Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Pressurized Liquid Extraction
2.4. Design of Experiments
2.5. HPLC-ESI-TOF-MS Analysis
3. Results
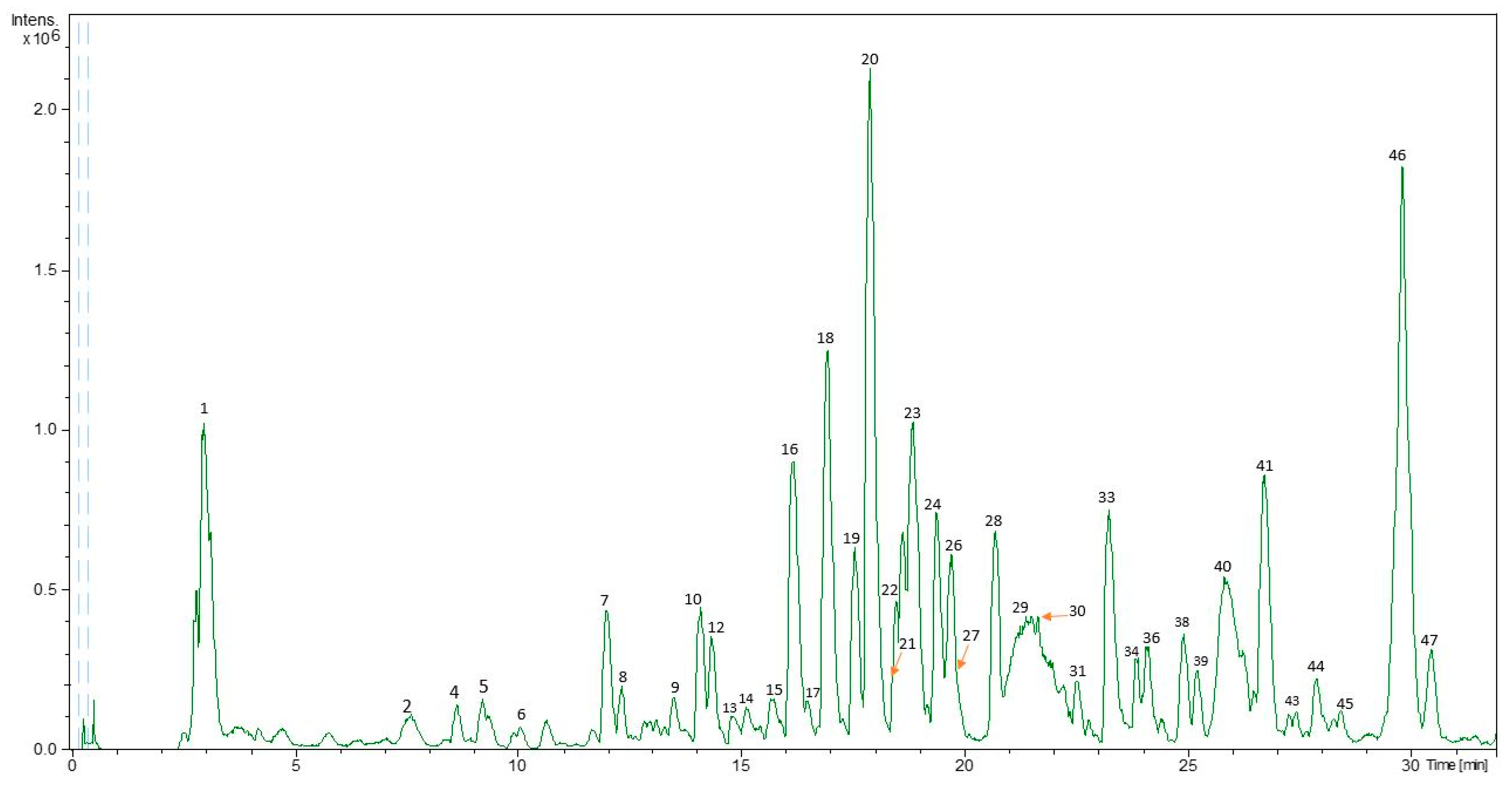
3.1. Identification of Polar Compounds in PLE Extracts of C. grandis by HPLC-ESI-TOF-MS
3.1.1. Disaccharides
3.1.2. Hydroxybenzoic Acids
3.1.3. Flavonoids
3.1.4. Other Polar Compounds
3.2. Quantification of Polar Compounds in C. grandis Seed PLE Extracts by HPLC-ESI-TOF-MS
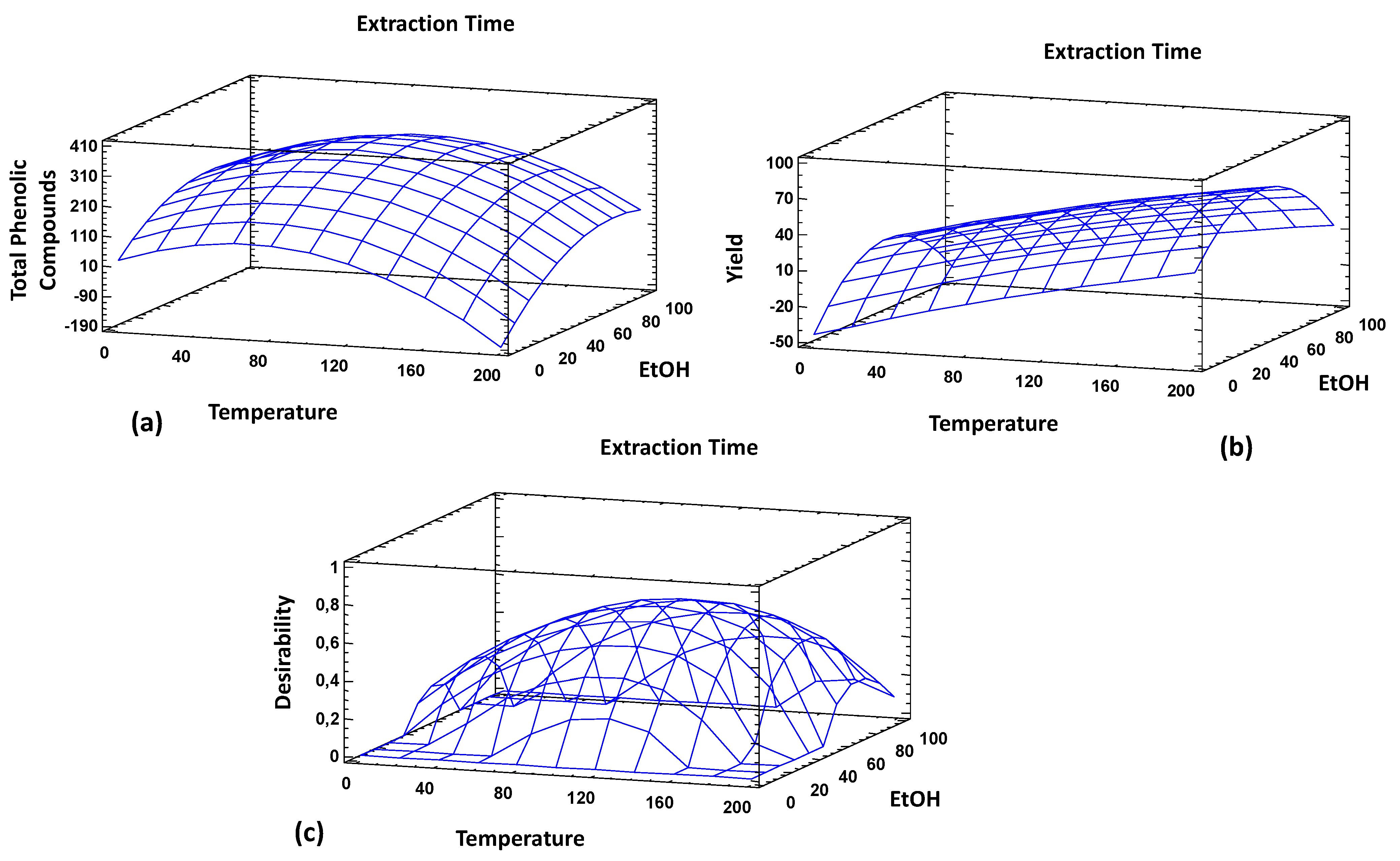
3.3. PLE Extraction Design Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A. Polyphenols and the Modulation of Gene Expression Pathways: Can We Eat Our Way Out of the Danger of Chronic Disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef]
- Frankel, E.; Bakhouche, A.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Literature Review on Production Process to Obtain Extra Virgin Olive Oil Enriched in Bioactive Compounds. Potential Use of Byproducts as Alternative Sources of Polyphenols. J. Agric. Food Chem. 2013, 61, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.L.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships Between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef]
- Villegas-Aguilar, M.D.C.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.D.L.L.; Pimentel-Moral, S.; Lozano-Sánchez, J.; Arráez-Román, D.; Segura-Carretero, A. Pleiotropic Biological Effects of Dietary Phenolic Compounds and their Metabolites on Energy Metabolism, Inflammation and Aging. Molecules 2020, 25, 596. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Jauregui, A.M.; Ramos Escudero, F. Componentes Fenólicos de la Dieta y sus Propiedades Biomedicinales—Phenolics Compounds of the Diet and his Biomedicinal Properties. Horiz. Med. 2007, 7, 23–38. [Google Scholar]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.L.L.; Arráez-Román, D.; Segura-Carretero, A. Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus. Nutrients 2019, 11, 1646. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. Comparative Study of Conventional and Pressurized Liquid Extraction for Recovering Bioactive Compounds from Lippia citriodora Leaves. Food. Res. Int. 2018, 109, 213–222. