Theobromacacao Criollo var. Beans: Biological Properties and Chemical Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection and Extraction Procedure
2.3. Antiradical Activity
2.3.1. ABTS Assay
2.3.2. DPPH Assay
2.4. Antimutagenicity and Antigenotoxicity Activity
2.4.1. Salmonella Mutagenicity Assay (Ames Test)
2.4.2. Salmonella Genotoxicity Assay (Umu Test)
2.5. Cell Viability Inhibition
2.5.1. Cultivation of Human Cancer Cell Lines
2.5.2. MTT Assay
2.6. UV-Vis Spectroscopy for ICB and PCB Extracts’ Analysis
2.7. UHPLC-ESI-QqTOF-MS/MS Analysis
2.8. Statistical Analysis
3. Results
3.1. Antiradical Activity
3.2. Mutagenicity/Genotoxicity
3.3. Antimutagenicity
3.4. Antigenotoxicity
3.5. Cell Viability
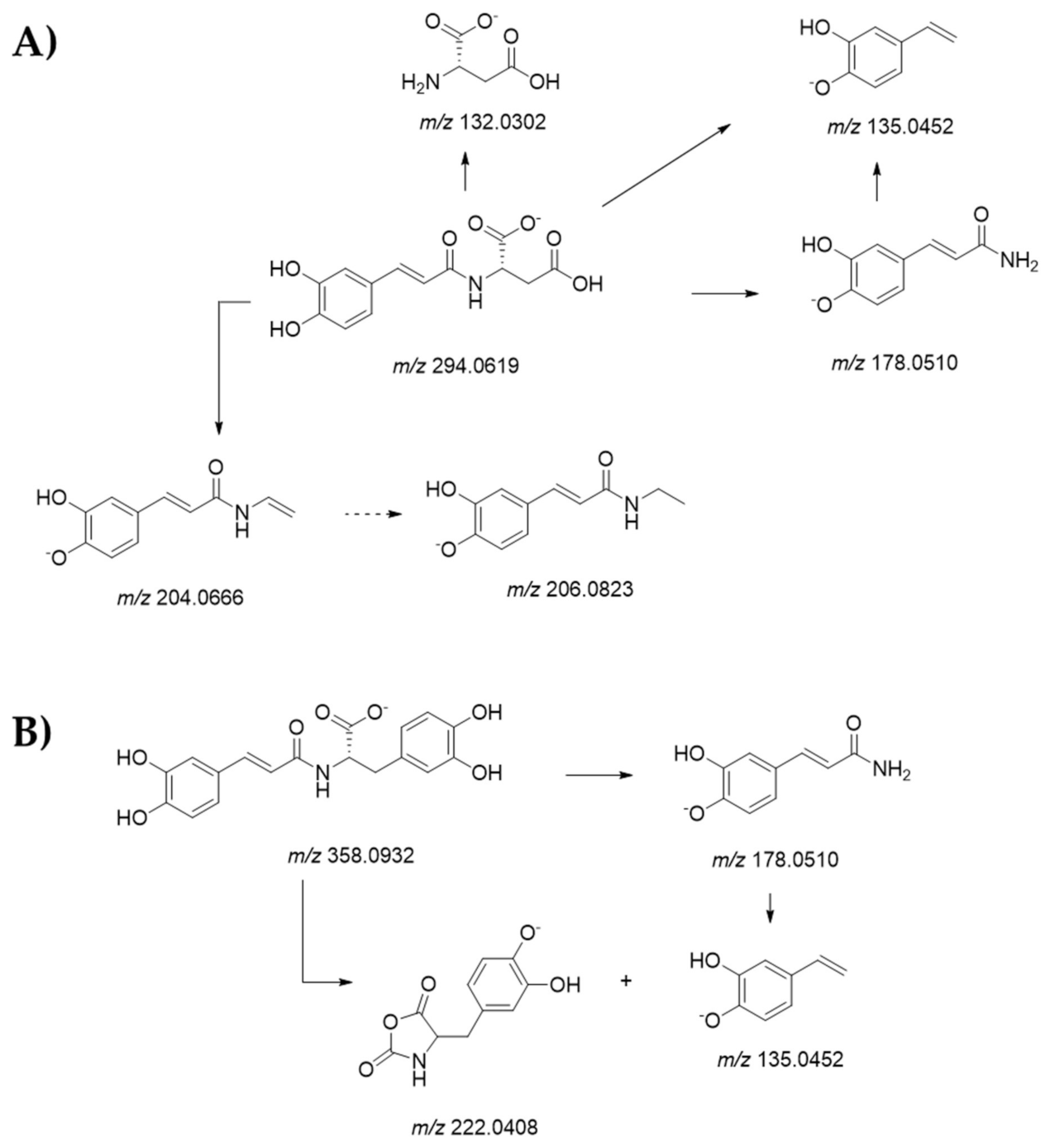
3.6. Compounds in Cocoa Beans Extracts from UV-Vis and UHPLC-ESI-QqTOF-MS/MS Analysis
3.6.1. UHPLC-ESI-QqTOF-MS/MS Analyses of Hydroalcoholic Extracts
3.6.2. UHPLC-ESI-QqTOF-MS/MS Analyses of Lipophilic Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagarathna, P.K.M.; Johnson Wesley, M.; Sriram Reddy, P.; Reena, K. Review on genotoxicity, its molecular mechanisms and prevention. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 236–243. [Google Scholar]
- Słoczyńska, K.; Powroźnik, B.; Pękala, E.; Waszkielewicz, A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014, 55, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Goya, L.; Ramos, S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem. Toxicol. 2013, 56, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, S.; Stehle, P. Impact of cocoa consumption on inflammation processes—A critical review of randomized controlled trials. Nutrients 2016, 8, 321. [Google Scholar] [CrossRef]
- Henderson, J.S.; Joyce, R.A.; Hall, G.R.; Hurst, W.J.; McGovern, P.E. Chemical and archaeological evidence for the earliest cacao beverages. Proc. Natl. Acad. Sci. USA 2007, 104, 18937–18940. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor chemistry of cocoa and cocoa products-an overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.M.; Chejne, F.; Ciro, H.; Montoya, J. Roasting impact on the chemical and physical structure of Criollo cocoa variety (Theobroma cacao L). J. Food Process Eng. 2020, 1–15. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lavorgna, M.; Orlo, E.; Nugnes, R.; Piscitelli, C.; Russo, C.; Isidori, M. Capsaicin in hot chili peppers: In vitro evaluation of its antiradical, antiproliferative and apoptotic activities. Plant Foods Hum. Nutr. 2019, 74, 164–170. [Google Scholar] [CrossRef]
- Rakholiya, K.; Kaneria, M.; Nagani, K.; Patel, A.; Chanda, S. Comparative analysis and simultaneous quantification of antioxidant capacity of four terminalia species using various photometric assays. World J. Pharm. Res. 2015, 4, 1280–1296. [Google Scholar]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives—Inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pacifico, S.; Gallicchio, M.; Lorenz, P.; Duckstein, M.; Potenza, N.; Galasso, S.; Marciano, S.; Fiorentino, A.; Stintzing, F.C.; Monaco, P. Neuroprotective potential of Laurus nobilis antioxidant polyphenol-enrichedleaf extracts. Chem. Res. Toxicol. 2014, 27, 611–626. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Resende, F.A.; Campos, D.L.; da Silva, V.C.; De Grandis, R.A.; Souza, L.P.; Leonardo Junior, C.S.; da Rocha, C.Q.; Dossantos, L.C.; Vilegas, W.; Varabda, E.A. Mutagenicity and chemopreventive activities of Astronium species ossesse by Ames test. Regul. Toxicol. Pharmacol. 2015, 72, 506–513. [Google Scholar] [CrossRef]
