1. Introduction
In an effort to improve wine quality, many smaller high-end wineries employ laborers to hand sort individual berries after destemming to remove unwanted material such as raisins, diseased berries, unripe berries, and materials other than grapes (MOG) such as leaves and stems. This can be costly, labor intensive, and it can slow down the process line. To reduce costs and increase throughput, many wineries have adopted optical sorting technology. Using this technology, MOG can be removed more efficiently, and parameters such as color, shape, and size can be used to sort individual berries. Depending on the type of sorter, processing speeds can range between 2 and 15 tons per hour. Furthermore, fewer workers are needed to operate an optical sorter than to hand sort the respective amount of fruit [
1].
In addition to saving time and money, optical sorters have the potential to decrease the impact of inconsistent ripening in grapes. One study successfully sorted Carlos Muscadine grapes into four different ripeness levels using light at two different wavelengths in the visible spectrum [
2]. The researchers found that with successive sorting levels, there was an increase in Brix and pH, along with a decrease in titratable acidity in grape samples. In the wines, an increase in tannin and pH and a decrease in titratable acidity was found with increasing sorting. In sensory analysis, the first and fourth sorting levels were found to be inferior compared to the middle two treatments. Even though this study used outdated equipment compared to today’s standards, it shows that white grapes can be sorted into different ripeness levels and this can affect the quality of the wine produced. A recent study used visible near-infrared spectroscopy to classify table grapes into different groups based on soluble solid and phenolic content [
3]. The researchers were able to differentiate berries of different classes with accuracy ranging from 77% to 94%. Another study found that wine made from optically sorted Chardonnay grapes had higher residual sugar, pH, and total phenols than the unsorted control [
4]. The wines were analyzed sensorially with descriptive analysis and the judges scored the sorted wines significantly higher in tropical fruit and sweetness. However, with only two significant attributes out of twenty, the wines were determined to be similar in character. Another study investigating the effect of mechanical harvesting and optical berry sorting on Pinot noir grapes found that, in general, wines made from optically sorted fruit were significantly lower in total phenol and tannin, potentially due to the removal of MOG during sorting [
5]. In sensory analysis only two significant attributes out of eighteen were found (tropical fruit and hue saturation) and it was concluded that the wines were similar in character. A study published in 2014 used an optical sorter on Riesling, Müller-Thurgau, and Pinot gris grapes infected with Botrytis cinerea to investigate the effect of optical sorting on sulfur binding compounds in the finished wine [
6]. The researchers found that wine made from optically sorted fruit contained significantly less 2-oxoglutaric acid and pyruvic acid (both are common sulfur binding compounds that can be higher in wines made from grapes infected with Botrytis cinerea). They concluded that optical sorting is an effective method for reducing the amount of sulfur dioxide (SO
2) needed in the winemaking process using these varieties. There is a lack of published research investigating the impact of optical berry sorting on wine composition and only a few cultivars of
Vitis vinifera have been tested. The objective of the current study was to provide more information on the effect of optical berry sorting on different varieties and investigate the capabilities of today’s optical sorters to sort for different ripeness levels using red grapes and using color as a sorting parameter. The current study found that although optical sorting can efficiently replace hand sorting, the overall impact on wine sensory attributes was minimal. Therefore, in general, the study supported the findings of previous researchers.
2. Materials and Methods
Three varieties were tested in 2016: Barbera (BA), Cabernet Sauvignon (CS), and Grenache (GN). BA was harvested on 19 August 2016, CS was harvested 30 August 30 2016, and GN was harvested 8 September 2016. All three varieties were hand harvested early in the morning from UC Davis campus vineyards and processed the same day. Fruit condition was good with seemingly little variation, although GN fruit showed more variation in color than the other cultivars. Half-ton bins were dumped by a forklift into a receiving hoper. Clusters were carried by a Delta TR elevator (Bucher-Vaslin, Santa Rosa, CA, USA) into a Delta E2 destemmer (Bucher-Vaslin, Santa Rosa, CA, USA). Destemmed berries fell onto a moving belt and were carried onto a ChromaxHD Berrytek Optical Sorter (Woodside Electronics Corporation, WECO, Woodland, CA, USA). Rejection parameters were established by capturing color profiles of optimal berries, suboptimal berries (green/underripe berries and raisins), and MOG. These parameters were optimized with the assistance of a WECO technician for removing suboptimal berries and MOG while rejecting as few optimal berries as possible. This process was repeated, and parameters were adjusted for each variety. The must was pumped directly into 200 L stainless steel research fermentors [
7], which were filled incrementally to reduce vineyard variation. The rejected material was collected in buckets and transferred into research fermentors. The grapes were processed in three treatments, control (no sorting), sort (accepted material), and reject (material rejected by the optical sorter). The rejection rates were 14.9%, 3.9%, and 1.5% (
w/
w) for GN, BA, and CS, respectively. Juice collected in trays from the rolling belts during processing operations was added back to each treatment in proportional amounts. This was done to maintain a consistent solid-to-juice ratio in the must among treatments.
