | |
| Galactomannans-rich extract | Galactomannans | Heterogeneous and rigid films with light-brownish coloration | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods 2021, 10, 683. https://doi.org/10.3390/foods10030683
Oliveira G, Passos CP, Ferreira P, Coimbra MA, Gonçalves I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods. 2021; 10(3):683. https://doi.org/10.3390/foods10030683
Chicago/Turabian StyleOliveira, Gonçalo, Cláudia P. Passos, Paula Ferreira, Manuel A. Coimbra, and Idalina Gonçalves. 2021. "Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials" Foods 10, no. 3: 683. https://doi.org/10.3390/foods10030683
APA StyleOliveira, G., Passos, C. P., Ferreira, P., Coimbra, M. A., & Gonçalves, I. (2021). Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods, 10(3), 683. https://doi.org/10.3390/foods10030683