- ISO/DIS 13829. Water Quality Determination of the Genotoxicity of Water and Waste Water Using the Umu-Test; International Standards Organisation: Brussels, Belgium, 2000. [Google Scholar]
- Skrzypczak, A.; Przystupa, N.; Zgadzaj, A.; Parzonko, A.; Sykłowska-Baranek, K.; Paradowska, K.; Nałęcz-Jawecki, G. Antigenotoxic, anti-photogenotoxic and antioxidant activities of natural naphthoquinone shikonin and acetylshikonin and Arnebia euchroma callus extracts evaluated by the Umu-test and EPR method. Toxicol. In Vitro 2015, 30, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Caillet, S.; Lessard, S.; Lamoureux, G.; Lacroix, M. Umu test applied for screening natural antimutagenic agents. Food Chem. 2011, 124, 1699–1707. [Google Scholar] [CrossRef]
- Baharum, Z.; Akim, A.M.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Nocera, P.; Pacifico, F.; Manti, L.; Pacifico, S. Ultrasound-assisted aqueous extraction, LC-MS/MS analysis and radiomodulating capability of autochthonous Italian sweet cherry fruits. Food Funct. 2018, 9, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Masike, K.; Mhlongo, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 29. [Google Scholar] [CrossRef]
- Locatelli, M.; Travaglia, F.; Lorella Giovannelli, L.; Coïsson, J.; Bordiga, M.; Pattarino, F.; Arlorio, M. Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int. 2013, 50, 129–134. [Google Scholar] [CrossRef]
- Bouchez, P.; Teixeira, B.V.; Baidoo, E.E.K.; Mortimer, J.C.; Sullivan, M.L.; Scheller, H.V.; Eudes, A. Production of clovamide and its analogues in Saccharomyces cerevisiae and Lactococcus lactis. Lett. Appl. Microbiol. 2019, 69, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl flavonol glycosides and more in marketed green teas: An intrinsic value beyond mMuch-lauded catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schubel, B.; Thiele, B.; Fett, R.; Colensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Inter. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- De Taeye, C.; Caullet, G.; Eyamo Evina, V.J.; Collin, S. Procyanidin A2 and its degradation products in raw, fermented, and roasted cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef]
- de Souza, L.M.; Cipriani, T.R.; Iacomini, M.; Gorin, P.A.; Sassaki, G.L. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J. Pharm. Biomed. Anal. 2008, 47, 59–67. [Google Scholar] [CrossRef]
- Jalil, A.M.; Ismail, A. Polyphenols in cocoa and cocoa products: Is there a link between antioxidant properties and health? Molecules 2008, 13, 2190–2219. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Lavorgna, M.; Scognamiglio, M.; Russo, C.; Graziani, V.; Piscitelli, C.; Fiorentino, A.; Isidori, M. 2D-NMR investigation and in vitro evaluation of antioxidant, antigenotoxic and estrogenic/antiestrogenic activities of strawberry grape. Food Chem. Toxicol. 2017, 105, 52–60. [Google Scholar] [CrossRef]