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Pressurized GRAS Solvents for the Green Extraction of Phenolic Compounds from Hibiscus sabdariffa Calyces. Food Res. Int. 2020, 137, 109466. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Cea-Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; Segura-Carretero, A. Enhancing the Yield of Bioactive Compounds from Sclerocarya birrea Bark by Green Extraction Approaches. Molecules 2019, 24, 966. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Castro-Puyana, M.; Mendiola, J.A.; Segura-Carretero, A.; Cifuentes, A.; Ibáñez, E. Recovering Bioactive Compounds from Olive Oil Filter Cake by Advanced Extraction Techniques. Int. J. Mol. Sci. 2014, 15, 16270–16283. [Google Scholar] [CrossRef]
- Nastić, N.; Lozano-Sánchez, J.; Borrás-Linares, I.; Švarc-Gajić, J.; Segura-Carretero, A. New Technological Approaches for Recovering Bioactive Food Constituents from Sweet Cherry (Prunus avium L.) Stems. Phytochem. Anal. 2020, 31, 119–130. [Google Scholar]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Comparative Assessment of Phytochemical Profiles of Comfrey (Symphytum officinale L.) Root Extracts Obtained by Different Extraction Techniques. Molecules 2020, 25, 837. [Google Scholar] [CrossRef]
- Joshi, H.; Kapoor, V.P. Cassia grandis Linn. f. Seed Galactomannan: Structural and Crystallographical Studies. Carbohydr. Res. 2003, 338, 1907–1912. [Google Scholar] [CrossRef]
- Macía Fuentes, J.A.; Fernández, I.M.; Fernández, H.Z.; Sánchez, J.L.; Alemán, R.S.; Navarro-Alarcon, M.; Borrás-Linares, I.; Saravia Maldonado, S.A. Quantification of Bioactive Molecules, Minerals and Bromatological Analysis in Carao (Cassia grandis). J. Agric. Sci. 2020, 12, 88–94. [Google Scholar]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura- Carretero, A. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-inflammatory Proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Gabaldón-Hernández, J.A.; Segura-Carretero, A.; Fernández- Gutiérrez, A. RP-HPLC-ESI-QTOF/MS2 based Strategy for the Comprehensive Metabolite Profiling of Sclerocarya birrea (Marula) Bark. Ind. Crops Prod. 2015, 71, 214–234. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A Proanthocyanidin-rich Extract from Cassia abbreviata Exhibits Antioxidant and Hepatoprotective Activities In Vivo. J. Ethnopharmacol. 2018, 213, 38–47. [Google Scholar] [CrossRef]
