Antimicrobial Effect of Zn2+ Ions Governs the Microbial Quality of Donor Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacteria from HM
2.2. Growth Medium and Conditions
2.3. Growth Analysis of General Viable Bacterial Population in HM
2.4. Growth Analysis for HM Isolates
2.5. Scanning Electron Microscopy (SEM) Analysis for Visualization the Morphological Changes of Bacterial Cells
2.6. Statistical Analysis
3. Results
3.1. Isolation and Identification of Bacterial Species Contaminating in HM
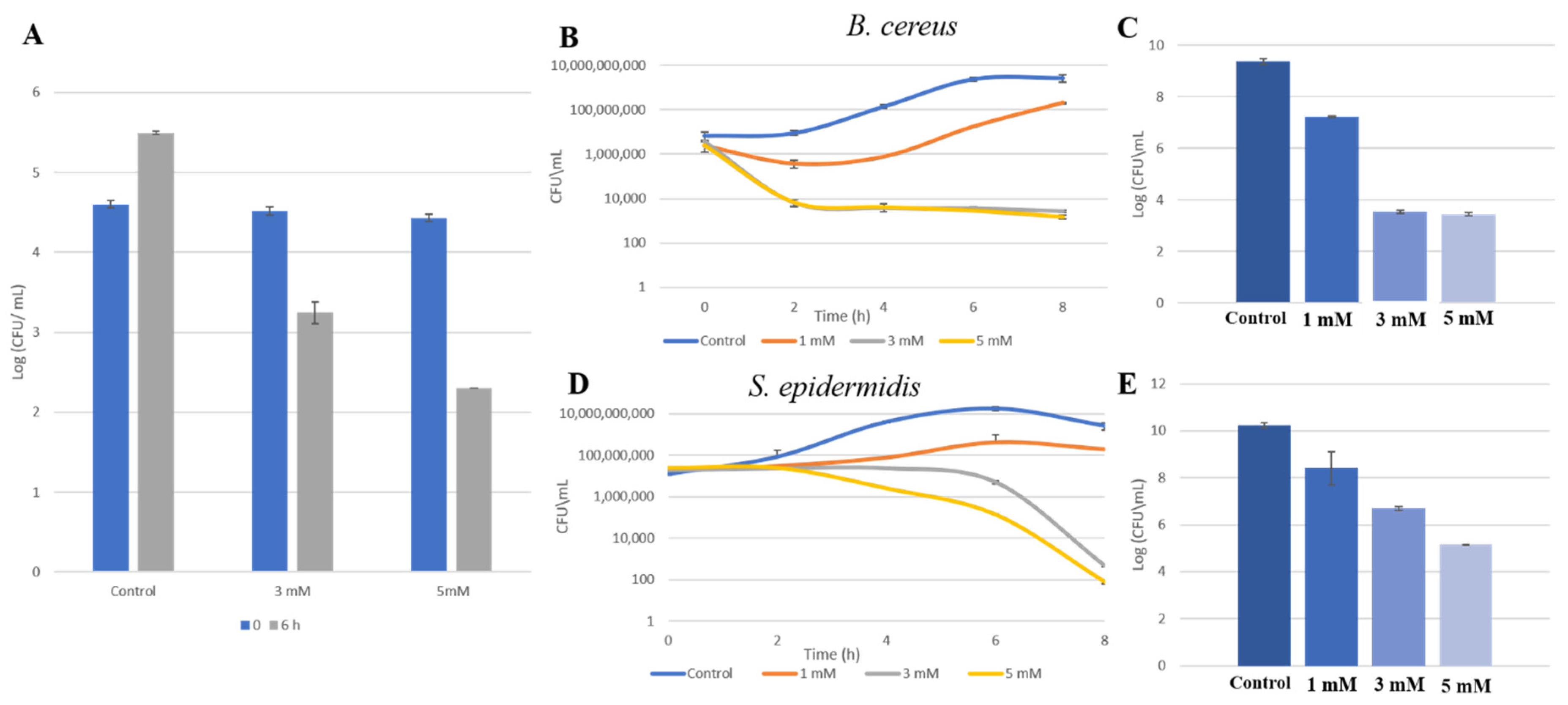
3.2. Zn2+ Ions Inhibit Bacterial Growth in HM
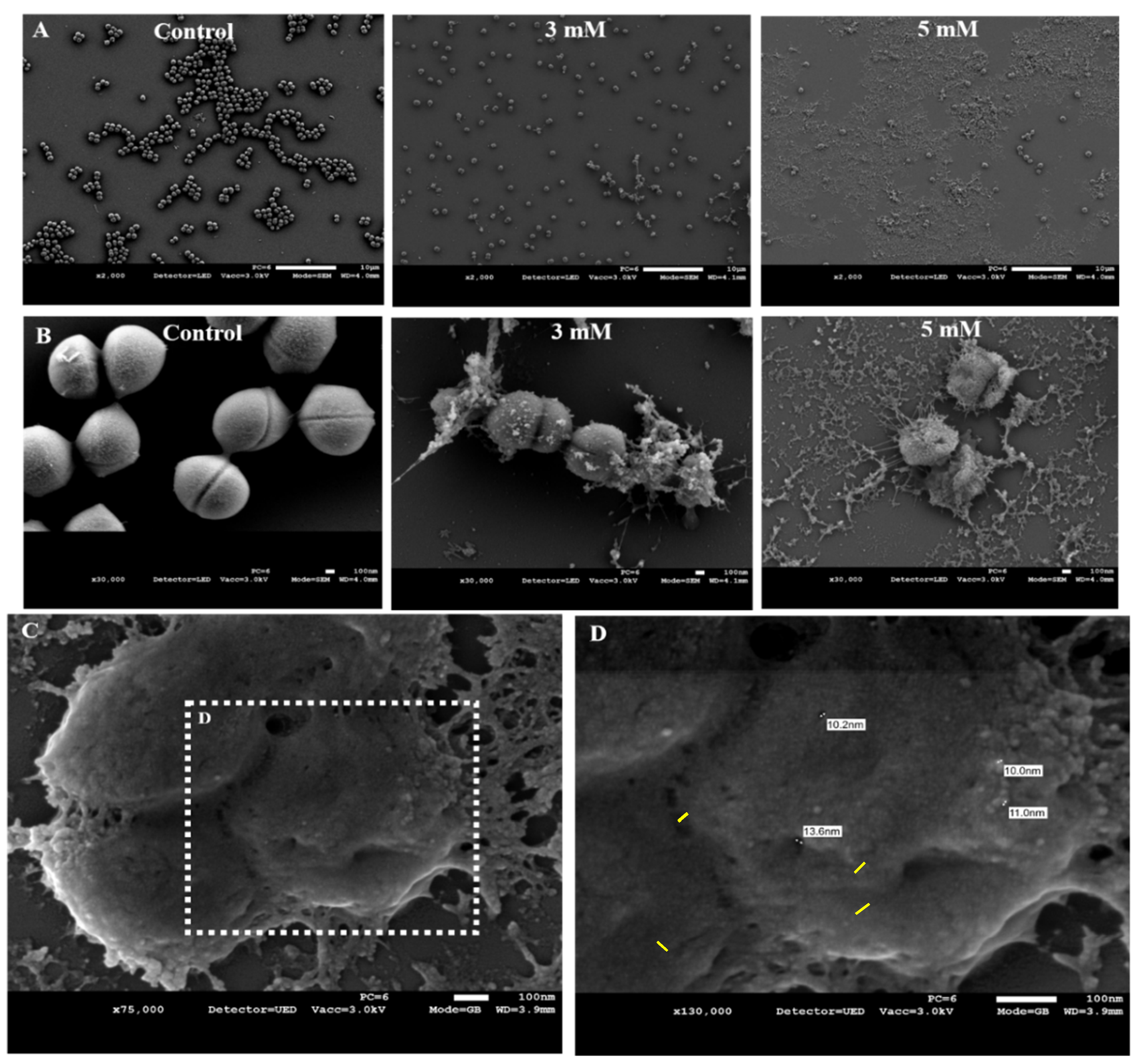
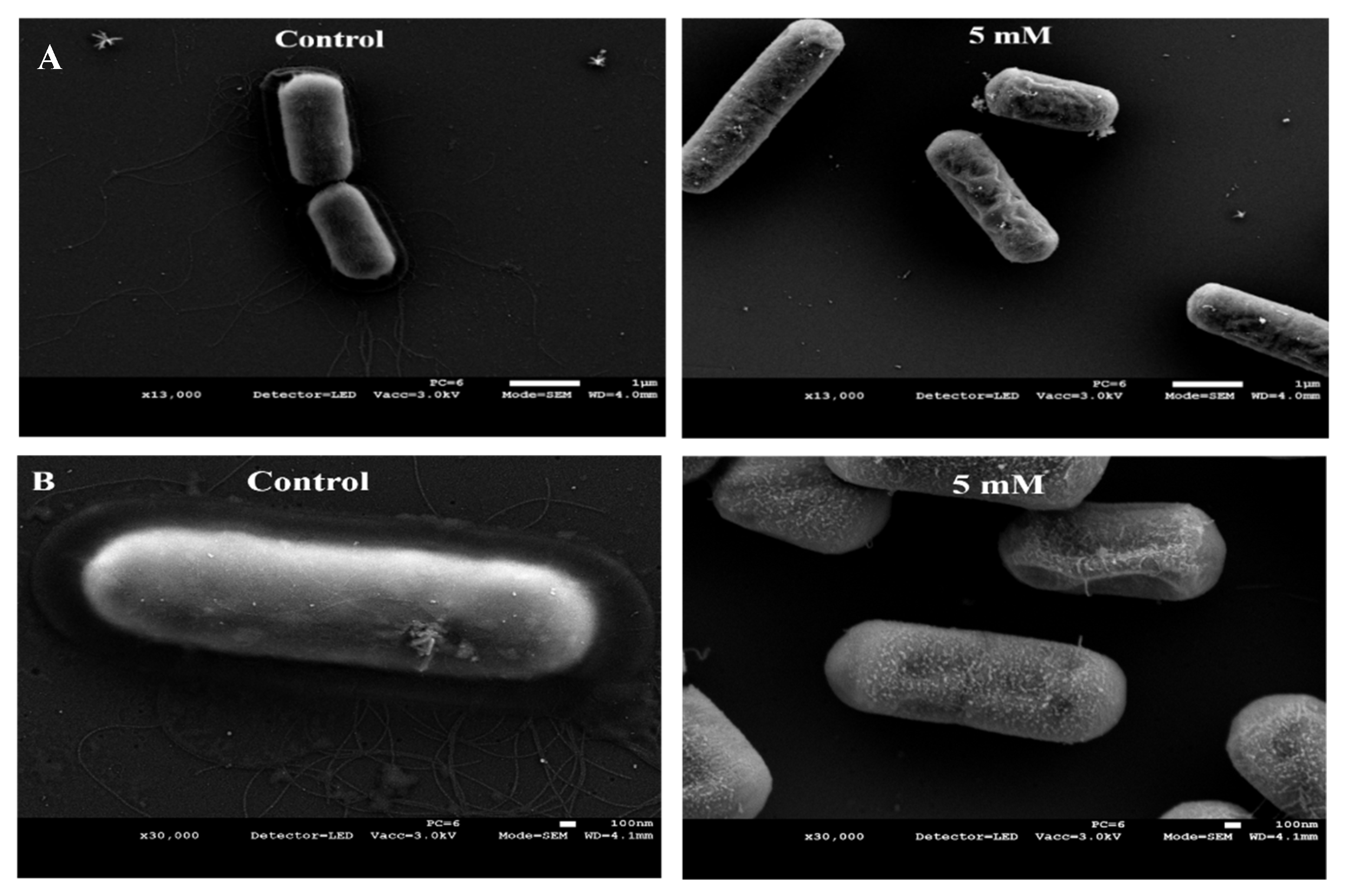
3.3. Zn2+ Ions Cause Morphological Deformation in B. cereus and Cell Lysis in S. epidermidis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, O. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. 2014, 60, 1–24. [Google Scholar]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized Trial of Exclusive Human Milk Versus Preterm Formula Diets in Extremely Premature Infants. J. Pediatr. 2013, 163, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newborn. Donor Human Milk for the High-Risk Infant: Preparation, Safety, and Usage Options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef] [PubMed]
- Meinzen-Derr, J.; Poindexter, B.; Wrage, L.; Morrow, A.L.; Stoll, B.; Donovan, E.F. Role of Human Milk in Extremely Low Birth Weight Infants’ Risk of Necrotizing Enterocolitis or Death. J. Perinatol. 2009, 29, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Shulman, R.J.; Lau, C. Feeding Strategies for Premature Infants: Beneficial Outcomes of Feeding Fortified Human Milk Versus Preterm Formula. Pediatrics 1999, 103, 1150–1157. [Google Scholar] [CrossRef]
- Landers, S.; Updergrove, K. Bacteriological Screening of Donor Human Milk Bafore and After Holder Pasteurization. Breastfeed Med. 2010, 5, 117–121. [Google Scholar] [CrossRef]
- Underwood, M.A. Human Milk for the Premature Infant Human milk Premature infant Necrotizing enterocolitis Donor milk Lactation. Pediatr. Clin. 2013, 60, 189–207. [Google Scholar]
