5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bread-Making and Baking Protocol
2.3. Bread Characterization and Sample Homogenization
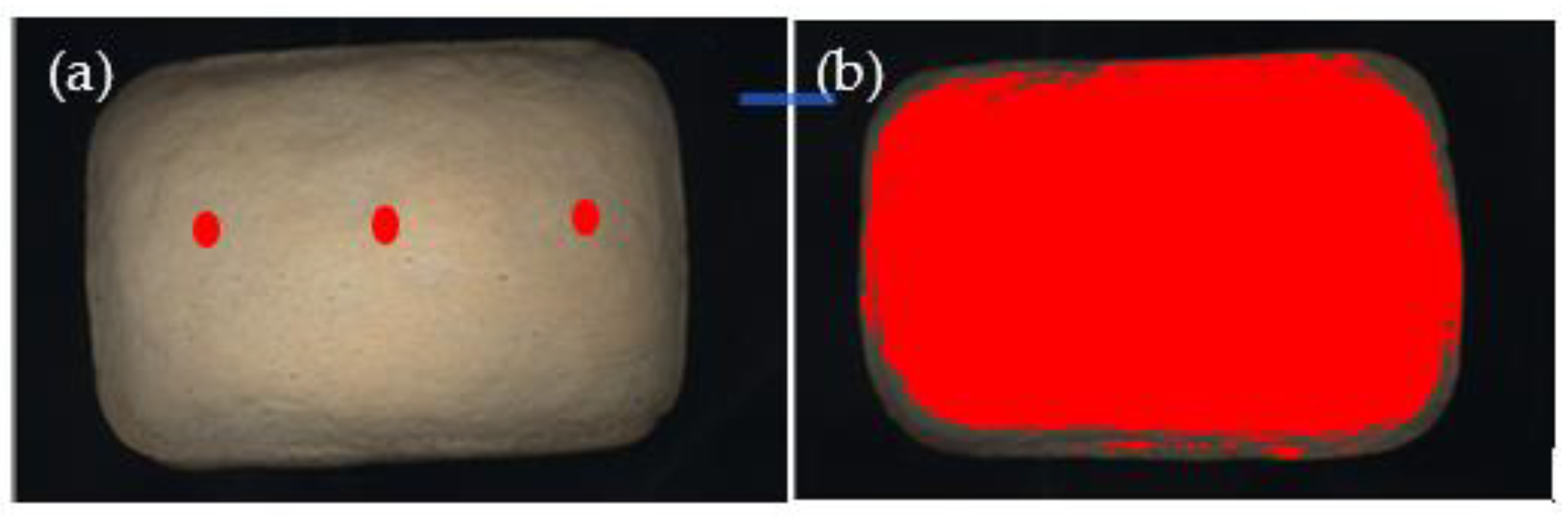
2.4. Colour Evaluation
2.5. Determination of Maillard Reaction Products
2.6. Determination of 5-hydroxymethylfurfural
2.7. Statistical Analysis
3. Results and Discussion
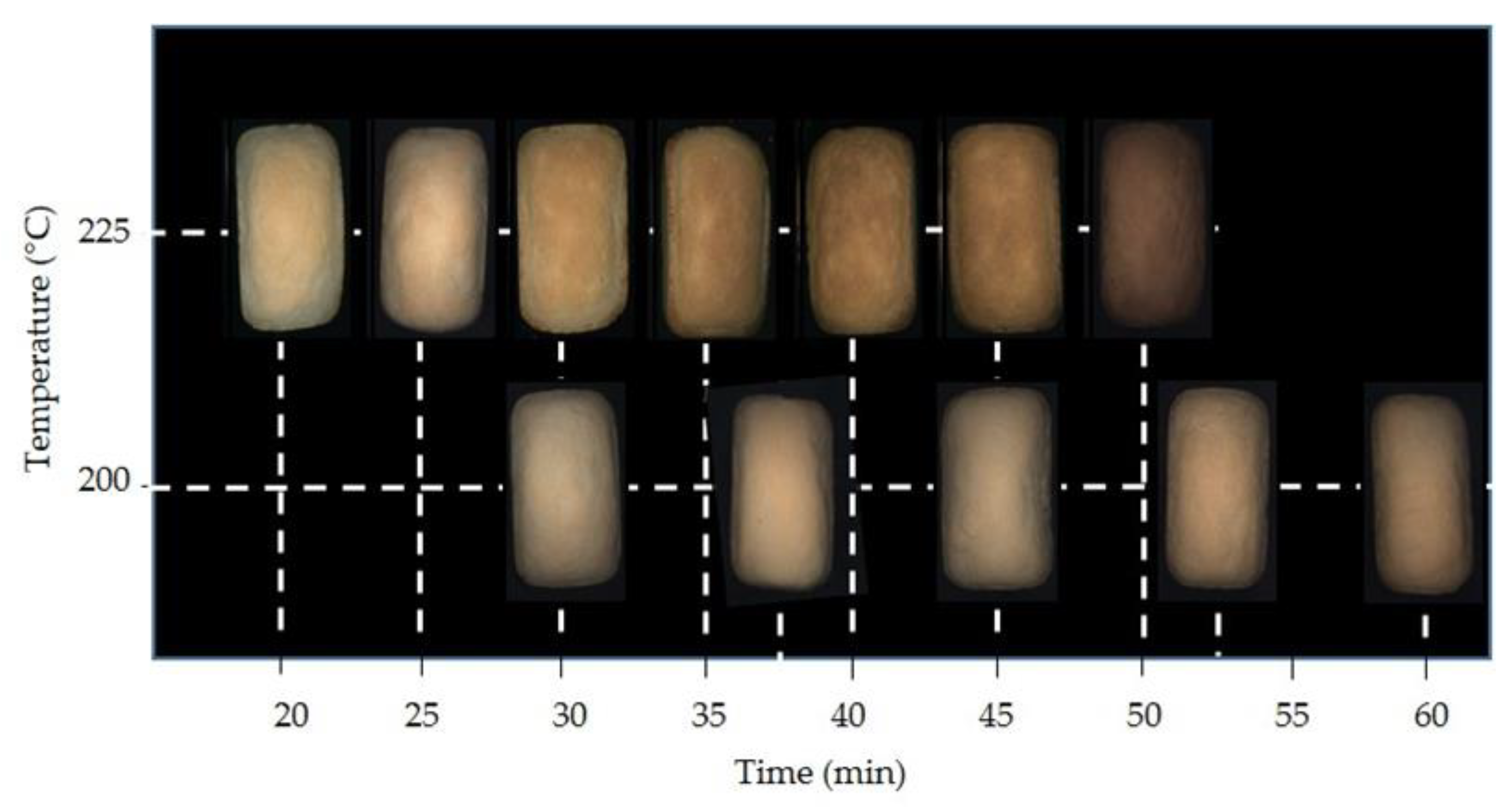
3.1. Bread Characterization
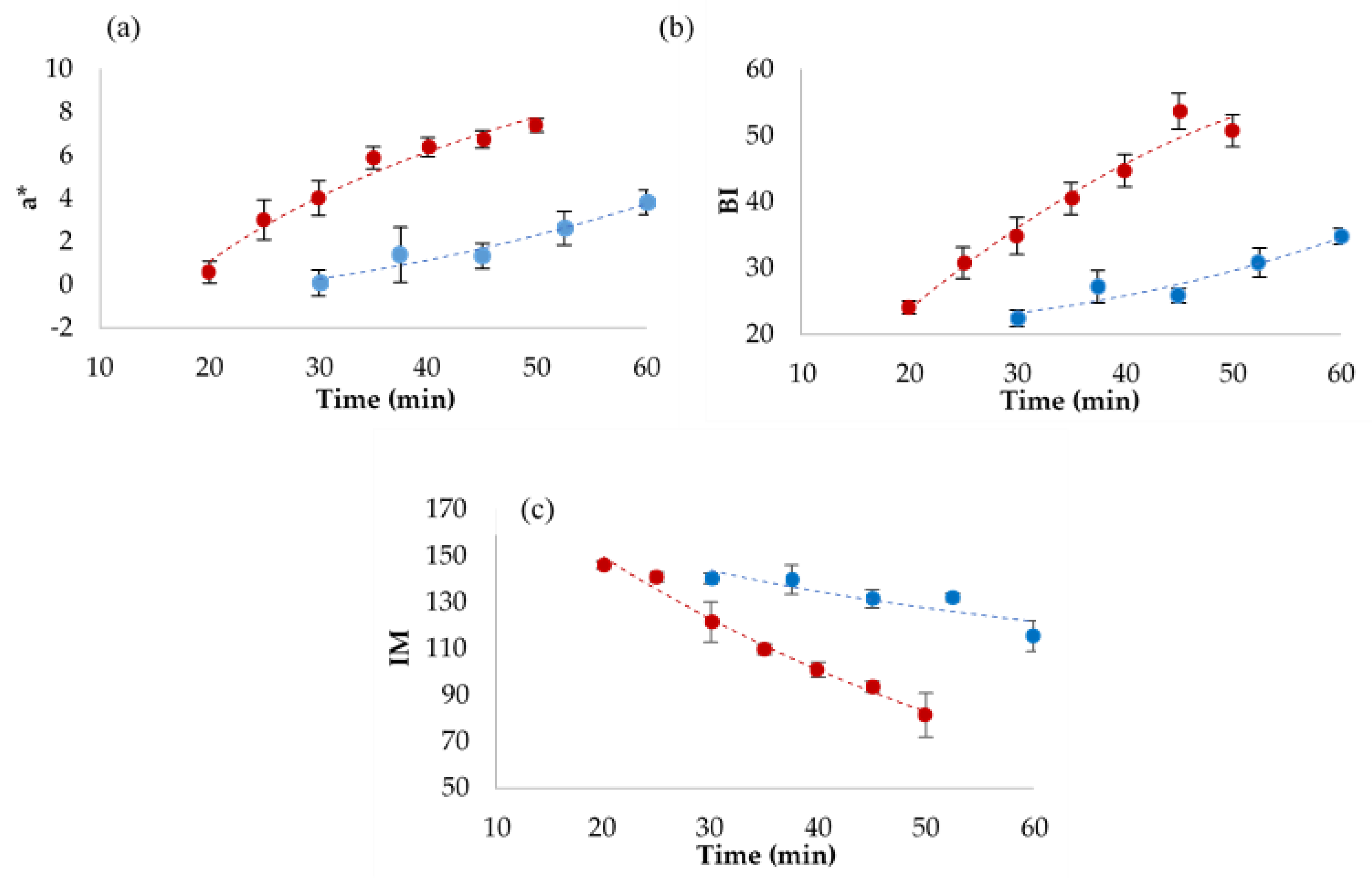
3.2. Colour Development