- Da Costa Silva, T.; Justino, A.B.; Prado, D.G.; Koch, G.A.; Martins, M.M.; de Souza Santos, P.; Lemos de Morais, S.A.; Goulart, L.R.; Scalon Cunha, L.C.; Ferreira de Sousa, R.M.; et al. Chemical Composition, Antioxidant Activity and Inhibitory Capacity of α-Amylase, α-Glucosidase, Lipase and Non-Enzymatic Glycation, In Vitro, of the Leaves of Cassia bakeriana Craib. Ind. Crops Prod. 2019, 140, 111641. [Google Scholar] [CrossRef]
- Ucar, M.B.; Ucar, G.; Pizzi, A.; Gonultas, O. Characterization of Pinus brutia Bark Tannin by MALDI-TOF MS and 13C NMR. Ind. Crops Prod. 2013, 49, 697–704. [Google Scholar] [CrossRef]
- Xu, X.; Xie, H.; Hao, J.; Jiang, Y.; Wei, X. Flavonoid Glycosides from the Seeds of Litchi chinensis. J. Agric. Food Chem. 2011, 59, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lou, G.; Ma, Z.; Liu, X. Chemical Constituents with Antioxidant Activities from Litchi (Litchi chinensis Sonn.) Seeds. Food Chem. 2011, 126, 1081–1087. [Google Scholar] [CrossRef]
- Demirkiran, O.; Topcu, G.; Hussain, J.; Uddin Ahmad, V.; Choudhary, M.I. Structure Elucidation of Two New Unusual Monoterpene Glycosides from Euphorbia decipiens, by 1D and 2D NMR Experiments. Magn. Reson. Chem. 2011, 49, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, G.; Luo, B.; Wang, L.; Lu, Y.; Liu, W. Screening and Isolation of Natural Antioxidants from: Ziziphora clinopodioides Lam. with High Performance Liquid Chromatography coupled to a Post-column Ce(IV) Reduction Capacity Assay. RSC Adv. 2016, 6, 62378–62384. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Li, N.; Zhou, Y.; Jiang, Y.; Tu, P.F. Simultaneous Qualitative and Quantitative Determination of Major Polymethoxylated Flavonoids in the Leaves of Murraya paniculata by RRLC-DAD-ESI-MSn. Anal. Methods 2012, 4, 3399–3406. [Google Scholar] [CrossRef]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast Separation and Sensitive Quantitation of Polymethoxylated Flavonoids in the Peels of Citrus using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, J.; Zhang, Q.; Dai, L.; Liu, Y.; Tu, P.; Qiao, Y. Simultaneous Screening and Identifying Four Categories of Particular Flavonoids in the Leaves of Murraya exotica L. by HPLC-DAD-ESI-MS-MS. J. Chromatogr. Sci. 2014, 52, 1–12. [Google Scholar] [CrossRef]