- Picaud, J. Human Milk—Treatment and Quality of Banked Human Milk. Clin. Perinatol. 2017, 44, 95–119. [Google Scholar] [CrossRef]
- Israel Minestry of Health. Human Milk Bank- Guidelines for the Establishment and Operation of Human Milk Bank in Israel, Statement 4/2016. 2016. Available online: https://www.health.gov.il/hozer/BZ04_2016.pdf (accessed on 28 February 2018).
- Clifford, V.; Klein, L.D.; Sulfaro, C.; Karalis, T.; Hoad, V.; Gosbell, I.; Pink, J. What are Optimal Bacteriological Screening Test Cut-Offs for Pasteurized Donor Human Milk Intended for Feeding Preterm Infants? J. Hum. Lact. 2021, 37, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, C.; Courdent, P.; Charlet, C.; Dumoulin, D.; Courcol, R.; Pierrat, V. Contamination of Human Milk With Aerobic Flora: Evaluation of Losses for a Human Milk Bank. Arch. Pediatr. 2015, 22, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Simmer, K.; Hartmann, B. The Knowns and Unknowns of Human Milk Banking. Early Hum. Dev. 2009, 85, 701–704. [Google Scholar] [CrossRef]
- Gomez-gallego, C.; Garcia-mantrana, I.; Salminen, S.; Carmen, M. The human milk microbiome and factors influencing its composition and activity. Semin. Fetal Neonatal Med. 2016, 6, 400–405. [Google Scholar] [CrossRef]
- Wills, M.E.; Han, V.E.; Harris, D.A.; Baum, J.D. Short-time low-temperature pasteurisation of human milk. Early Hum. Dev. 1982, 7, 71–80. [Google Scholar] [CrossRef]
- Aires, G.S.; Walter, E.H.; Junqueira, V.C.; Roig, S.M.; Faria, J.A. Bacillus cereus in refrigerated milk submitted to different heat treatments. J. Food Prot. 2009, 72, 1301–1305. [Google Scholar] [CrossRef]
- Lin, S.; Schraft, H.; Odumeru, J.A.; Griffiths, M.W. Identification of contamination sources of Bacillus cereus in pasteurized milk. Int. J. Food Microbiol. 1998, 43, 159–171. [Google Scholar] [CrossRef]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 2000, 51, 451–460. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Stremick, C.A.; Turner, R.J. Biofilm susceptibility to metal toxicity. Environ. Microbiol. 