Wines were made in the UC Davis Teaching and Research Winery using 200 L stainless steel research fermentors. The control and sort treatments were fermented in triplicate and the reject treatments were fermented in duplicate. Duplicate fermentations were used for the reject treatment wines because only a small amount of reject material was obtained during grape processing. Fermentation replications were kept separate through the entire experiment. Juice samples were taken from each fermentation vessel after mixing. Fifty milligrams per liter of SO2 was added to the must after processing using a 15% potassium metabisulfite solution. Yeast assimilable nitrogen (YAN) was adjusted to 250 mg/L using diammonium phosphate (DAP), titratable acidity (TA) was adjusted (if necessary) to 6 g/L using tartaric acid. The must was heated to 25 °C before inoculation with Lalvin EC1118 yeast (Lallemand, Inc., Petaluma, CA, USA) using the manufacturers rehydration procedure. One tank volume was pumped over twice per day using automated pump overs for all wines except for the reject treatment for CS. The volume in these tanks was too low for the automated pumps to create suction, therefore, the wines were punched down manually once per day during the fermentation. Once wines were dry, they were pressed using a basket press and allowed to settle for 5 days before being racked and transferred to a temperature-controlled room held at 20 °C. The wines were then inoculated with Viniflora CH16 Oenococcus oeni bacteria by Chr. Hansen (Milwaukee, WI, USA). Upon finishing the malolactic (ML) fermentation, 50 mg/L SO2 was added to the wines and they were held in a 9 °C cold room until bottling. Free SO2 was adjusted to 30 mg/L before bottling. All samples for basic wine chemical analyses were taken at the time of bottling. Ethanol (v/v) was measured using an Alcolyzer (Anton Paar, Ashland, VA, USA) and pH was measured using an Orion 5-star pH meter (Thermo Scientific, Waltham, MA, USA). Titratable acidity (TA) and free SO2 were measured using a Mettler Toledo DL50 auto titrator (Mettler Toledo, Columbus, OH, USA). Residual sugar, malic acid, and volatile acidity were measured using a Thermo Fisher Scientific Gallery automated analyzer (Thermo Scientific, Waltham, MA, USA). Wines were sterile filtered using 0.45 μm membrane filters (Millipore, Burlington, MA, USA) prior to bottling using green Bordeaux style bottles and screw cap closures with Saranex liners by Amcor (Yuba City, CA, USA).
Samples for phenolic analysis were taken from bottles at the time of sensory analysis. The modified Adams-Harbertson [
8] assay was used to determine levels of anthocyanin (expressed as malvidin-3-glucoside equivalents), tannin (expressed as catechin equivalents), and total iron-reactive phenolics (expressed as catechin equivalents) [
8]. A Genesis 10S UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used for this assay.
Phenolic compounds were also analyzed by a method using reverse-phase high-performance liquid chromatography (RP-HPLC) previously described in the literature [
9]. Briefly, compounds were measured at four wavelengths; 280 nm (gallic acid, (+)-catechin, dimer B1, (−)-epicatechin, epicatechin gallate, and polymeric phenols); 320 nm (caftaric acid, caffeic acid, coutaric acid, and p-coumaric acid); 360 nm (quercetin-3-galactoside, quercetin-3-glucoside, quercetin-3-glucuronide, quercetin-3-rhamnoside, and quercetin); and 520 nm (delphinidin-3-glucoside, petunidin-3-glucoside, peonidin-3-glucoside, malvidin-3-glucoside, malvidin-3-acetylglucoside, malvidin-3-p-coumglucoside, and polymeric pigments). Wine samples were stored in HPLC vials in a −20 °C freezer until analysis. An Agilent 1260 Infinity HPLC (Agilent Technologies, Santa Clara, CA, USA) with a diode array detector (DAD) was used. An Agilent PLRP-S (150 × 4.6 mm, 100 A, and 3 µm pore size) column with an Agilent PLRP-S guard cartridge (5 × 3 mm) was maintained at 35 °C. Agilent CDS ChemStation software (version B.04) was used for instrument control and data analysis. The injection volume was 20 µL and a gradient mobile phase of water with 0.3% phosphoric acid (
v/
v) (88%, Fisher Scientific, Pittsburgh, PA, USA) (mobile phase A) and acetonitrile (HPLC grade, Sigma-Aldrich, St. Louis, MO, USA) with 0.2% phosphoric acid (
v/
v) (mobile phase B) was used at a flow rate of 1.0 mL/min. The solvent gradient was 6–31% B at 0–73 min, 31–62% B at 73–78 min, isocratic 62% B at 78–86 min, 62–6% B at 86–90 min. Compounds were identified using retention time and spectral comparison to standards. An external calibration was used for the quantification of phenolic compounds and curves were made for gallic acid, (+)-catechin (98%, Sigma-Aldrich, St. Louis, MO, USA), (−)-epicatechin (95%, Sigma-Aldrich, St. Louis, MO, USA), caffeic acid (98%, Sigma-Aldrich, St. Louis, MO, USA), quercetin-3-rhamnoside, and malvidin-3-glucoside. Caftaric acid was quantified as caffeic acid equivalents, quercetin-glycosides as quercetin-3-O-rhamnoside (98.5%, Indofine Chemical Company, Hillsborough, NJ, USA) units and all pigments as malvidin-3-glucoside (95%, Extrasynthese, Genay, France) units. Bottle duplicates for each fermentation replication were analyzed and the sequence was randomized.
Wine aroma compounds were analyzed using head-space solid-phase microextraction gas chromatography mass spectrometry (HS-SPME-GC-MS). The method used was adapted from a previous study [
5]. Samples used for wine volatile analysis were taken at the time of sensory analysis and stored at 4 °C for no more than one month. Identified volatile peaks are normalized against an internal standard and the obtained data is thus semiquantitative only. Twenty mL amber glass headspace vials (Agilent Technologies) were used, containing 10 mL milliliters of wine sample, 3 g of NaCl salt and 50 µL of a 10 mg/L solution of 2-undecanone (internal standard, 99%, Sigma-Aldrich, St. Louis, MO, USA). Twenty millimeter green magnetic caps with 3 mm PTFE silicone septa (Supelco, St. Louis, MO, USA) were crimped onto the vials and the samples were mixed by vortexing. The analysis was done using an Agilent Technologies 7890A GC system (Agilent Technologies, Santa Clara, CA, USA) with a Gerstel MPS2 multipurpose sampler (Mülheim an der Ruhr, Deutschland). The mass analyzer was an Agilent Technologies 5975C inert XL EI/CI MSD. The column used was an Agilent Technologies DB-Waxetr with a temperature range of 30 °C to 260 °C. The dimensions of the column were 30 m, 0.250 mm, and 0.25 µm. Maestro software (version 1.2.3.1; Gerstel) was used to control the instrument and data were collected using ChemStation software (version E.01.01.335; Agilent). During the analysis, the oven was held at 40 °C for 5 min and then increased 3 °C/min to 180 °C, followed by 30 °C/min to 250 °C and held for 7.67 min. The MSD interface was kept at 260 °C. HS-SPME-GC-MS conditions were as previously described [
5]. Shortly, samples were heated to 30 °C for five minutes while agitating with a speed of 500 rpm prior to exposing the fiber (1 cm polydimethylsiloxane 23-gauge SPME fiber, Supelco, St. Louis, MO, USA) to the sample for 45 min at 30 °C with agitation at 250 rpm. The SPME fiber was desorbed in split mode with a 10:1 split ratio and the inlet temperature was kept at 260 °C. Bottle duplicates were analyzed in triplicate for each treatment. Compound details are provided in
Table S1.