- Thabit, S.; Handoussa, H.; Roxo, M.; El Sayed, N.S.; de Azevedo, B.C.; Wink, M. Evaluation of Antioxidant and Neuroprotective Activities of Cassia fistula (L.) using the Caenorhabditis elegans model. PeerJ 2018, 13, 1–33. [Google Scholar] [CrossRef]
- Zheng, Y.; Luan, L.; Chen, Y.; Ren, Y.; Wu, Y. Characterization of Physalins and Fingerprint Analysis for the Quality Evaluation of Physalis alkekengi L. var. franchetii by Ultra-Performance Liquid Chromatography combined with Diode Array Detection and Electrospray Ionization Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2012, 71, 54–62. [Google Scholar] [CrossRef]
- Chen, X.; Tong, L.; Chu, Y.; Wang, X.; Zhang, L.; Ma, X.; Zhou, S.; Liu, C. Identification and Characterization of Anthraquinones in Cassia tora L. by Liquid Chromatography connected with Time of Flight Mass Spectrometry and Ion Trap Mass Spectrometry. Asian J. Chem. 2013, 25, 7840–7842. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of Drying Process and Pressurized Liquid Extraction for Recovery of Bioactive Compounds from Avocado Peel By-product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed Fluids for the Extraction of Bioactive Compounds. Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-Assisted Extraction for Hibiscus sabdariffa Bioactive Compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Manca, M.L.; Manconi, M.; Caddeo, C.; Vázquez, J.A.; Lozano-Sánchez, J.; Escribano-Ferrer, E.; Arráez-Román, D.; Segura-Carretero, A. Incorporation of Lippia citriodora Microwave Extract into Total-Green Biogelatin-Phospholipid vesicles to Improve its Antioxidant Activity. Nanomaterials 2020, 10, 765. [Google Scholar] [CrossRef] [PubMed]
| Experimental Condition | Temperature (°C) | %EtOH | Static Cycle (min) |
|---|---|---|---|
| PLE 1 | 40 | 15 | 20 |
| PLE 2 | 40 | 85 | 5 |
| PLE 3 | 110 | 5 | 12.5 |
| PLE 4 | 110 | 50 | 22 |
| PLE 5 | 40 | 15 | 5 |
| PLE 6 | 20 | 50 | 12.5 |
| PLE 7 | 110 | 50 | 3 |
| PLE 8 | 110 | 50 | 12.5 |
| PLE 9 | 110 | 50 | 12.5 |
| PLE 10 | 40 | 85 | 20 |
| PLE 11 | 180 | 85 | 5 |
| PLE 12 | 180 | 85 | 20 |
| PLE 13 | 110 | 95 | 12.5 |
| PLE 14 | 200 | 50 | 12.5 |