2004, 6, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Oknin, H.; Steinberg, D.; Shemesh, M. Magnesium ions mitigate biofilm formation of Bacillus species via downregulation of matrix genes expression. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ben-ishay, N.; Oknin, H.; Steinberg, D.; Berkovich, Z.; Reifen, R.; Shemesh, M. Enrichment of milk with magnesium provides healthier and safer dairy products. Biofilms Microbiomes 2017, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.; Rajasekharan, S.K.; Reifen, R.; Shemesh, M. Mitigating Milk-Associated Bacteria through Inducing Zinc Ions Antibiofilm Activity. Foods 2020, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Sheng, J.; Nguyen, P.T.M.; Marquis, R.E. Co-operative inhibition by fluoride and zinc of glucosyl transferase production and polysaccharide synthesis by mutans streptococci in suspension cultures and biofilms. FEMS Microbiol. Lett. 2006, 254, 134–140. [Google Scholar] [CrossRef]
- Phan, T.N.; Buckner, T.; Sheng, J.; Baldeck, J.D.; Marquis, R.E. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol. Immunol. 2004, 19, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Serafini, A.B.; André, M.C.; Rodrigues, M.A.; Kipnis, A.; Carvalho, C.O.; Campos, M.R.; Monteiro, E.C.; Martins, F.; Jubé, T.F. Microbiological quality of human milk from a Brazilian milk bank. Rev. Saude Publica 2003, 37, 775–779. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, P.H.; Surana, A.U.; Chaudhari, V. Bacteriological Analysis of Donor Human Milk in Milk Bank in an Indian Setting. Indian J. Child Health 2017, 4, 7–9. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, Z.; Lotfi, M.H.; Nori, S.; Zandi, H.; Taghipour-Zahir, S.; Akhondzardaini, R. Bacterial Contamination of Expressed Breast Milk in Neonatal Intensive Care Unit. Zahedan J. Res. Med. Sci. 2013, 15, 48–52. [Google Scholar]
- El-Mohandes, A.E.; Schatz, V.; Keiser, J.F.; Jackson, B.J. Bacterial contaminants of collected and frozen human milk used in an intensive care nursery. AJIC Am. J. Infect. Control 1993, 5, 226–230. [Google Scholar] [CrossRef]