- Piscitelli, C.; Lavorgna, M.; De Prisco, R.; Coppola, E.; Grilli, E.; Russo, C.; Isidori, M. Tomato plants (Solanum lycopersicum L.) grown in experimental contaminated soil: Bioconcentration of potentially toxic elements and free radical scavenging evaluation. PLoS ONE 2020, 15, e0237031. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Formato, M.; Pacifico, S. A cup of hemp coffee by moka pot from Southern Italy: An UHPLC-HRMS investigation. Foods 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Tokede, O.A.; Gaziano, J.M.; Djoussé, L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur. J. Clin. Nutr. 2011, 65, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Tan, L.; Wu, H.; Fang, Y.; Xu, F.; Chu, Z.; Wang, Q. Comparison of cocoa beans from China, Indonesia and Papua New Guinea. Foods 2013, 2, 183–197. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef]
- Mustiga, G.M.; Morrissey, J.; Stack, J.C.; DuVal, A.; Royaert, S.; Jansen, J.; Bizzotto, C.; Villela-Dias, C.; Mei, L.; Cahoon, E.B.; et al. Identification of climate and genetic factors that control fat content and fatty acid composition of Theobroma cacao L. Beans. Front. Plant Sci. 2019, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [CrossRef]
- Grzelczyk, A.; Gendaszewska-Darmach, E. Novel bioactive glycerol-based lysophospholipids: New data-new insight into their function. Biochimie 2013, 95, 667–679. [Google Scholar] [CrossRef]
- Arifin, S.A.; Paternoster, S.; Carlessi, R.; Casari, I.; Ekberg, J.H.; Maffucci, T.; Newsholme, P.; Rosenkilde, M.M.; Falasca, M. Oleoyl-lysophosphatidylinositol enhances glucagon-like peptide-1 secretion from enteroendocrine L-cells through GPR119. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1863, 1132–1141. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, B.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Cartron, E.; Carbonneau, M.A.; Fouret, G.; Descomps, B.; Léger, C.L. Specific antioxidant activity of caffeoyl derivatives and other natural phenolic compounds: LDL protection against oxidation and decrease in the proinflammatory lysophosphatidylcholine production. J. Nat. Prod. 2001, 64, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Locatelli, M.; Bardelli, C.; Amoruso, A.; Coisson, J.D.; Travaglia, F.; Arlorio, M.; Brunelleschi, S. Anti-inflammatory properties of clovamide and Theobroma cacao phenolic extracts in human monocytes: Evaluation of respiratory burst, cytokine release, NF-κB activation, and PPARγ modulation. J. Agric. Food Chem. 2011, 59, 5342–5350. [Google Scholar] [CrossRef]
- Tsunoda, T.; Takase, M.; Shigemori, H. Structure-activity relationship of clovamide and its related compounds for the inhibition of amyloid β aggregation. Bioorg. Med. Chem. 2018, 26, 3202–3209. [Google Scholar] [CrossRef]