| Peak | RT (min) | Proposed Compound | m/z | m/z Exp | Molecular Formula | Error (ppm) | mSigma | PLE |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.96 | Sucrose | 341.1089 | 341.1171 | C12H22O11 | −6.1 | 36.4 | * |
| 2 | 7.59 | Galloyl glucoside | 331.0671 | 331.0696 | C13H16O10 | 2.0 | 11.8 | * |
| 3 | 8.40 | Galloyl glucoside derivative | 315.0722 | 315.0675 | C13H16O9 | 3.3 | 5.7 | 1, 3, 4, 5, 6, 7, 8, 11 |
| 4 | 8.66 | UK1 | 397.1715 | 397.1736 | C16H29O11 | −4.1 | 13.3 | 2, 10 |
| 5 | 9.20 | UK2 | 380.1562 | 380.1574 | C15H26NO10 | −0.5 | 6.0 | 2, 10 |
| 6 | 10.05 | UK3 | 371.0925 | 371.0993 | C23H16O5 | −13.4 | 32.6 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 |
| 7 | 11.99 | UK4 | 443.1923 | 443.1963 | C21H32O10 | −8.8 | 3.6 | * |
| 8 | 12.33 | (Epi)gallocatechin–(epi)catechin or isomer 1 | 593.1301 | 593.1511 | C30H26O13 | −19.6 | 5.1 | * |
| 9 | 13.48 | Theaflavin derivative | 771.2353 | 771.2391 | C34H44O20 | 5.8 | 3.9 | * |
| 10 | 14.10 | UK5 | 541.2173 | 541.2179 | C40H29O2 | 6.5 | 72.4 | * |
| 11 | 14.23 | Catechin | 289.0718 | 289.0734 | C15H14O6 | −9.6 | 9.5 | 3, 4, 5, 8, 9, 11, 12, 14 |
| 12 | 14.33 | (Epi)gallocatechin–(epi)catechin or isomer 2 | 593.1301 | 593.1523 | C30H26O13 | −18.9 | 28.4 | * |
| 13 | 14.82 | Procyanidin derivative | 579.1508 | 579.1728 | C30H27O12 | −19.5 | 11.8 | 1, 2, 3, 4, 7, 8, 9, 10, 12, 13 |
| 14 | 15.12 | (Epi)catechin–(epi)catechin | 577.1351 | 577.1368 | C30H26O12 | 5.8 | 8.4 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13 |
| 15 | 15.72 | UK6 | 401.1089 | 401.1096 | C17H21O11 | −1.9 | 10.6 | 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13 |
| 16 | 16.17 | (Epi)-catechin | 289.0718 | 289.0786 | C15H14O6 | −10.3 | 2.1 | * |
| 17 | 16.47 | Astilbin | 449.1089 | 449.1109 | C21H22O11 | 2.9 | 6.4 | * |
| 18 | 16.94 | Pinocembrin 7-neohesperidoside | 563.1770 | 563.1743 | C27H32O13 | 5.3 | 14.1 | * |
| 19 | 17.54 | UK7 | 563.1864 | 563.1822 | C38H28O5 | 7.3 | 10.6 | * |
| 20 | 17.91 | Pinocembrin 7-rutinoside | 563.1770 | 563.1778 | C27H32O13 | −14.5 | 12.8 | * |
| 21 | 18.41 | (Epi)-afzelechin or isomer 1 | 273.0768 | 273.0777 | C15H14O5 | −1.8 | 36.9 | * |
| 22 | 18.50 | Cassanidin A | 817.2138 | 817.2133 | C45H38O15 | 6.8 | 9.8 | * |
| 23 | 18.85 | (Epi)-guibourtinidol-(epi)-catechin or isomer 1 | 545.1453 | 545.1547 | C30H26O10 | −13.1 | 40.4 | * |