Wines were analyzed sensorially using descriptive analysis in the J. Lohr Wine Sensory Room, University of California, Davis, CA. GN, BA, and CS wines were analyzed approximately two, three, and four months, respectively, after bottling. Three separate descriptive analysis panels were utilized, one for each variety. Eleven panelists were recruited for GN, and ten each for BA and CS. The panelists were offered $30 gift certificates for completion of the study. The study was approved by the International Review Board (571923-1) and all participants reviewed and agreed to the terms of the experiment. None of the panelists knew details of the experiment.
Two fermentation replicates were selected from each treatment totaling six wines for each descriptive analysis study. There were six training sessions and three evaluation sessions. The panelists were given 30 mL of each wine sample for both the training sessions and the evaluation sessions. The wines were presented blind using black wine glasses (ISO 3591:1977) and the order was randomized for each session. In the first training session, panelists generated descriptors used for differentiating the wines. In subsequent sessions, the reference standards for each descriptor were optimized through panel discussions until there was general agreement. The list of descriptors was narrowed down until there were twenty descriptors for GN (12 aroma, 4 taste, and 4 mouthfeel), twenty-six for BA (14 aroma, 4 taste, and 8 mouthfeel), and twenty-two for CS (11 aroma, 5 taste, and 4 mouthfeel;
Table 1,
Table 2 and
Table 3). Panelists were asked to rate the intensity of each attribute using an unmarked line scale. Reference standards were given as an anchor for the high end of the intensity scale of each attribute. In addition to these attributes, panelists also analyzed color by matching each wine with a color chart (Les Couleurs Du Vin, Bouchard Aîné & Fils). Panelists were given 30 mL of wine in a clear glass and instructed to hold the glass at arm’s length with a white background and match with the closest color on the poster. Scores were reported by assigning number values to each color on the poster. Perceived color differences from sensory analysis were compared to wine colors determined using a CR-400 Chroma Meter (Konica Minolta, Ramsey, NJ, USA) using the CIELAB color space.
Wines were analyzed by the panelists in triplicate using a randomized block design over a one-week period. All analyses were completed in isolated booths with positive air flow and red lighting. Randomized three-digit codes were assigned to the wines (unique for each panelist for each session) to eliminate biases. Panelists were given breaks in between each wine and were encouraged to drink water and eat an unsalted cracker as a pallet cleanser. All samples were expectorated. Data were collected using FIZZ software (version 2.00L, Biosystems, Dijon, France).
All statistical analyses were carried out using XLSTAT (Microsoft Office Professional Plus 2010, version 14.0.7194.5000, Redmond, WA, USA). Univariate analysis of variance (ANOVA) was used for all data in determining significant differences. For descriptive analysis data, multivariate analysis of variance (MANOVA) was used prior to ANOVA to determine the main treatment effect. ANOVA was used for judge, treatment, and replicate effects along with a pseudo mixed model. Fisher’s least significant difference (LSD) was used for pairwise comparisons of means. Statistical significance was set at 5% for all tests.
4. Discussion
The purpose of this study was to determine what effects, if any, optical berry sorting had on wine made from different red grape varieties, and to investigate the potential to use optical sorters to sort for different ripeness levels using color as a main criterion. Given the observed differences in Brix and final ethanol content, optical sorting seemed to be successful in removing underripe berries for CS and possibly for BA; however, this did not result in a significant difference in the final ethanol content between the sort and control treatments. The removal of underripe berries was also evident by the difference in color among treatments. For BA, the rejected treatments were significantly lighter in color; however, the color of the sort and control treatments was very similar, whereas a similar trend was observed in the CS treatments. Wines made from GN generally did not follow these trends; possibly because sorting parameters were too aggressive for this cultivar, resulting in a high percent rejection of optimal berries. This may have minimized potential differences between reject wine with the other treatments. Another possibility is that color differences in the GN fruit did not correspond to differences in sugar content. From these results, it may be concluded that, when using color as a criterion, optical sorting based on ripeness level was successful but may be dependent on variety and fruit variability. Additionally, the impact on the resulting wine is likely dependent on the initial variability in grape ripeness. The optical sorter was successful in removing MOG. This result was reflected in the phenolic analyses; reject treatments were generally higher in total phenolics and tannin, most likely due to the greater proportion of MOG included in the must. The decrease in anthocyanins is likely due to the higher percentage of green, underripe berries in the reject treatment musts. A study that made wine with the addition of MOG found that this addition significantly increased the phenolic and tannin content in the resulting wines [
21]. Despite the differences observed in the phenolic composition of the reject wines, the control and sort treatments were very similar for all three varieties. This is in contrast with some previous studies that have found wine made from optical sorted fruit had significantly different levels of phenolics. One study found that optical sorting led to wines with higher levels of total phenolics [
5]. It should be mentioned that the researchers here did whole cluster pressing for their control wines (Chardonnay), whereas the sorted wines were destemmed. It is possible that higher levels of phenolics were extracted due to the damage caused by the destemming process on the seeds and skins. Another study found that wine made from optically sorted grapes that were machine harvested generally had lower levels of phenolics; levels that were similar to the same wines made from a handpick treatment [
6]. Given that the rejects were, in general, significantly higher in total phenolics and tannin than the control and sort treatments, it can be suggested that optical sorting has the potential to decrease the phenolic content in wine; however, there was not enough MOG to show a large impact in the current study. Optical sorting likely has a greater impact on mechanically harvest fruit due to generally higher levels of MOG observed from this harvest method.