- Fallarini, S.; Miglio, G.; Paoletti, T.; Minassi, A.; Amoruso, A.; Bardelli, C.; Brunelleschi, S.; Lombardi, G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br. J. Pharmacol. 2009, 157, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, B.; Cao, S.; Yang, E.; Xu, X.; Guo, S. A-type procyanidins from Litchi chinensis pericarp with antioxidant activity. Food Chem. 2007, 105, 1446–1451. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Ham, H.; Jeong, H.S.; Lee, J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem. 2013, 137, 136–141. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, A.; Madrigal-Santillán, E. Evidence of some natural products with antigenotoxic effects. Part 2: Plants, vegetables, and natural resin. Nutrients 2018, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Osakabe, N.; Natsume, M.; Adachi, T.; Takizawa, T.; Kumon, H.; Osawa, T. Anticlastogenic activity of cacao: Inhibitory effects of cacao liquor polyphenols against mitomycin C-induced DNA damage. Food Chem. Toxicol. 2001, 39, 1279–1283. [Google Scholar] [CrossRef]
- Cajurao, E.C.; Revale, I.F.H. Antigenotoxicity screening of Coffee (Coffea arabica Linn) and Cacao (Theobroma cacao Linn.). APJEAS 2016, 3, 94–97. [Google Scholar]
- Simon Luca, V.; Miron, A.; Aprotosoaie, A.C. The antigenotoxic potential of dietary flavonoids. Phytochem. Rev. 2016, 15, 591–625. [Google Scholar] [CrossRef]
- Bauer, D.; Pimentel de Abreu, J.; Salete, H.; Oliveira, S.; Goes-Neto, A.; Bello Koblitz, M.G.; Junger Teodoro, A. Antioxidant activity and cytotoxicity effect of cocoa beans subjected to different processing conditions in human lung carcinoma cells. Oxid. Med. Cell Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Ramljak, D.; Romanczyk, L.J.; Metheny-Barlow, L.J.; Thompson, N.; Knezevic, V.; Galperin, M.; Ramesh, A.; Dickson, R.B. Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol. Cancer Ther. 2005, 4, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Carnésecchi, S.; Schneider, Y.; Lazarus, S.A.; Coehlo, D.; Gossé, F.; Raul, F. Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells. Cancer Lett. 2002, 175, 147–155. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Tückmantel, W.; Böttcher, G.; Romanczyk, L.J.J. Studies in polyphenol chemistry and bioactivity. 4. Synthesis of trimeric, tetrameric, pentameric, and higher oligomeric epicatechin-derived procyanidins having all-4β,8-interflavan connectivity and their inhibition of cancer cell growth through cell cycle arrest. J. Org. Chem. 2003, 68, 1641–1658. [Google Scholar]
- Choi, J.S.; Piao, Y.J.; Kang, K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition of quercetin. Arch. Pharm. Res. 2011, 34, 607–613. [Google Scholar] [CrossRef]
- Ramos, S.; Rodriguez-Ramiro, I.; Martin, M.A.; Goya, L.; Bravo, L. Dietary flavanols exert different effects on antioxidant defenses and apoptosis/proliferation in Caco-2 and SW480 colon cancer cells. Toxicol. In Vitro 2011, 25, 1771–1781. [Google Scholar] [CrossRef]
- Witjaksono, J.A. Cocoa farming system in Indonesia and its sustainability under climate change. Agric. For. Fish. 2016, 5, 170–180. [Google Scholar] [CrossRef][Green Version]
- Keller, M.; Echeverria, D.; United Nations Development Programme (UNDP), Bureau for Crisis Prevention and Recovery (BCPR). Climate Risk Management for Agriculture in Peru: Focus on the Regions of Junín and Piura; United Nations Development Programme (UNDP), Bureau for Crisis Prevention and Recovery (BCPR): New York, NY, USA, 2012. [Google Scholar]