- Heikkilä, M.P.; Saris, P.E.J. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J. Appl. Microbiol. 2003, 95, 471–478. [Google Scholar] [CrossRef]
- Chen, P.W.; Lin, Y.L.; Huang, M.S. Profiles of commensal and opportunistic bacteria in human milk from healthy donors in Taiwan. J. Food Drug Anal. 2018, 26, 1235–1244. [Google Scholar] [CrossRef]
- Fey, P.D.; Olson, M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010, 5, 917–933. [Google Scholar] [CrossRef]
- Rogers, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. N. Am. 2009, 23, 73–98. [Google Scholar] [CrossRef]
- Begović, J.; Jovčić, B.; Papić-Obradović, M.; Veljović, K.; Lukić, J.; Kojić, M.; Topisirović, L. Genotypic diversity and virulent factors of Staphylococcus epidermidis isolated from human breast milk. Microbiol. Res. 2013, 168, 77–83. [Google Scholar] [CrossRef]
- Serra, V.V.; Teves, S.; De Volder, A.L.; Ossorio, F.; Aguilar, N.; Armadans, M. Comparison of the risk of microbiological contamination between samples of breast milk obtained at home and at a healthcare facility. Arch. Argent Pediatr. 2013, 111, 115–119. [Google Scholar] [CrossRef]
- Mullié, C.; Obin, O.; Outurquin, G.; Grognet, S.; Léké, A.; Adjidé, C. Breastmilk donations: Bacteriological assessment, analysis of causes of non-compliance and suggestions for improvement. Arch. Pediatr. 2018, 25, 263–268. [Google Scholar] [CrossRef]
- Almutawif, Y.; Hartmann, B.; Lloyd, M.; Erber, W.; Geddes, D. A retrospective audit of bacterial culture results of donated human milk in Perth, Western Australia. Early Hum. Dev. 2017, 105, 1–6. [Google Scholar] [CrossRef]
- Lima, H.K.; Wagner-Gillespie, M.; Perrin, M.T.; Fogleman, A.D. Bacteria and bioactivity in Holder pasteurized and shelf-stable human milk products. Curr. Dev. Nutr. 2017, 1, e001438. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.L.O.; Ewaschuk, J.B.; Unger, S. Human milk pasteurization: Benefits and risks. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 269–275. [Google Scholar]