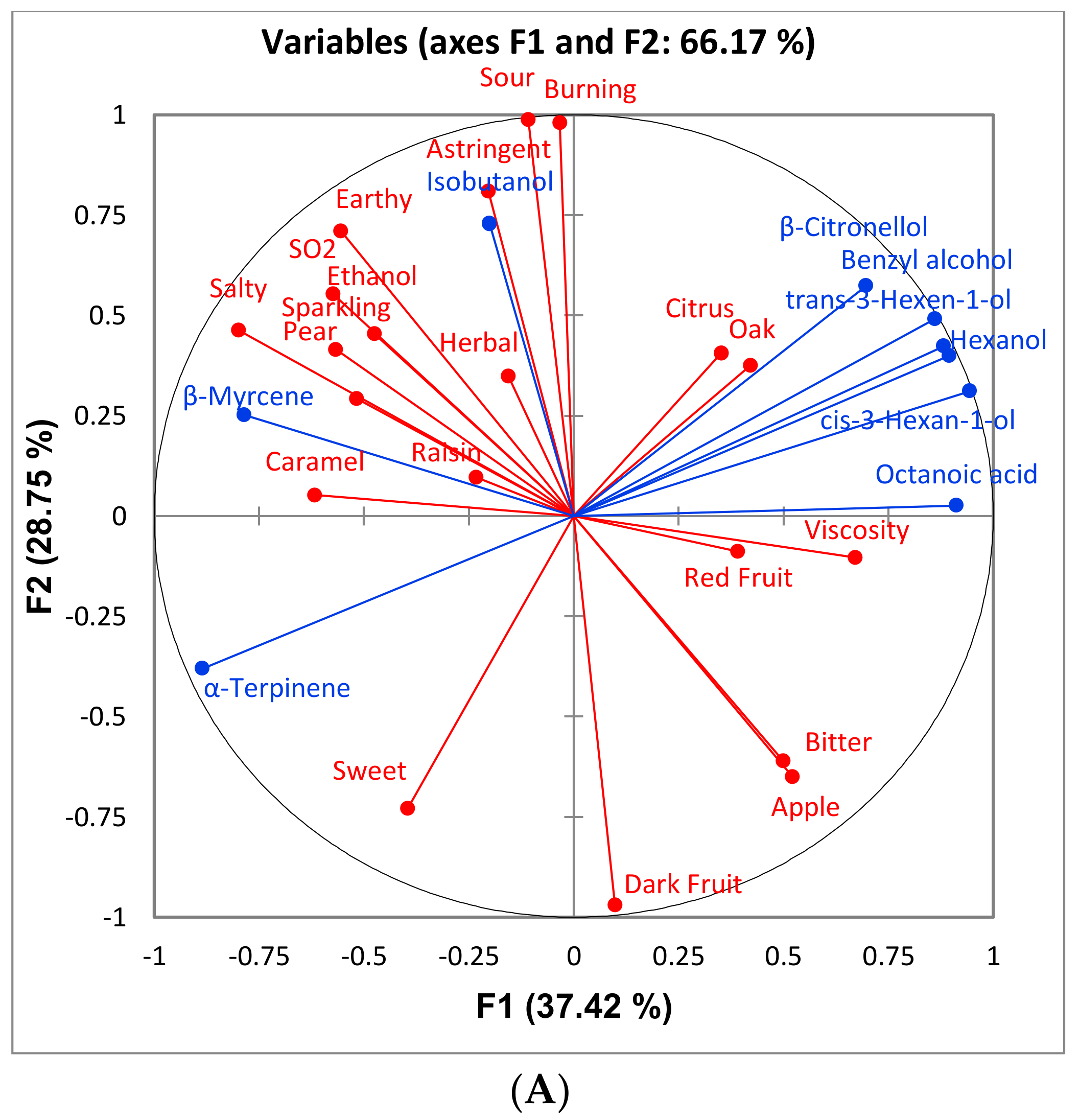
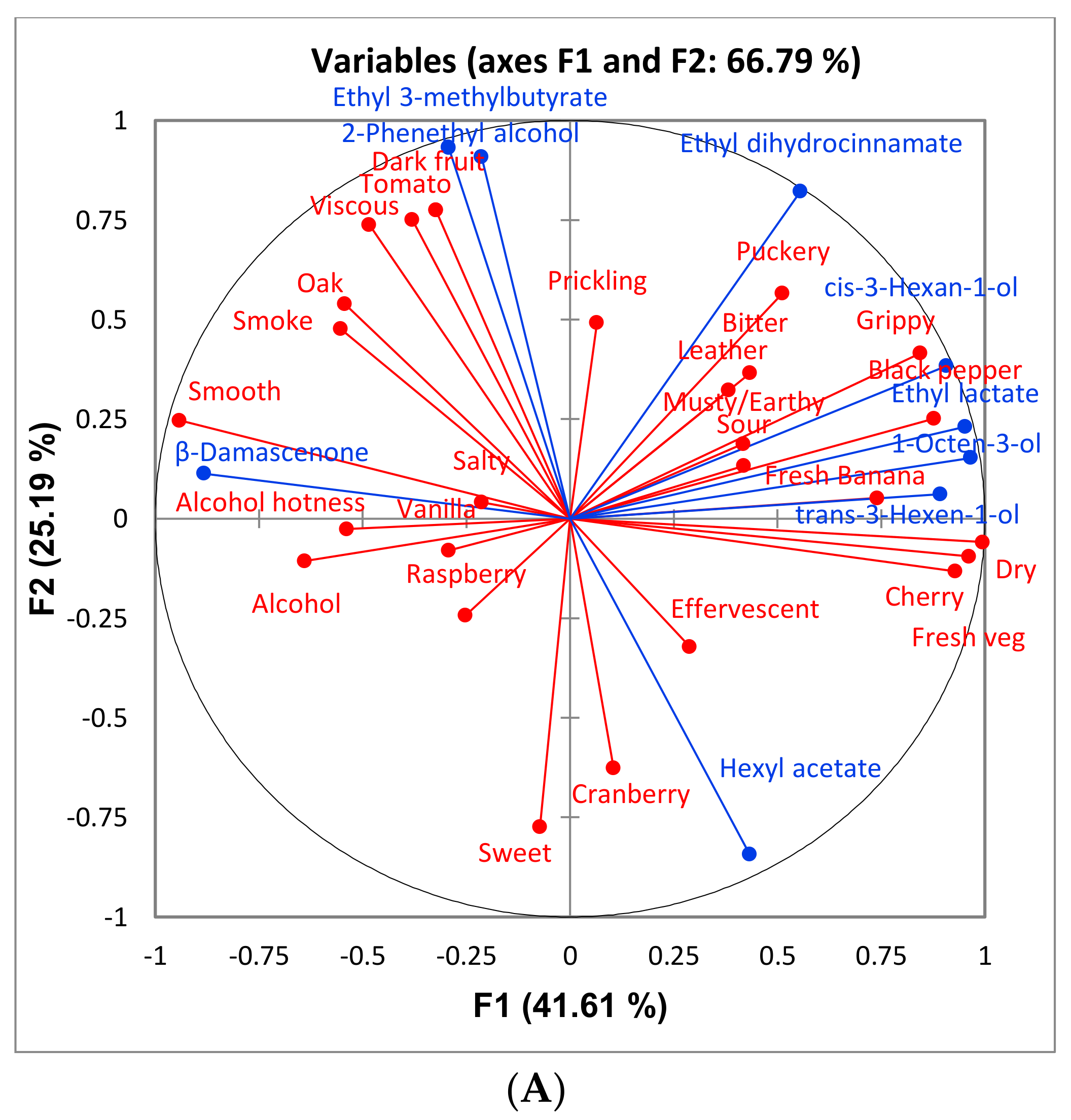
Some differences were found among treatments in the aroma profiles of the wines. Few compounds differed significantly between sort and control treatment and, in general, the reject treatments had greater concentrations of higher alcohols and control and sort treatments had greater concentrations of ethyl esters. The higher ethanol content of the sort and control treatments as well as their lower pH (
Table 5) can lead to a higher production of esters [
15]. In general, reject treatments contained significantly more suspended solids then the control and sort treatments for all varieties studied. Research has shown that high levels of suspended solids during fermentation can lead to greater production of higher alcohols [
16,
17,
18].