- Kelloff, G.J.; Lippman, S.M.; Dannenberg, A.J.; Sigman, C.C.; Pearce, H.L.; Reid, B.J.; Szabo, E.; Jordan, V.C.; Spitz, M.R.; Mills, G.B.; et al. Progress in Chemoprevention Drug Development: The Promise of Molecular Biomarkers for Prevention of Intraepithelial Neoplasia and Cancer—A Plan to Move Forward. Clin. Cancer Res. 2006, 12, 3661–3697. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H.; Feskens, E.J.M.; Bueno de Mesquita, H.B.; Kromhout, D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 2001, 55, 76–81. [Google Scholar] [CrossRef]
- Tabernero, M.; Serrano, J.; Saura-Calixto, F. The antioxidant capacity of cocoa products: Contribution to the Spanish diet. Int. J. Food Sci. Technol. 2006, 4, 28–32. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Brillo, E.; Romanelli, M.; Porcaro, G.; Capanna, F.; Kanninen, T.T.; Gerli, S.; Clerici, G. Potential effects of chocolate on human pregnancy: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2012, 25, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, B.; Derewiaka, D.; Lenart, A.; Kowalska, J. Changes in the composition and content of polyphenols in chocolate resulting from pre-treatment method of cocoa beans and technological process. Eur. Food Res. Technol. 2019, 245, 2101–2112. [Google Scholar] [CrossRef]
- Sudarma, V.; Sukmaniah, S.; Siregar, P. Effect of dark chocolate on nitric oxide serum levels and blood pressure in prehypertension subjects. Acta Med. Indones. 2011, 43, 224–228. [Google Scholar] [PubMed]
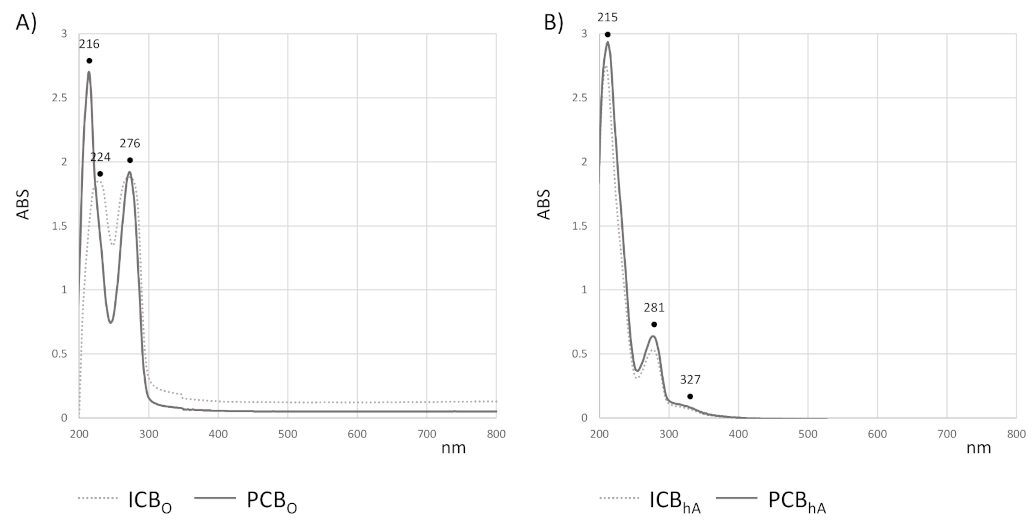

| EC50 | TEAC | |||
|---|---|---|---|---|
| DPPH | ABTS | DPPH | ABTS | |
| Trolox | 74.97 (64.31–87.40) | 14.23 (12.40–16.30) | - | - |
| ICBhA | 186.00 (165.00–209.80) | 72.63 (67.66–77.84) | 0.40 | 0.20 |
| PCBHa | 289.30 (258.60–323.80) | 322.20 (257.60–403.10) | 0.26 | 0.04 |
| Treatment [μg/mL] | TA98 Revertants/Plate (Mean ± SD) | Inhibition Rate (% Mean ± SD) | TA100 Revertants/Plate (Mean ± SD) | Inhibition Rate (% Mean ± SD) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2-NF 3 2.5 μg/mL | 2-NF 10 μg/mL | 2-NF 2.5 μg/mL | 2-NF 10 μg/mL | SOD 4 5 μg/mL | SOD 20 μg/mL | SOD 5 μg/mL | SOD 20 μg/mL | ||
| NC 1 | 69 ± 7 | - | - | 232 ± 0 | - | - | |||
| M 2 | 157 ± 8 | 646 ± 1 | - | - | 471 ± 9 | 1075 ± 7 | - | - | |
| ICBhA | 10 | 39 ± 7 *** | 391 ± 78 ** | 75 ± 3 b | 39 ± 12 a | 119 ± 10 *** | 594 ± 37 *** | 75 ± 2 b | 45 ± 3 b |
| 50 | 27 ± 13 *** | 352 ± 83 ** | 83 ± 7 b | 45 ± 13 b | 183 ± 13 *** | 558 ± 36 *** | 61 ± 3 b | 48 ± 3 b | |
| 100 | 17 ± 4 *** | 217 ± 8 *** | 89 ± 2 b | 66 ± 1 b | 142 ± 31 *** | 530 ± 17 *** | 70 ± 7 b | 51 ± 1 b | |
| ICBO | 10 | 40 ± 0 *** | 267 ± 34 *** | 75 ± 1 b | 59 ± 5 b | 149 ± 1 *** | 636 ± 66 *** | 68 ± 0 b | 41 ± 6 b |
| 50 | 31 ± 6 *** | 246 ± 22 *** | 80 ± 3 b | 62 ± 3 b | 114 ± 0 *** | 621 ± 72 *** | 76 ± 0 b | 42 ± 6 b | |
| 100 | 22 ± 4 *** | 172 ± 15 *** | 86 ± 2 b | 73 ± 2 b | 170 ± 28 *** | 543 ± 18 *** | 64 ± 7 b | 49 ± 1 b | |
| PCBhA | 10 | 35 ± 3 *** | 418 ± 82 ** | 78 ± 1 b | 35 ± 13 a | 143 ± 35 *** | 630 ± 85 *** | 70 ± 7 b | 41 ± 8 b |