- Atmaca, S. The Effect of Zinc on Microbial Growth. Turk. J. Med. Sci. 1998, 28, 595–597. [Google Scholar]
- Yarawsky, A.E.; Johns, S.L.; Schuck, P.; Herr, A.B. The biofilm adhesion protein Aap from Staphylococcus epidermidis forms zinc-dependent amyloid fibers. J. Biol. Chem. 2020, 295, 4411–4427. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Ramasamy, S.N.; Balachandran, C. Size-dependent antimicrobial response of zinc oxide nanoparticles. IET Nanobiotechnol. 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Haase, H.; Overbeck, S.; Rink, L. Zinc supplementation for the treatment or prevention of disease: Current status and future perspectives. Exp. Gerontol. 2008, 43, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Luchinat, C. The Reaction Pathways of Zinc Enzymes and Related Biological Catalysts. In Bioorganic Chemistry, Caltech Book; University Science Book: Mill Valley, CA, USA, 1994; pp. 37–106. [Google Scholar]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Colloids and Surfaces B: Biointerfaces Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2014, 115, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.D.; Anthony, J.R.; Donohue, T.J. The importance of zinc-binding to the function of Rhodobacter sphaeroides ChrR as an anti-sigma factor. J. Mol. Biol. 2001, 313, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bottrill, A.R.; Bibb, M.J.; Buttner, M.J.; Paget, M.S.B.; Kleanthous, C. The Role of Zinc in the Disulphide Stress-regulated Anti-sigma Factor RsrA from Streptomyces coelicolor. J. Mol. Biol. 2003, 333, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Francke, C.; Kormelink, T.G.; Hagemeijer, Y.; Overmars, L.; Sluijter, V.; Moezelaar, R.; Siezen, R.J. Comparative analyses imply that the enigmatic sigma factor 54 is a central controller of the bacterial exterior. BMC Genom. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, H.; Tempelaars, M.; Groot, M.N.; Abee, T. Bacillus cereus ATCC 14579 RpoN (Sigma 54) is a pleiotropic regulator of growth, carbohydrate metabolism, motility, biofilm formation and toxin production. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Yoshida, K.; Yasuda, Y.; Tsutsui, T. Infantile zinc deficiency: Association with autism spectrum disorders. Sci. Rep. 2011, 1, 1–5. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Terrin, G.; Berni Canani, R.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; De Curtis, M. Zinc supplementation reduces morbidity and mortality in very-low-birth-weight preterm neonates: A hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Optimal Feeding of Low-Birth-Weight Infants. 2006. Available online: https://www.who.int/maternal_child_adolescent/documents/9241595094/en/ (accessed on 8 February 2021).
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Rossander-Hultén, L.; Brune, M.; Sandström, B.; Lönnerdal, B.; Hallberg, L. Competitive inhibition of iron absorption by manganese and zinc in humans. Am. J. Clin. Nutr. 1991, 54, 152–156. [Google Scholar] [CrossRef] [PubMed]

| Pre-Pasteurization | Post-Pasteurization |
|---|---|
| Total colony count < 105 CFU/mL | Total colony count < 10 CFU/mL |
| Enterobacteriaceae < 104 CFU/mL | |
| Staphylococcus aureus < 104 CFU/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchings, C.; Prokocimer Yair, Z.; Reifen, R.; Shemesh, M. Antimicrobial Effect of Zn2+ Ions Governs the Microbial Quality of Donor Human Milk. Foods 2021, 10, 637. https://doi.org/10.3390/foods10030637
Hutchings C, Prokocimer Yair Z, Reifen R, Shemesh M. Antimicrobial Effect of Zn2+ Ions Governs the Microbial Quality of Donor Human Milk. Foods. 2021; 10(3):637. https://doi.org/10.3390/foods10030637
Chicago/Turabian StyleHutchings, Carmel, Zafnat Prokocimer Yair, Ram Reifen, and Moshe Shemesh. 2021. "Antimicrobial Effect of Zn2+ Ions Governs the Microbial Quality of Donor Human Milk" Foods 10, no. 3: 637. https://doi.org/10.3390/foods10030637
APA StyleHutchings, C., Prokocimer Yair, Z., Reifen, R., & Shemesh, M. (2021). Antimicrobial Effect of Zn2+ Ions Governs the Microbial Quality of Donor Human Milk. Foods, 10(3), 637. https://doi.org/10.3390/foods10030637