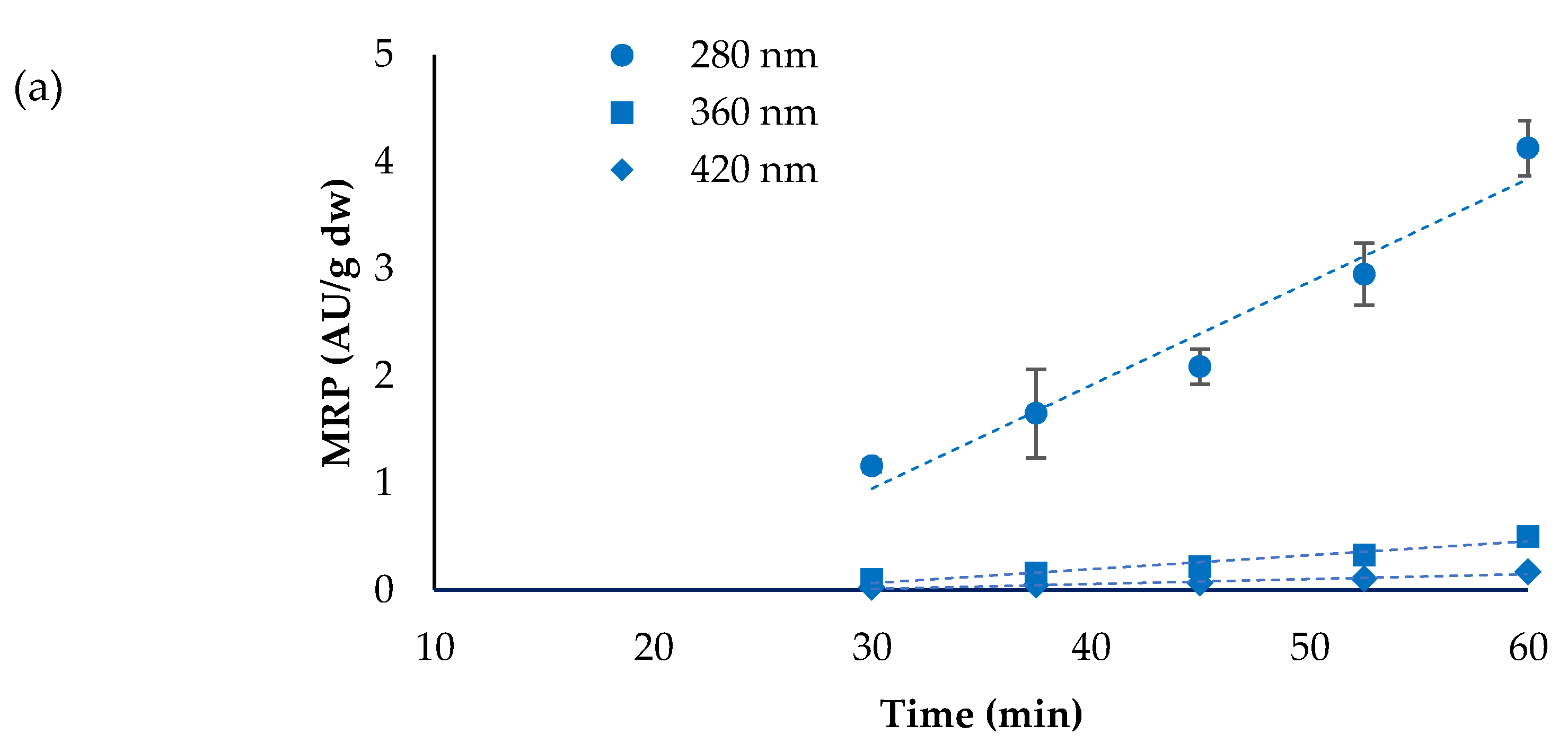
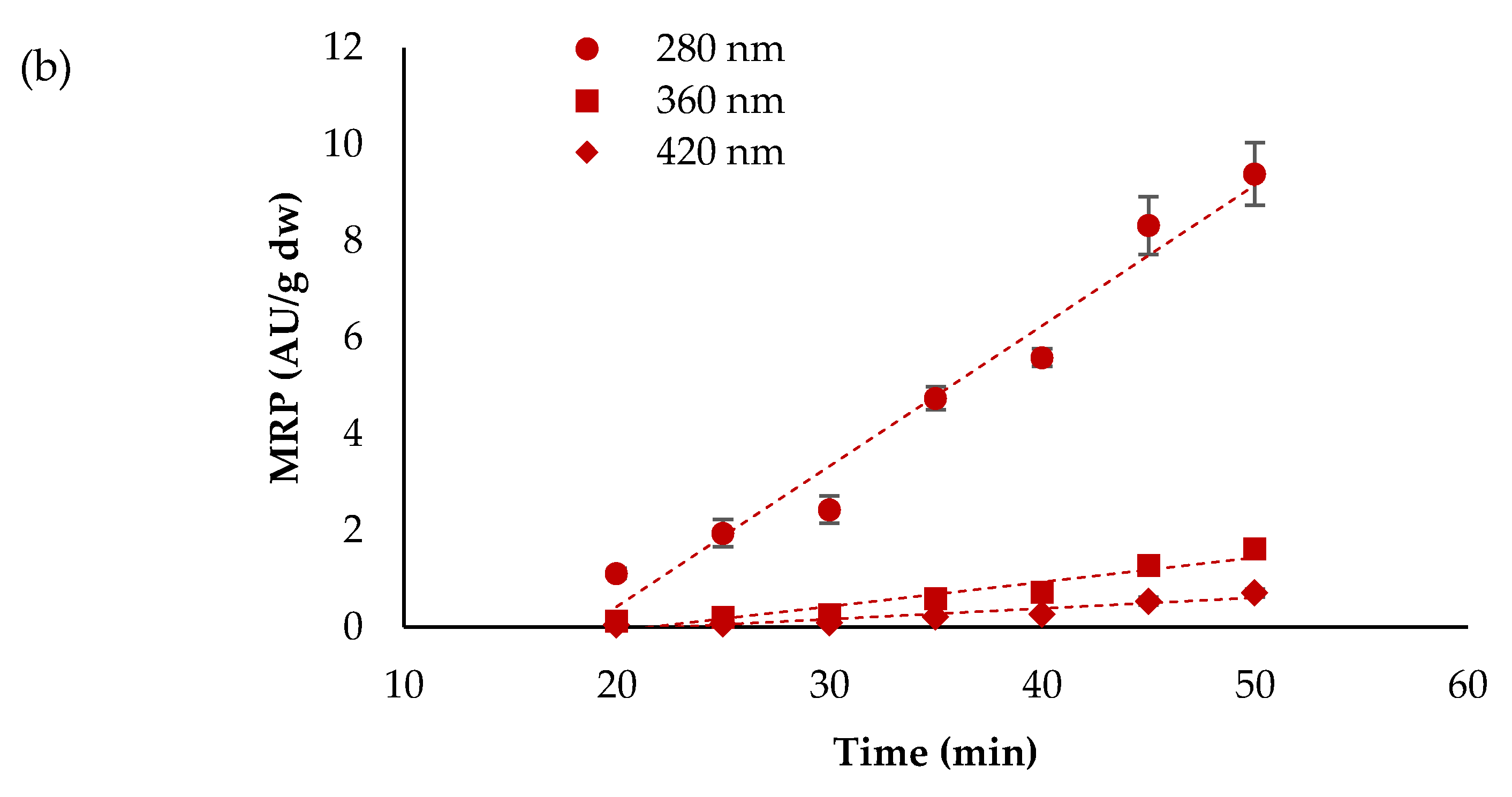
3.3. Maillard Reaction Products (MRP)
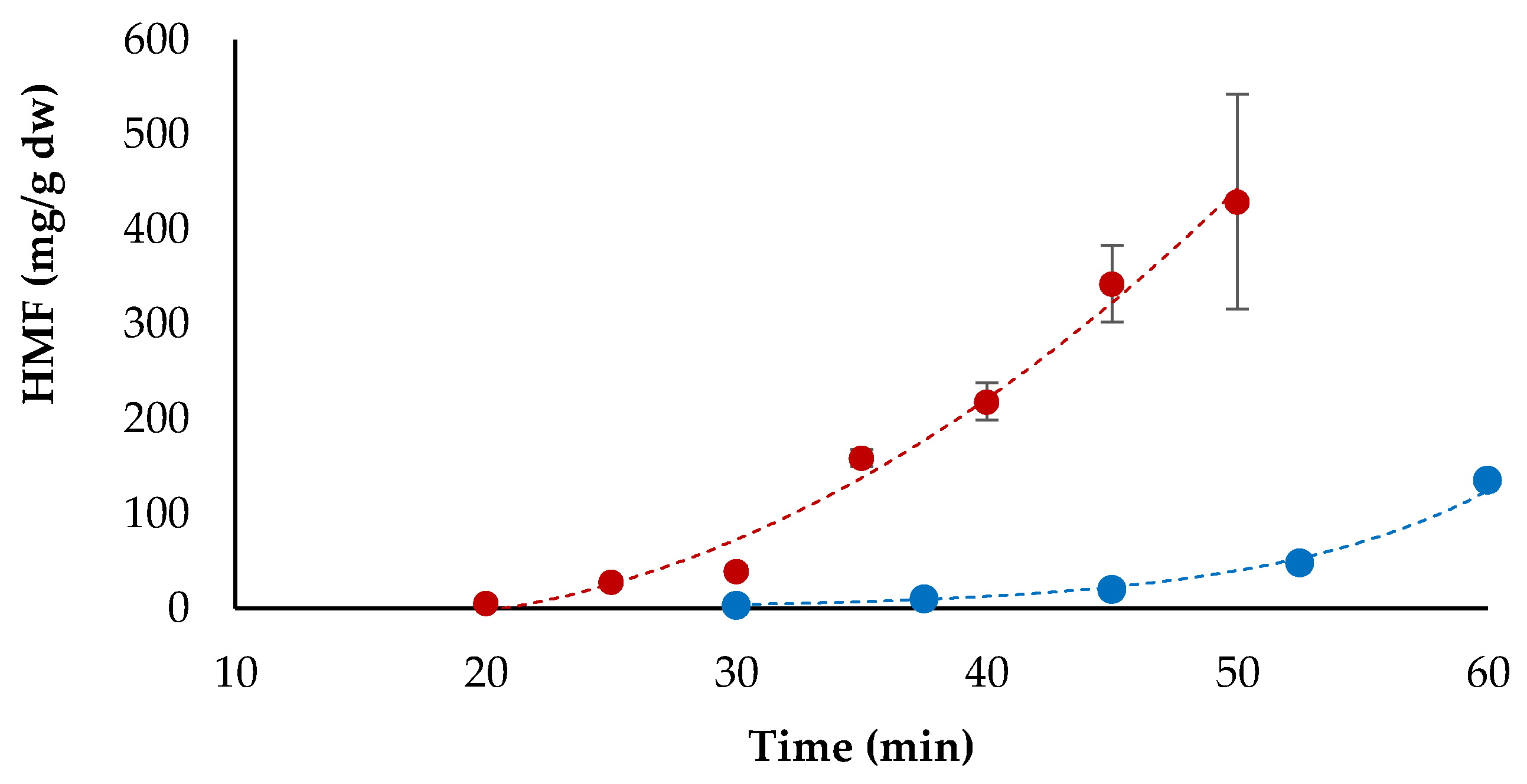
3.4. HMF Formation
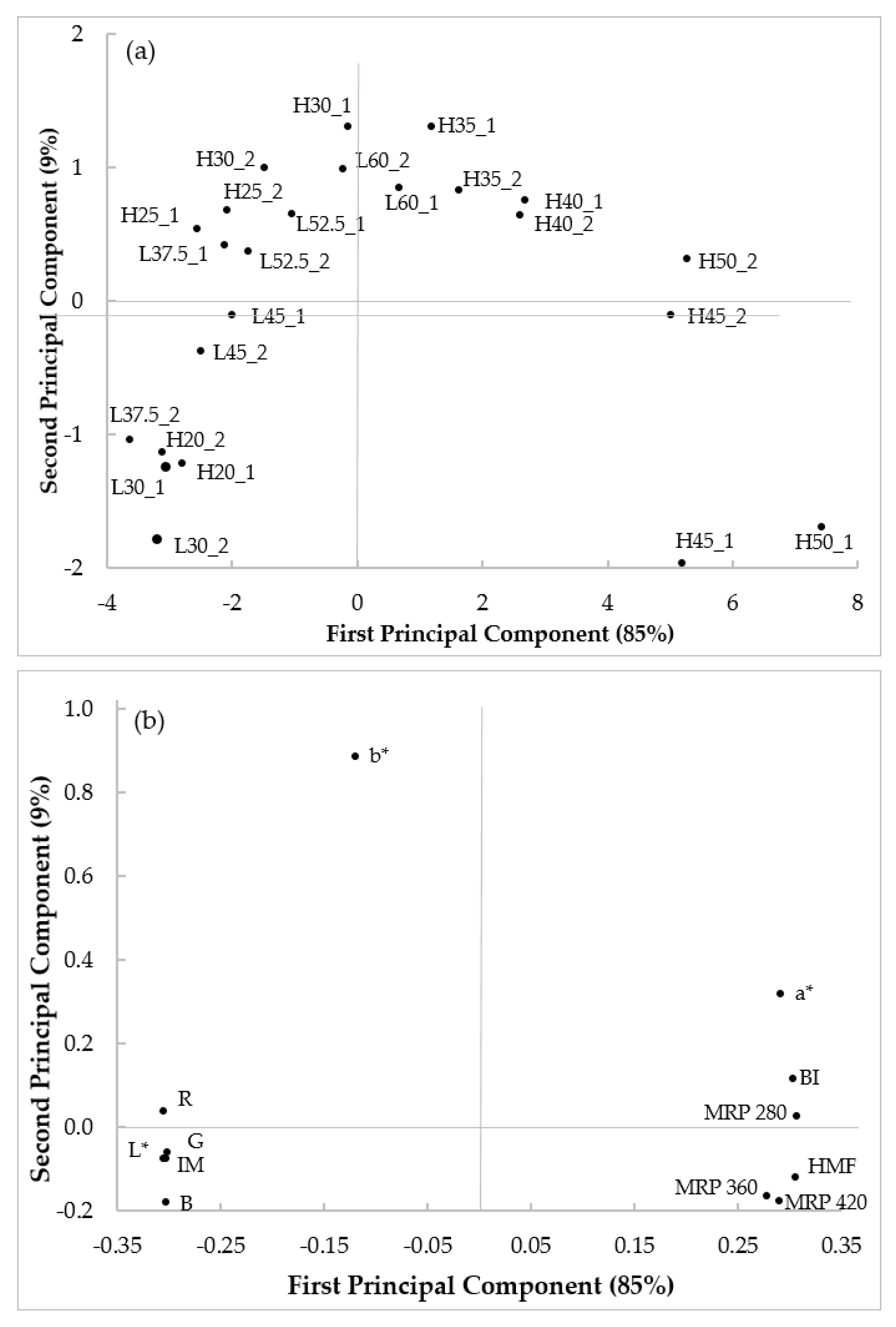
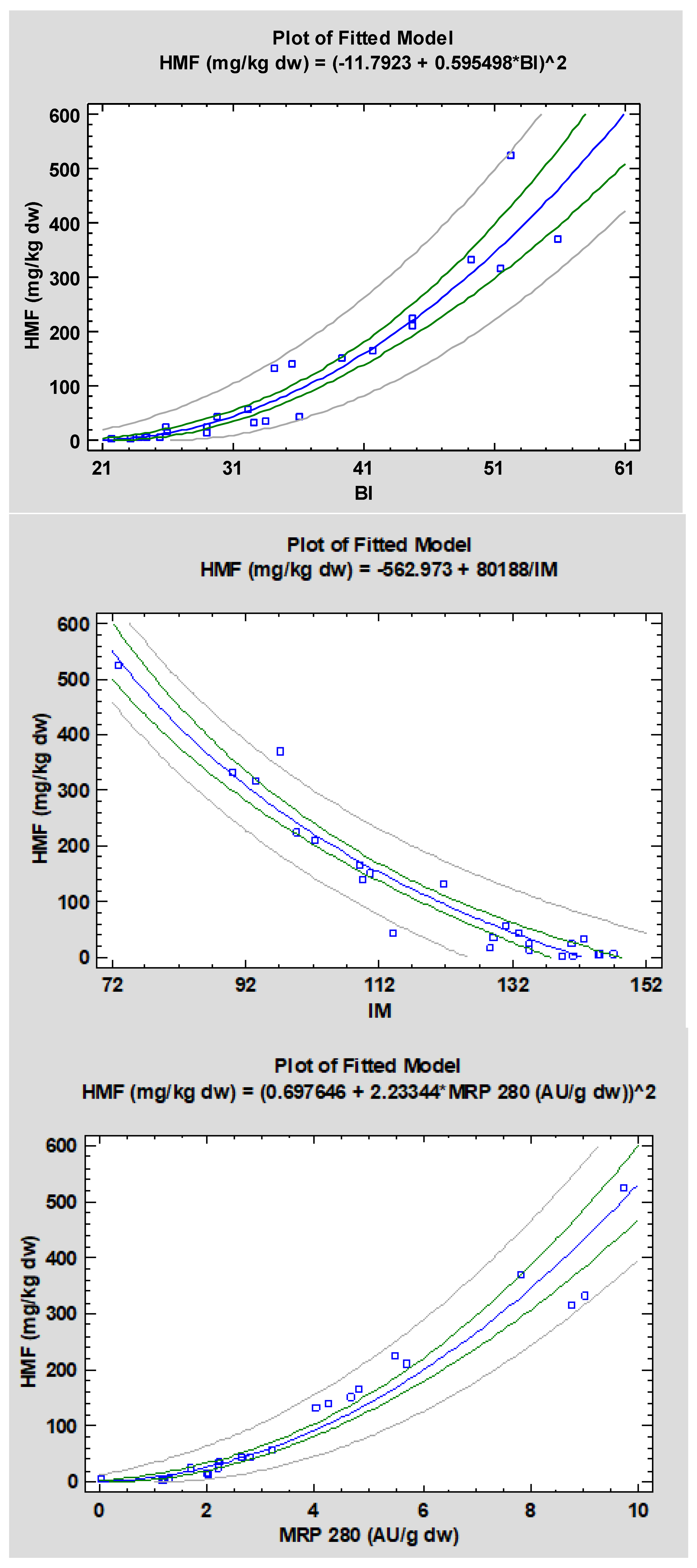
3.5. Principal Component Analysis and Regression Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Purlis, E. Browning development in bakery products—A review. J. Food Eng. 2010, 99, 239–249. [Google Scholar] [CrossRef]