| 50 | 29 ± 6 *** | 330 ± 79 *** | 82 ± 3 b | 49 ± 12 b | 150 ± 57 *** | 610 ± 70 *** | 68 ± 11 b | 43 ± 6 b | |
| 100 | 20 ± 3 *** | 169 ± 24 *** | 87 ± 1 b | 74 ± 4 b | 188 ± 11 *** | 544 ± 26 *** | 60 ± 3 b | 49 ± 2 b | |
| PCBO | 10 | 42 ± 11 *** | 417 ± 38 ** | 73 ± 6 b | 35 ± 6 a | 141 ± 10 *** | 638 ± 52 *** | 70 ± 2 b | 41 ± 4 b |
| 50 | 30 ± 8 *** | 367 ± 87 ** | 81 ± 4 b | 43 ± 13 b | 112 ± 1 *** | 586 ± 8 *** | 76 ± 0 b | 46 ± 1 b | |
| 100 | 18 ± 6 *** | 171 ± 16 *** | 89 ± 3 b | 74 ± 2 b | 175 ± 18 *** | 547 ± 35 *** | 63 ± 5 b | 49 ± 4 b | |
| [μg/mL] | IR ± DS | Antigenotoxicity (% Mean ± SD) | |
|---|---|---|---|
| 4-NQO 1 | 0.05 | 2.31 ± 0.40 | - |
| ICBhA | 25 | 1.76 ± 0.39 | 24.15 ± 3.54 a |
| 50 | 1.55 ± 0.21 | 32.40 ± 2.70 a | |
| 100 | 1.01 ± 0.16 ** | 56.26 ± 1.01 b | |
| ICBO | 25 | 2.03 ± 0.32 | 26.05 ± 3.20 a |
| 50 | 1.59 ± 0.28 | 36.11 ± 2.29 a | |
| 100 | 1.23 ± 0.18 * | 58.30 ± 2.69 b | |
| PCBhA | 25 | 1.72 ± 0.37 | 11.98 ± 1.35 a |
| 50 | 1.48 ± 0.31 | 31.32 ± 0.01 a | |
| 100 | 0.96 ± 0.11 ** | 46.68 ± 1.78 b | |
| PCBO | 25 | 1.93 ± 0.45 | 16.80 ± 5.30 a |
| 50 | 1.59 ± 0.28 | 31.07 ± 0.24 a | |
| 100 | 1.18 ± 0.24 * | 48.85 ± 1.42 b |
| Cell Lines | IC50 | |||
|---|---|---|---|---|
| ICBhA | ICBO | PCBhA | PCBO | |
| MCF-7 | 254.20 (225.80–286.20) | >1000 | 708.30 (532.60–942.00) | >1000 |
| OE19 | 903.30 (606.00–1347.00) | >1000 | >1000 | >1000 |
| Hep-G2 | 122.00 (99.91–149.00) | >1000 | 199.70 (156.10–255.00) | >1000 |
| Caco-2 | 104.90 (73.06–150.60) | 181.50 (122.40–269.10) | 133.90 (88.92–201.70) | 234.00 (189.80–288.50) |
| RT (min) | Tentative Assignment | Formula | [M-H]− calc. (m/z) | [M-H]− Found (m/z) | Error (ppm) | RDB | MS/MS Fragment Ions (m/z) and Relative Intensity (%) | ICBhA Content (%) | PCBhA Content (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.275 | Hexytol | C6H14O6 | 181.0718 | 181.0716 | −0.9 | 0 | 113.0281, 101.0248 (100) | 0.97 | 0.19 |
| 2 | 0.295 | Disaccharide | C12H22O11 | 341.1094 | 341.1094 | 1.3 | 2 | 341.1094; 236.9674; 198.1265; 179.0553; 161.0459; 119.0363; 113.0252; 101.0253; 89.0254 (100) | 1.4 | 1.66 |
| 3 | 0.360 | Isocitric acid | C6H8O7 | 191.0197 | 191.0198 | 0.4 | 3 | 111.0087 (100); 87.0091 | 8.94 | 4.47 |
| 4 | 0.435 0.486 | Caffeoyl aspartic acid | C13H13NO7 | 294.0619 | 294.0618 [2M-H]− 589.1321 | −0.4 | 8 | 206.0828; 204.0697; 178.0527; 161.0236; 135.0466; 134.0384; 132.0318 (100); 115.0048; 88.0416 | 6.98 | 4.62 |
| 5 | 0.636 | Gallo(epi)catechin | C15H14O7 | 305.0667 | 305.0671 | 1.4 | 9 | 305.0671; 219.0667; 167.0353; 139.0403; 137.0248; 125.0248; 109.0299 | 1.42 | 1.65 |
| 6 | 0.615 | Procyanidin B type (e.g., B3) | C30H26O12 | 577.1352 | 577.1361 | 1.6 | 18 | 577.1377; 559.1285; 451.1040; 425.0886; 407.0777 (100); 339.0856; 299.0572; 289.0705 (98); 287.0556; 245.0825; 221.0810; 167.0377; 161.0240; 125.0141 | 2.02 | 1.03 |
| 7 | 0.749 | Coumaroyl aspartic acid | C13H13NO6 | 278.0670 | 278.0671 | 0.3 | 8 | 278.0672; 216.0653; 190.0857; 163.0382; 162.0560; 146.0610; 119.0503 (100); 117.0356; 115.0032; 93.0367 | 4.35 | 2.93 |
| 8 | 1.260 | Catechin | C15H14O6 | 289.0718 | 289.0720 [2M-H]− 579.1510 | 0.8 | 9 | 289.0718 (100); 245.0821; 203.0726; 179.0357; 125.0247; 109.0300 | 22.07 | 3.5 |