| 24 | 19.32 | UK8 or isomer 1 | 553.2232 | 553.2200 | C34H33O7 | 2.4 | 6.6 | 2, 3, 5, 6, 10 |
| 25 | 19.37 | (Epi)-afzelechin or isomer 2 | 273.0768 | 273.0840 | C15H14O5 | −15.8 | 7.2 | 11, 12 |
| 26 | 19.70 | UK8 or isomer 2 | 553.2232 | 553.2195 | C34H33O7 | 4.9 | 9.8 | 2, 3, 6, 10 |
| 27 | 19.77 | Quercentin-3-glucoside | 463.0882 | 463.0894 | C21H20O12 | −2.1 | 34.0 | 1, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 |
| 28 | 20.67 | Physalin A | 525.1766 | 525.1672 | C28H30O10 | 17.0 | 17.3 | * |
| 29 | 21.46 | (Epi)-guibourtinidol-(epi)-afzelechin or isomer 1 | 529.1504 | 529.1534 | C30H26O9 | −0.2 | 42.5 | * |
| 30 | 21.62 | Quercetin- rhamnoside | 447.0933 | 447.0951 | C21H20O11 | −7.7 | 38.6 | * |
| 31 | 22.49 | (Epi)-guibourtinidol-(epi)-catechin or isomer 2 | 545.1453 | 545.1465 | C30H26O10 | 9.0 | 10.0 | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 |
| 32 | 22.56 | (Epi)-guibourtinidol-(epi)-afzelechin or isomer 2 | 529.1504 | 529.1559 | C30H26O9 | −4.7 | 9.1 | 3, 12 |
| 33 | 23.20 | (Epi)-guibourtinidol-(epi)-afzelechin or isomer 3 | 529.1504 | 529.1574 | C30H26O9 | −6.5 | 29.5 | * |
| 34 | 23.81 | (Epi)-guibourtinidol-(epi)-catechin or isomer 3 | 545.1453 | 545.1470 | C30H26O10 | 6.6 | 3.8 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13 |
| 35 | 23.96 | (Epi)-guibourtinidol-(epi)-afzelechin or isomer 4 | 529.1504 | 529.1501 | C30H26O9 | 7.3 | 34.8 | 1, 2, 3, 4, 5, 7, 8, 9, 11, 12, 13, 14 |
| 36 | 24.07 | Kaempferol- rhamnoside or isomer 1 | 431.0984 | 431.0999 | C21H20O10 | −3.5 | 19.9 | 1, 2, 3, 6, 10, 11, 12, 13, 14 |
| 37 | 24.35 | (Epi)-guibourtinidol-(epi)-afzelechin or isomer 5 | 529.1504 | 529.1498 | C30H26O9 | 1.2 | 29.1 | 1, 2, 4, 6, 7, 8, 9, 10, 12, 13 |
| 38 | 24.87 | Kaempferol- rhamnoside or isomer 2 | 431.0984 | 431.1004 | C21H20O10 | 9.7 | 6.1 | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 |
| 39 | 25.19 | Catechin-guibourtinidol-cassiaflavan | 785.2240 | 785.2271 | C45H38O13 | 7.4 | 3.2 | * |
| 40 | 25.80 | Diflavanoid or isomer 1 | 513.1555 | 513.1599 | C30H26O8 | −0.4 | 22.4 | * |
| 41 | 26.67 | Diflavanoid or isomer 2 | 513.1555 | 513.1619 | C30H26O8 | −13.1 | 23.3 | * |
| 42 | 27.08 | Chrysophanol | 253.0506 | 253.0521 | C15H10O4 | −8.4 | 5.4 | 3, 5 |