- Ames, J.M. The Maillard reaction. In Biochemistry of Food Proteins; Hudson, B.J.F., Ed.; Elsevier: London, UK, 1992; pp. 95–153. [Google Scholar]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Aljahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Hardy, J.; Parmentier, M.; Fanni, J. Functionality of nutrients and thermal treatments of food. Proc. Nutr. Soc. 1999, 58, 579–585. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 2019, 60, 1677–1692. [Google Scholar] [CrossRef]
- Gökmen, V.; Açar, Ö.Ç.; Köksel, H.; Acar, J. Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formation in cookies. Food Chem. 2007, 104, 1136–1142. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Van Der Fels-Klerx, H. (Ine); Peters, R.J.; Van Boekel, M.A. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chem. 2016, 192, 575–585. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bin-Jumah, M.; Othman, S.I.; Khafaga, A.F.; Shaheen, H.M.; Samak, D.; Shehata, A.M.; Allam, A.A.; El-Hack, M.E.A. The Toxicological Aspects of the Heat-Borne Toxicant 5-Hydroxymethylfurfural in Animals: A Review. Molecules 2020, 25, 1941. [Google Scholar] [CrossRef]
- Fogliano, V.; Morales, F.J. Estimation of dietary intake of melanoidins from coffee and bread. Food Funct. 2011, 2, 117–123. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 12th ed.; Association of Official Analytica Chemists: Arlington, VA, USA, 2002. [Google Scholar]
- Ramírez-Jiménez, A.; Guerra-Hernández, E.; García-Villanova, B. Browning Indicators in Bread. J. Agric. Food Chem. 2000, 48, 4176–4181. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- DPR 30 Novembre 1998 n° 502, Regolamento recante norme per la revisione della normativa in materia di lavorazione e di commercio del pane, a norma dell’articolo 50 della legge 22 febbraio 1994, n. 146. Gazzetta Ufficiale Serie Generale n. 25 del 01–02–1999.