| 9 | 1.784 | Epicatechin | C15H14O6 | 289.0718 | 289.0714 | 0.8 | 9 | 289.0723; 245.0825; 221.0833; 203.0730; 187.0414; 175.0418; 125.0252; 123.0461; 109.0308 (100); 97.0305 | 4.67 | 32.75 |
| 10 | 4.217 | Clovamide | C18H17NO7 | 358.0932 | 358.0929 | −0.9 | 11 | 358.0899; 222.0396; 178.0503; 161.0237; 135.0448 (100); 134.6163; 133.0301; 86.1043 | 2.07 | 1.07 |
| 11 | 0.800 | Procyanidin B type (e.g., B1) | C30H26O12 | 577.1352 | 577.1363 | 2.0 | 18 | 577.1415; 559.1338; 451.1076; 425.0915; 407.0812 (100); 339.0900; 299.0568; 289.0738; 287.0578; 245.0837; 221.0829; 167.0361; 161.0255; 125.0254 | 2.08 | 10.25 |
| 12 | 4.255 | ((Epi)catechin trimer | C45H38O18 | 865.1985 | 865.1977 | −0.9 | 27 | 739.1742; 713.1592; 695.1486; 577.1418; 559.1302; 543.0984; 525.0872; 451.1069; 425.0919; 413.0909; 407.0808; 381.1008; 341.0693; 289.0740; 287.0581; 261.0427; 243.0311; 217.0522; 175.0417; 161.0259; 125.0260 | 2.24 | 1.5 |
| 13 | 5.190 | (Epi)catechin trimer | C45H38O18 | 865.1985 | 865.1977 | −0.9 | 27 | 739.1739; 713.1583; 695.1451; 587.1247; 577.1409; 561.1076; 543.0971; 525.0885; 449.0892; 425.0908; 407.0787; 381.1008; 341.0687; 289.0728; 287.0575; 261.0417; 245.0463; 217.0530; 175.0402; 161.0256; 125.0249 | 4.46 | 3.86 |
| 14 | 5.661 | Caffeoyl tyrosine | C18H17NO6 | 342.0983 | 342.0988 | 1.4 | 11 | 342.2398; 298.1083; 256.2008; 206.0473; 180.0675; 161.0242; 135.0452 (100); 119.0509; 107.0499; 93.0360 | 1.29 | 0.65 |
| 15 | 6.208 | Procyanidin B type (e.g., B4) | C30H26O12 | 577.1352 | 577.1367 | 1.6 | 18 | 577.1374; 451.1046; 425.0892; 407.0774; 339.0871; 299.0554; 289.0716 (100); 287.0559; 245.0818; 161.0244; 125.02416 | 4.64 | 7.04 |
| 16 | 6.247 | Isoquercetrin | C21H20O12 | 463.0882 | 463.0887 | 1.1 | 12 | 463.0884; 301.0340; 300.0267 (100); 271.0243; 255.0293; 243.0289 | 1.74 | 0.67 |
| 18 | 6.386 | Procyanidin dimer A type hexoside isomer | C36H34O17 | 737.1737 | 737.1737 | 0.1 | 25 | 737.1780; 611.1448; 539.1018; 449.0899 (100); 448.0808; 407.0783; 388.0595; 327.0509; 307.0605; 287.0557 | 2.51 | 2.26 |
| 19 | 6.398 | (Epi)catechin tetramer | C60H50O24 | 1153.2619 | 1153.2624 | 0.4 | 36 | 1153.2621; 1135.2792; 1001.2021; 983.2019; 575.1135; 289.0671; 287.0491; 245.0639; 161.0194; 125.0202 | 0.77 | 0.88 |
| 20 | 6.444 | (Epi)catechin trimer | C45H38O18 | 865.1985 | 865.1977 | −0.9 | 27 | 739.1762; 713.1582; 695.1488; 577.1423; 561.1118; 543.0994; 525.0885; 451.1085; 425.0915; 407.0814 (100); 381.1004; 299.0584; 289.0740; 287.0573; 245.0466; 161.0258; 125.0255 | 1.29 | 1.2 |
| 21 | 6.583 | Proanthocyanidin A type [(epi)catechin-(epi)gallocatechin] | C31H28O12 | 591.1508 | 591.1523 | 2.5 | 18 | 591.2831; 591.1525; 591.2002; 547.1555; 439.1032; 301.0703; 289.0698 (100); 245.0814; 215.0702; 203.0698; 149.0226; 137.0236; 109.0280 | 1.22 | 1.6 |
| 22 | 6.623 | p-Coumaroyl tyrosine | C18H17NO5 | 326.1034 | 326.1035 | 0.3 | 11 | 326.1046; 282.1143; 239.1091; 206.0461; 180.0664; 163.0401; 145.0292; 119.0498 (100); 117.0352; 93.0345 | 1.73 | 0.89 |