Descriptive analysis indicated only one significantly different attribute among GN treatments and only two significantly different attributes among BA treatments. BA control and sort wines were associated with the “alcohol” descriptor which correlated with the higher ethanol levels in these treatments compared to the reject treatment. Similarly, there were only three significant attributes among the CS treatments. “Alcohol hotness” related to ethanol content as previously described. The control and sort treatments were also rated significantly higher in “apple” and “sweet” aromas compared to the reject treatment. Some studies have shown that higher levels of ethanol can increase the perception of sweetness in a wine [
22,
23]. However, as King et al. [
24] noted, there is disagreement in this regard, as other studies have shown that ethanol content can either decrease or have no effect on the perception of sweetness [
25,
26]. Thus, this may not be a sufficient explanation as to why the control and sort wines were rated significantly higher in sweetness. Perhaps the higher concentration of total phenolics and tannin in reject wines could explain the difference (
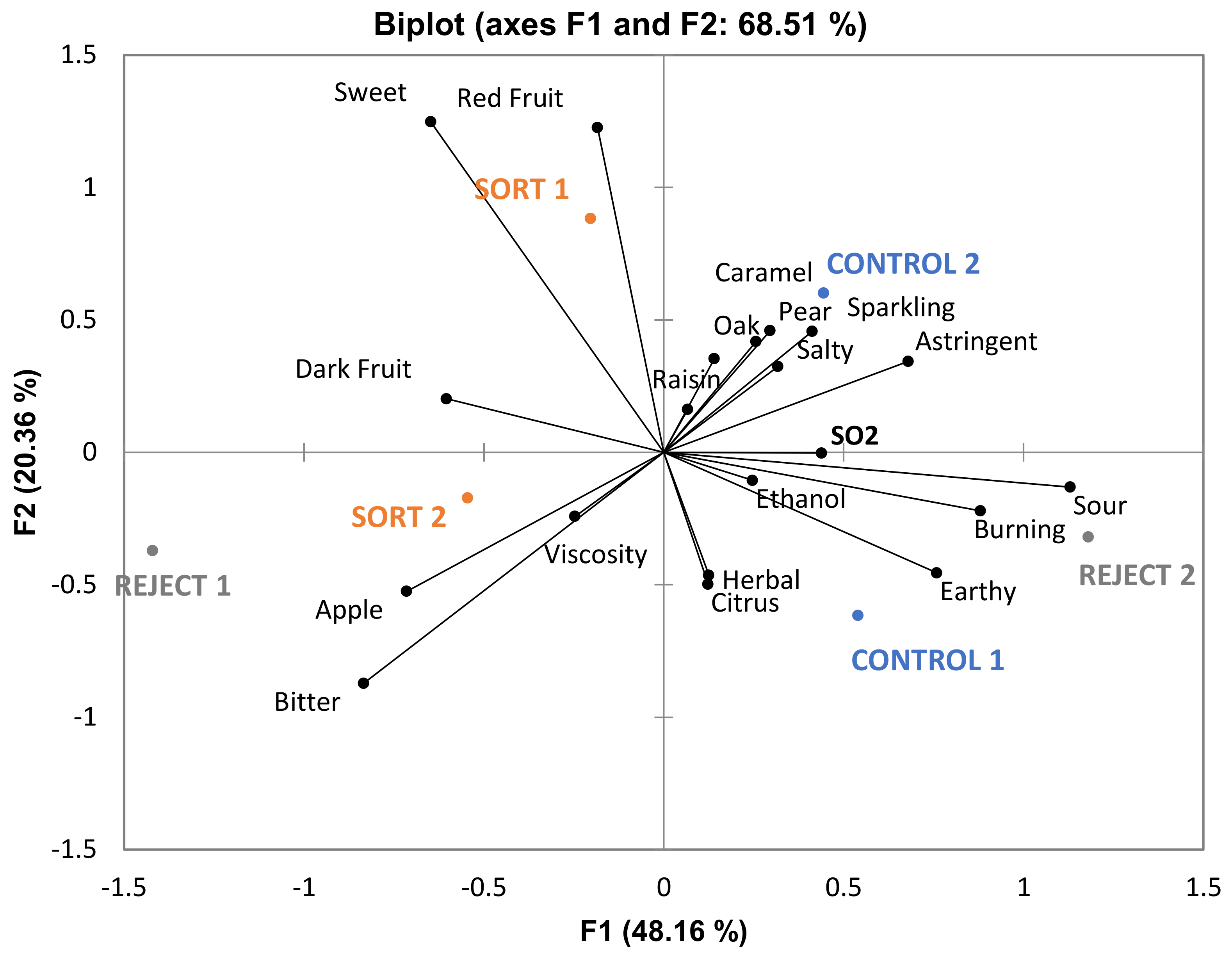
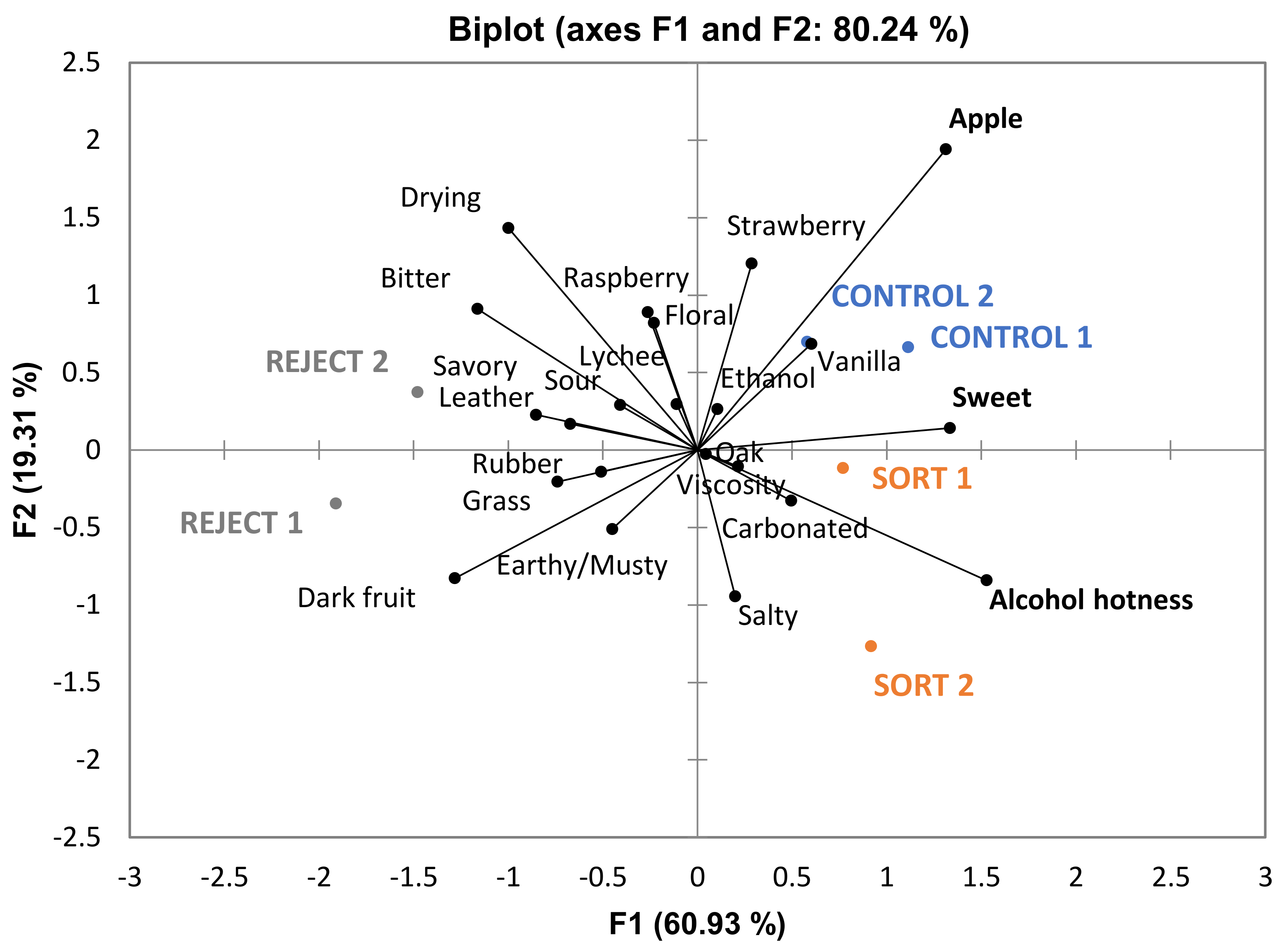
Table 6) given that phenolics in wine contribute to bitterness and astringency. From the PCA in
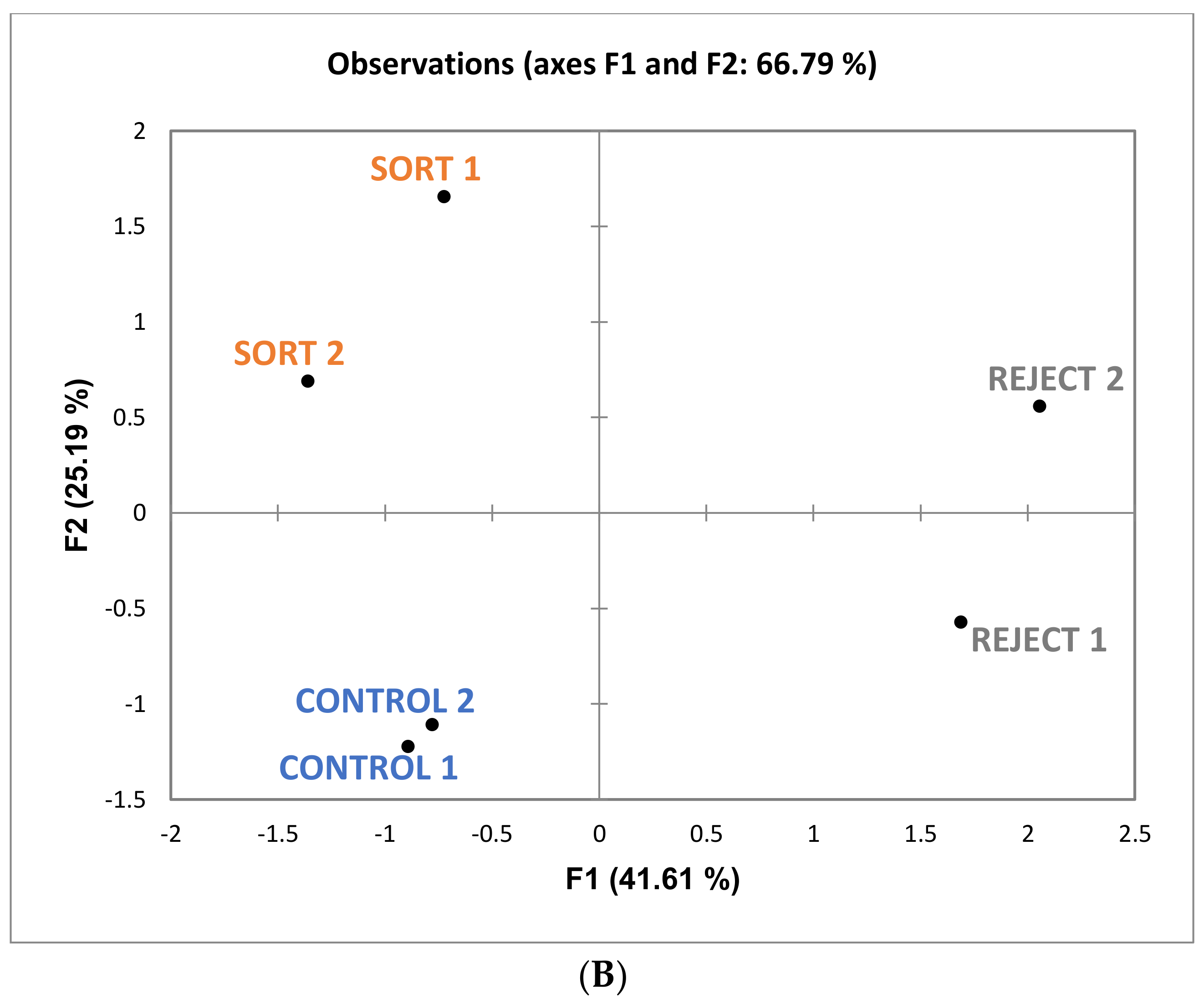
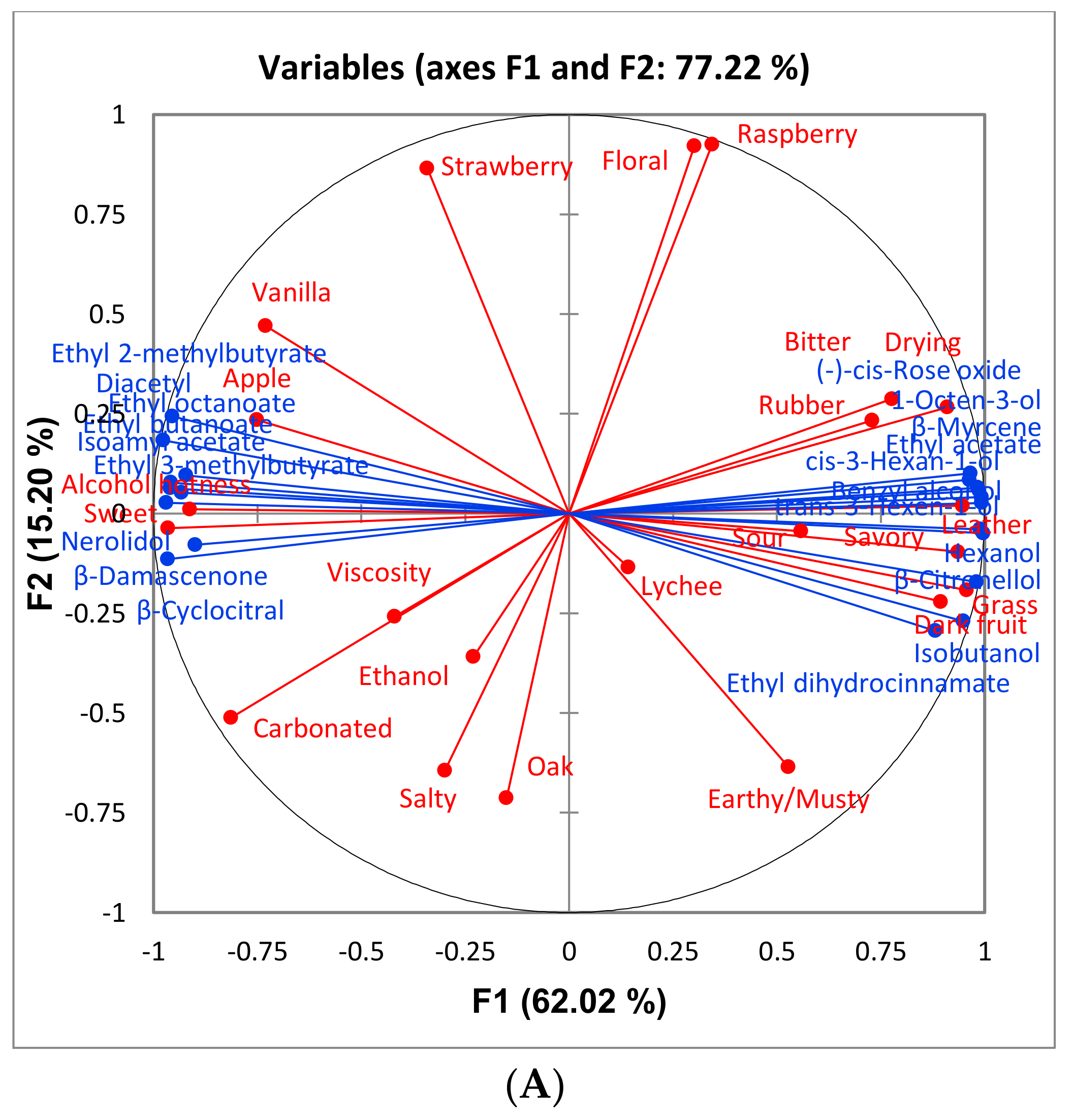
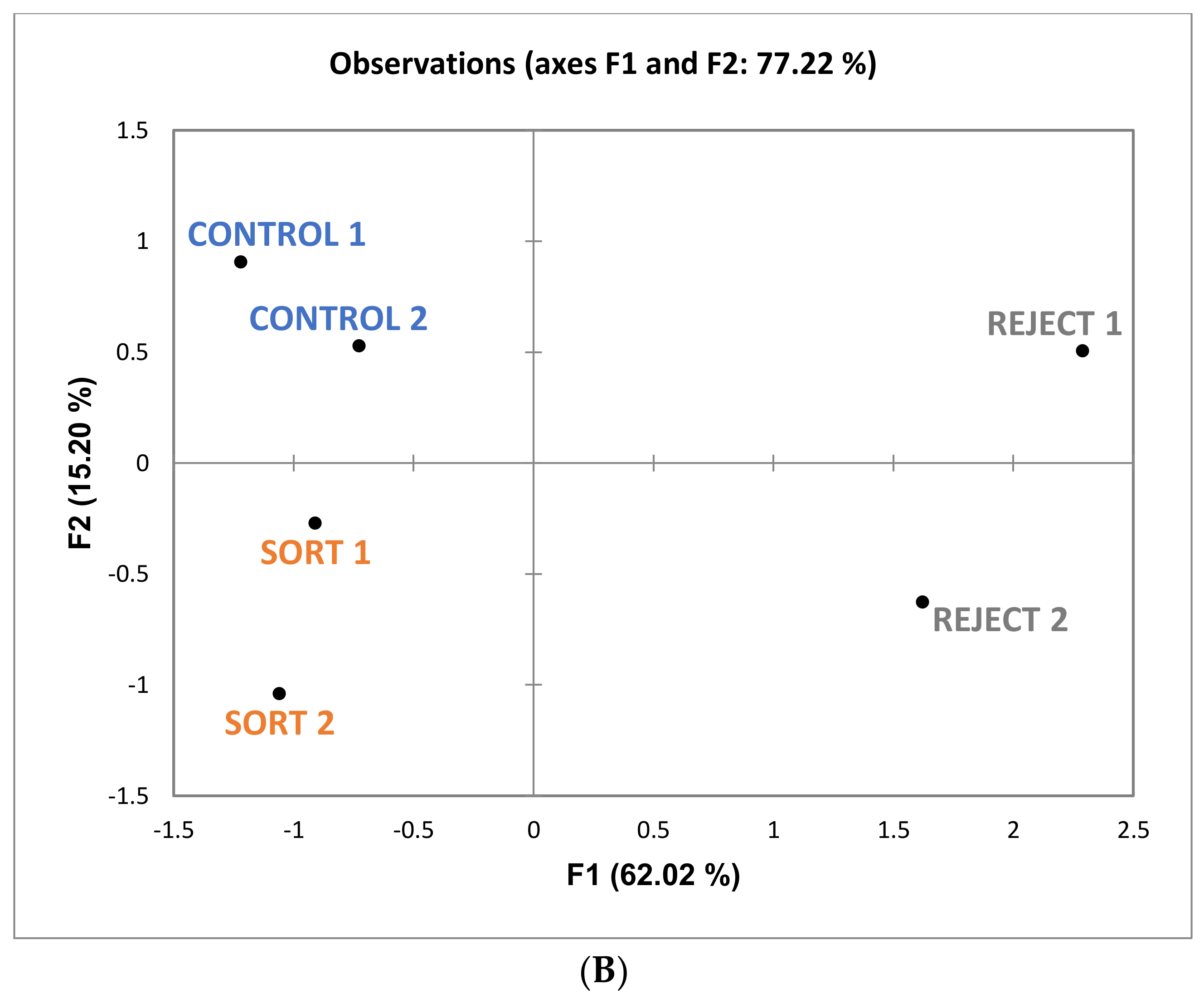
Figure 6, it can be noted that “bitter” and “drying” (with “drying” being a component of astringency) are more associated with reject wines. Although these attributes are not significantly different among the treatments there appears to be a trend which could impact the perception of sweetness. One study found that increasing bitterness in coffee (by the addition of caffeine) decreased the perception of sweetness [
27]. It is possible that reject wines were rated lower in “sweet” due to the higher concentration of phenolic compounds (which can contribute to bitterness perception) thus decreasing the perception of sweetness. The higher perception of sweetness in the control and sort wines may also be attributed to the higher intensity of the “apple” aroma, which the judges could have associated with a sweet taste. One study found that retronasal aroma perception of fruity compounds increased with an increasing level of sweetness in a model wine solution [
28]. The authors also noted several other studies which found that aroma compounds can enhance the perception of sweetness in different foods and beverages. Another study found that samples described as “fruity” were also often associated with a “sweet” aroma [
29]. This provides further evidence that the judges in the current study may have associated these attributes together. The overall sensory differences were minimal, and the wines were determined to be similar.
The results from this study largely agree with results from previous studies investigating the effects of optical sorters. It is possible that there was not enough variation in the starting material of the current study for optical sorting to have a large impact. Optical sorters may be used to greater effect during vintages with inconsistent ripening, issues with raisining, or large amounts of berry damage, possibly caused by either birds and/or fungal infections. Future research should investigate the impact of optical sorters in these scenarios.