- Gökmen, V.; Açar, Ö.Ç.; Arribas-Lorenzo, G.; Morales, F.J. Investigating the correlation between acrylamide content and browning ratio of model cookies. J. Food Eng. 2008, 87, 380–385. [Google Scholar] [CrossRef]
- Helou, C.; Jacolot, P.; Niquet-Léridon, C.; Gadonna-Widehem, P.; Tessier, F.J. Maillard reaction products in bread: A novel semi-quantitative method for evaluating melanoidins in bread. Food Chem. 2016, 190, 904–911. [Google Scholar] [CrossRef]
- Mustafa, A.; Andersson, R.; Rosén, J.; Kamal-Eldin, A.; Åman, P. Factors Influencing Acrylamide Content and Color in Rye Crisp Bread. J. Agric. Food Chem. 2005, 53, 5985–5989. [Google Scholar] [CrossRef] [PubMed]
- Surdyk, N.; Rosén, J.; Andersson, R.; Åman, P. Effects of Asparagine, Fructose, and Baking Conditions on Acrylamide Content in Yeast-Leavened Wheat Bread. J. Agric. Food Chem. 2004, 52, 2047–2051. [Google Scholar] [CrossRef]
- Ertop, M.H.; Sarikaya, S.B.Ö. The relations between hydroxymethylfurfural content, antioxidant activity and colorimetric properties of various bakery products. GIDA/J. Food 2017, 42, 834–843. [Google Scholar] [CrossRef]
- Purlis, E.; Salvadori, V.O. Modelling the browning of bread during baking. Food Res. Int. 2009, 42, 865–870. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Reviews kinetic aspects of the Maillard reaction: A critical review. Nahrung/Food 2001, 45, 150–159. [Google Scholar] [CrossRef]
- Mogol, B.A.; Gökmen, V. Computer vision-based analysis of foods: A non-destructive colour measurement tool to monitor quality and safety. J. Sci. Food Agric. 2013, 94, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
| Sample Code | Baking Temperature (°C) | Baking Time (min) |
|---|---|---|
| L30 | 200 | 30 |
| L37.5 | 200 | 37.5 |
| L45 | 200 | 45 |
| L52.5 | 200 | 52.5 |
| L60 | 200 | 60 |
| H20 | 225 | 20 |
| H25 | 225 | 25 |
| H30 | 225 | 30 |
| H35 | 225 | 35 |
| H40 | 225 | 40 |
| H45 | 225 | 45 |
| H50 | 225 | 50 |
| Sample Code * | Maximum Height (mm) | Baking Weight Loss (%) | Moisture Content (g/100 g) |
|---|---|---|---|
| L30_1 | 43 ± 2 fgh | 23.1 ± 0.4 b | 25.9 ± 0.1 kl |
| L30_2 | 41 ± 1 cde | 23.4 ± 0.8 bc | 25.4 ± 0.5 k |
| L37.5_1 | 43 ± 2 fgh | 26.3 ± 0.7 d | 21.1 ± 0.3 h |
| L37.5_2 | 42 ± 1 ef | 26.6 ± 0.5 de | 23.2 ± 0.4 j |
| L45_1 | 38 ± 1 ab | 30.0 ± 0.2 gh | 20.0 ± 0.2 g |