| 23 | 6.655 | (Epi)catechin ethyl dimer | C32H30O12 | 605.1665 | 605.1685 | 3.4 | 18 | 605.1675; 453.1197; 315.0875 (100); 289.0719; 271.0961; 245.0819; 229.0875; 205.0503; 163.0404; 151.0396; 137.0244; 109.0300 | 1.71 | 2.58 |
| 24 | 6.670 | Procyanidin dimer A type pentoside isomer | C35H32O16 | 707.1618 | 707.1636 | 2.6 | 20 | 707.1663; 581.1345; 539.1020; 449.0895 (100); 448.0815; 407.0789; 325.0339; 287.0543; 125.0246 | 2.39 | 1.98 |
| 25 | 6.682 | Quercetin pentoside | C20H18O11 | 433.0776 | 433.0776 | 12 | −0.1 | 433.0776; 301.0344; 300.0274 (100); 271.0248; 255.0302; 227.0354; 199.0407; 178.9991; 151.0024; 107.0138 | ||
| 26 | 6.702 | Kaempferol rutinoside | C27H30O15 | 593.1512 | 593.1523 | 13 | 1.9 | 593.1512 (100); 593.2491; 549.2540; 447.0976; 429.0806; 285.0396; 284.0314 | 0.35 | 0.43 |
| 27 | 6.783 | Quercetin pentoside | C20H18O11 | 433.0776 | 433.0775 | 12 | −0.1 | 433.0775; 301.0345; 300.0272 (100); 271.0239; 255.0288; 243.0288; 227.0339; 151.0025 | 0.23 | 0.04 |
| 28 | 6.959 | Procyanidin dimer A type | C30H24O12 | 575.1195 | 575.1198 | 19 | 0.5 | 575.1236; 557.1144; 539.1008; 531.2619; 471.1195; 449.0897; 423.0719; 409.0994; 407.0784; 387.0591; 341.0620; 327.0501; 307.0631; 289.0729; 287.0547; 285.0414 (100); 267.0306; 241.0501; 217.0504; 163.0044; 161.0252; 137.0259; 125.0246; 109.0315 | 5.71 | 3.74 |
| 29 | 7.041 | Procyanidin B type (e.g., B2) | C30H26O12 | 577.1352 | 577.1360 | 1.5 | 18 | 577.1369; 425.0891; 407.0878; 289.0720 (100); 287.0565; 245.0819; 161.0239; 125.0244 | 0.55 | 0.67 |
| 30 | 7.144 | Quercetin deoxyhexoside (e.g., Q-rhamnoside) | C21H20O11 | 447.0933 | 447.0945 | 12 | 2.7 | 447.0959; 301.0361; 300.0285 (100) 283.0246; 271.0251; 255.0304; 243.0301; 227.0349; 211.0406; 178.9981; 151.0036; 121.0289 | 0.4 | 0.55 |
| 31 | 8.280 | (Epi)catechin ethyl dimer | C32H30O12 | 605.1665 | 605.1673 | 1.4 | 18 | 605.1693; 453.1168; 315.0867; 289.0714 (100); 271.0956; 245.0811; 229.0856; 205.0504; 179.0347; 163.0416; 151.0397; 137.0250; 109.0293 | 7.36 | 2.98 |
| 32 | 8.422 | Proanthocyanidin A type | C30H24O12 | 575.1195 | 575.1207 | 19 | 2.1 | 575.1215 (100); 449.0887; 407.0778; 394.0692; 287.0565; 271.0239; 243.0287; 229.0504; 161.0230; 137.0245; 125.0245 | 1.02 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavorgna, M.; Pacifico, S.; Nugnes, R.; Russo, C.; Orlo, E.; Piccolella, S.; Isidori, M. Theobromacacao Criollo var. Beans: Biological Properties and Chemical Profile. Foods 2021, 10, 571. https://doi.org/10.3390/foods10030571
Lavorgna M, Pacifico S, Nugnes R, Russo C, Orlo E, Piccolella S, Isidori M. Theobromacacao Criollo var. Beans: Biological Properties and Chemical Profile. Foods. 2021; 10(3):571. https://doi.org/10.3390/foods10030571
Chicago/Turabian StyleLavorgna, Margherita, Severina Pacifico, Roberta Nugnes, Chiara Russo, Elena Orlo, Simona Piccolella, and Marina Isidori. 2021. "Theobromacacao Criollo var. Beans: Biological Properties and Chemical Profile" Foods 10, no. 3: 571. https://doi.org/10.3390/foods10030571
APA StyleLavorgna, M., Pacifico, S., Nugnes, R., Russo, C., Orlo, E., Piccolella, S., & Isidori, M. (2021). Theobromacacao Criollo var. Beans: Biological Properties and Chemical Profile. Foods, 10(3), 571. https://doi.org/10.3390/foods10030571