| 43 | 27.39 | UK9 | 697.2138 | 697.2121 | C35H38O15 | 9.2 | 6.8 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 |
| 44 | 27.81 | Diflavanoid or isomer 3 | 513.1555 | 513.1552 | C30H26O8 | 6.7 | 16.5 | * |
| 45 | 28.24 | Diflavanoid or isomer 4 | 513.1555 | 513.1541 | C30H26O8 | −11.4 | 21.9 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14 |
| 46 | 29.82 | Hexametoxyflavone | 401.1242 | 401.1180 | C21H22O8 | 12.5 | 16.8 | * |
| 47 | 30.44 | Diflavanoid or isomer 5 | 513.1555 | 513.1556 | C30H26O8 | −0.3 | 12.3 | * |
| Pattern | Calibration Range (mg/L) | Calibration Curve | R2 |
|---|---|---|---|
| Gallic acid | 1–150 | y = 23,395x − 37,644 | 0.9926 |
| Catechin | 0.5 -20 | y = 262,318x + 23,166 | 0.9963 |
| Epi-catechin | 0.5–50 | y = 287,543x + 575,127 | 0.9925 |
| Epigallocatechin-gallate | 0.5–150 | y = 82,849x + 198,670 | 0.9918 |
| Quercetin-3-glucoside | 0.5–20 | y = 457,785x + 340,216 | 0.9916 |
| Kaempferol-rutinoside | 0.5–150 | y = 115,620x + 754,772 | 0.996 |
| Experimental Design Condition | Yield (%) | Total Polar Compound (mg Compound/g Extract) |
|---|---|---|
| PLE 1 | 4.44 | 262 ± 4 |
| PLE 2 | 3.82 | 236 ± 1 |
| PLE 3 | 5.16 | 87 ± 4 |
| PLE 4 | 19.9 | 266 ± 3 |
| PLE 5 | 3.14 | 136 ± 1 |
| PLE 6 | 11.15 | 200 ± 2 |
| PLE 7 | 20.19 | 327 ± 5 |
| PLE 8 | 28.45 | 286 ± 5 |
| PLE 9 | 29.91 | 279 ± 2 |
| PLE 10 | 4.82 | 349 ± 2 |
| PLE 11 | 15.68 | 217 ± 7 |
| PLE 12 | 23.51 | 114 ± 2 |
| PLE 13 | 3.64 | 271 ± 12 |
| PLE 14 | 29.97 | 46.8 ± 0.6 |
| PLE 1 | PLE 2 | PLE 3 | PLE 4 | PLE 5 | PLE 6 | PLE 7 | |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic Acids | |||||||
| Galloyl glucoside | 25 ± 5 | 11.1 ± 0.6 | 11.7 ± 0.7 | 13.4 ± 0.9 | 16.1 ± 0.4 | 14 ± 1 | 16 ± 1 |
| Galloyl glucoside derivative | 2.2 ± 0.1 | ND | 2.36 ± 0.05 | 1.8 ± 0.1 | 3.4 ± 0.1 | 1.7 ± 0.3 | 2.1 ± 0.3 |
| Total hydroxybenzoic acids | 27 ± 5 | 11.1 ± 0.6 | 14 ± 1 | 15.1 ± 0.9 | 19.6 ± 0.4 | 15 ± 1 | 18 ± 1 |
| Flavanols | |||||||
| Theaflavin derivative | 0.72 ± 0.02 | 1.12 ± 0.03 | 0.163 ± 0.005 | 0.86 ± 0.04 | 0.249 ± 0.004 | 0.69 ± 0.02 | 1.03 ± 0.02 |
| Catechin | ND | ND | 1.01 ± 0.09 | 2.3 ± 0.1 | 1.25 ± 0.03 | ND | ND |
| (Epi)-catechin | 8.3 ± 0.5 | 6.63 ± 0.05 | 2.1 ± 0.2 | 8.0 ± 0.4 | 3.0 ± 0.1 | 5.9 ± 0.3 | 8.4 ± 0.1 |