| L45_2 | 42 ± 1 ef | 31.9 ± 0.6 i | 18.3 ± 0.2 f |
| L52.5_1 | 45 ± 3 hi | 32.9 ± 0.6 j | 16.8 ± 0.1 e |
| L52.5_2 | 38 ± 1 ab | 34.2 ± 2.4 k | 16.6 ± 0.2 e |
| L60_1 | 41 ± 1 def | 35.6 ± 1.0 lm | 12.5 ± 0.1 b |
| L60_2 | 45 ± 1 ghi | 36.0 ± 0.7 m | 11.6 ± 0.1 a |
| H20_1 | 40 ± 1 bcd | 19.8 ± 0.5 a | 28.6 ± 0.4 m |
| H20_2 | 41 ± 1 cde | 19.8 ± 0.5 a | 28.5 ± 0.4 m |
| H25_1 | 39 ± 2 bc | 23.2 ± 0.5 b | 26.2 ± 0.5 l |
| H25_2 | 43 ± 1 fg | 24.0 ± 0.3 c | 25.7 ± 0.7 kl |
| H30_1 | 40 ±1 bcd | 27.0 ± 0.6 e | 23.0 ± 0.5 j |
| H30_2 | 40 ± 2 cd | 27.6 ± 0.3 f | 21.9 ± 0.6 i |
| H35_1 | 40 ± 1 bcd | 29.4 ± 0.5 g | 18.5 ± 0.4 f |
| H35_2 | 45 ± 1i | 30.5 ± 0.6 h | 19.8 ± 0.3 g |
| H40_1 | 39 ± 2bc | 31.8 ± 0.2 i | 16.5 ± 0.1 e |
| H40_2 | 41 ± 2 cde | 32.7 ± 0.2 j | 15.4 ± 0.3 d |
| H45_1 | 48 ± 1 j | 35.0 ± 0.4 l | 14.2 ± 0.4 c |
| H45_2 | 40 ± 2 cde | 35.0 ± 0.8 l | 14.1 ± 0.1 c |
| H50_1 | 39 ± 1 bc | 37.7 ± 0.8 m | 11.3 ± 0.2 a |
| H50_2 | 37 ± 2 a | 38.0 ± 0.3 n | 11.5 ± 0.1 a |
| Simple Regression * (Y; X) | Best Fitting Model | r | p-Values | Equation |
|---|---|---|---|---|
| HMF; BI | Square root-Y model | 0.966 | 0.0000 | Y = (−11.7923 + 0.595498X)2 |
| HMF; IM | Reciprocal-X model | 0.968 | 0.0000 | Y = −562.973 + 80188/X |
| HMF; MRP 280 | Square root-Y model | 0.978 | 0.0000 | Y = (0.697646 + 2.23344X)2 |
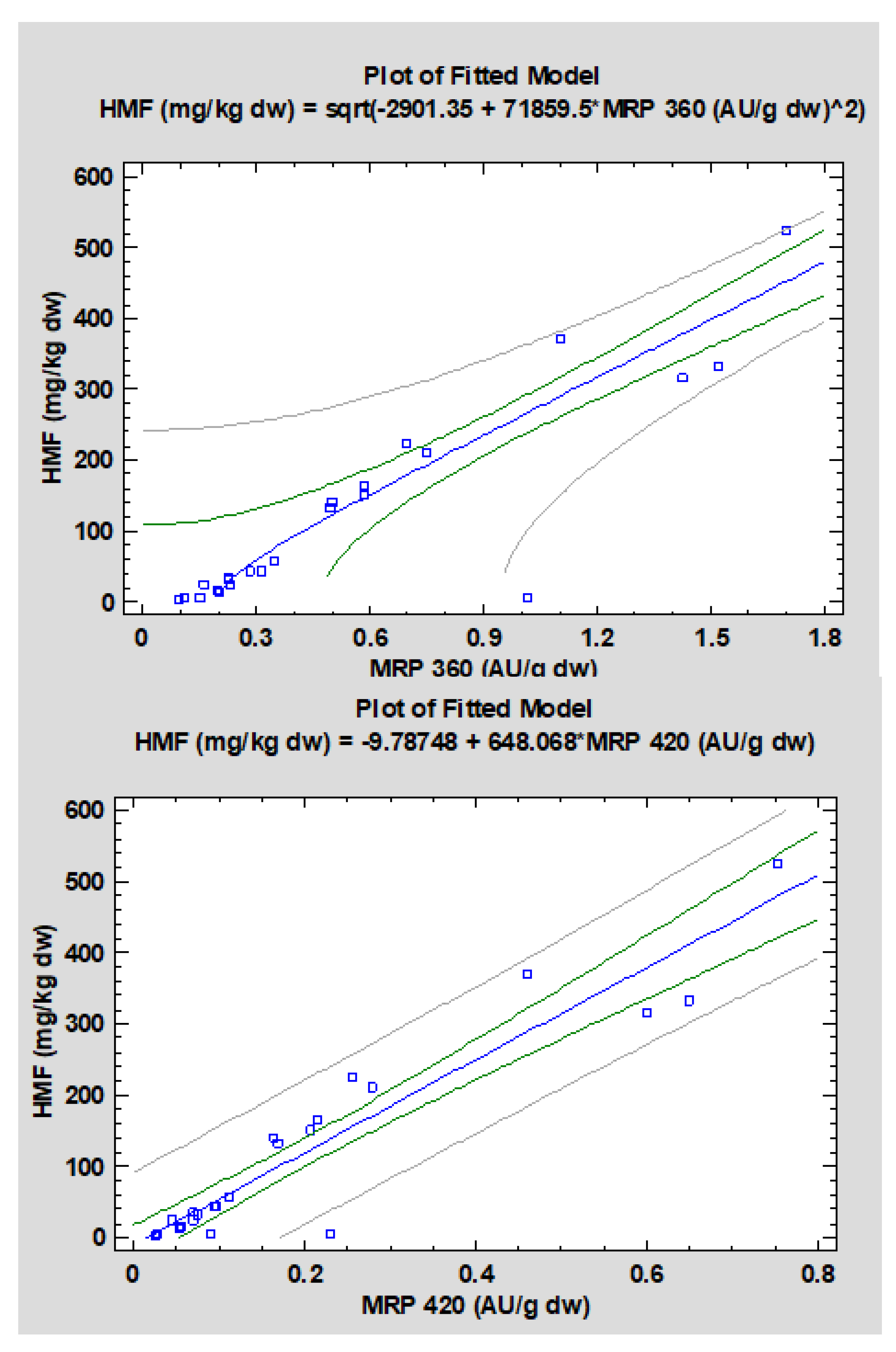
| HMF; MRP 360 | Double-squared | 0.900 | 0.0000 | Y = sqrt(−2901.35 + 71859.5X2) |
| HMF; MRP 420 | Linear | 0.945 | 0.0000 | Y = −9.78748 + 648.068X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanelli, G.; Cappa, C. 5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices. Foods 2021, 10, 417. https://doi.org/10.3390/foods10020417
Giovanelli G, Cappa C. 5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices. Foods. 2021; 10(2):417. https://doi.org/10.3390/foods10020417
Chicago/Turabian StyleGiovanelli, Gabriella, and Carola Cappa. 2021. "5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices" Foods 10, no. 2: 417. https://doi.org/10.3390/foods10020417
APA StyleGiovanelli, G., & Cappa, C. (2021). 5-Hydroxymethylfurfural Formation in Bread as a Function of Heat Treatment Intensity: Correlations with Browning Indices. Foods, 10(2), 417. https://doi.org/10.3390/foods10020417