| (Epi)-afzelechin or isomer 1 | 3.2 ± 0.5 | 2.1 ± 0.2 | 1.77 ± 0.06 | 4.0 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 | 4.7 ± 0.2 |
| (Epi)-afzelechin or isomer 2 | ND | ND | ND | ND | ND | ND | ND |
| Diflavanoid or isomer 1 | 14 ± 1 | 11.4± 0.4 | 3.0 ± 0.1 | 15.9 ± 0.6 | 6.2 ± 0.2 | 11.8 ± 0.3 | 10.3 ± 0.2 |
| Diflavanoid or isomer 2 | 11.5 ± 0.5 | 8.2 ± 0.4 | 1.94 ± 0.02 | 9.35 ± 0.03 | 3.1 ± 0.1 | 8.1 ± 0.4 | 13.6 ± 0.1 |
| Diflavanoid or isomer 3 | 1.41 ± 0.06 | 1.09 ± 0.08 | 0.78 ± 0.02 | 3.3 ± 0.2 | 1.26 ± 0.05 | 1.04 ± 0.02 | 2.54 ± 0.06 |
| Diflavanoid or isomer 4 | 0.18 ± 0.02 | 0.22 ± 0.01 | 0.197 ± 0.003 | 0.99 ± 0.08 | 0.23 ± 0.01 | 0.20 ± 0.01 | 0.476 ± 0.006 |
| Diflavanoid or isomer 5 | 2.01 ± 0.07 | 1.80 ± 0.04 | 0.236 ± 0.006 | 1.78 ± 0.06 | 0.404 ± 0.004 | 1.98 ± 0.06 | 4.1 ± 0.1 |
| Total flavanols | 41 ± 1 | 33 ± 1 | 11.2 ± 0.3 | 46 ± 1 | 18.1 ± 0.4 | 31.8 ± 0.9 | 45.2 ± 0.3 |
| Flavonols | |||||||
| Astilbin | 0.69 ± 0.04 | 0.30 ± 0.03 | 0.066 ± 0.004 | 0.70 ± 0.05 | 0.18 ± 0.01 | 0.30 ± 0.02 | 1.08 ± 0.05 |
| Quercentin-3-glucoside | 1.86 ± 0.08 | ND | 0.29 ± 0.02 | 2.03 ± 0.06 | 0.49 ± 0.03 | 0.66 ± 0.01 | 2.71 ± 0.1 |
| Quercetin-rhamnoside | 2.1 ± 0.2 | 0.91 ± 0.05 | 0.37 ± 0.01 | 2.07 ± 0.09 | 0.91 ± 0.05 | 1.23 ± 0.09 | 2.8 ± 0.1 |
| Kaempferol-rhamnoside or isomer 1 | 1.8 ± 0.1 | 2.74 ± 0.05 | 0.26 ± 0.01 | ND | ND | 1.9 ± 0.1 | ND |
| Kaempferol-rhamnoside or isomer 2 | 2.5 ± 0.2 | 2.5 ± 0.2 | ND | 1.13 ± 0.06 | 0.56 ± 0.02 | 2.2 ± 0.1 | 2.10 ± 0.06 |
| Total flavonols | 9.0 ± 0.3 | 6.5 ± 0.2 | 1.00 ± 0.02 | 5.94 ± 0.07 | 2.15 ± 0.03 | 6.3 ± 0.2 | 8.7 ± 0.2 |
| Flavanones | |||||||
| Pinocembrin 7-neohesperidoside | 18 ± 2 | 24.9 ± 0.4 | 3.7 ± 0.2 | 15.1 ± 0.2 | 5.7 ± 0.2 | 13.7 ± 0.3 | 19 ± 1 |
| Pinocembrin 7-rutinoside | 25 ± 1 | 35.9 ± 0.9 | 7.7 ± 0.1 | 20.8 ± 0.6 | 13.5 ± 0.5 | 20.5 ± 0.4 | 26 ± 1 |
| Total flavanones | 43 ± 3 | 61 ± 1 | 11.4 ± 0.3 | 36.0 ± 0.7 | 19.1 ± 0.7 | 34.3 ± 0.6 | 45 ± 2 |
| Flavones | |||||||
| Hexametoxyflavone | 2.9 ± 0.1 | 18.4 ± 0.8 | 1.38 ± 0.09 | 1.55 ± 0.04 | 2.8 ± 0.1 | 9.1 ± 0.3 | 3.00 ± 0.08 |
| Total flavones | 2.9 ± 0.1 | 18.4 ± 0.8 | 1.38 ± 0.09 | 1.55 ± 0.04 | 2.8 ± 0.1 | 9.1 ± 0.3 | 3.00 ± 0.08 |
| Proanthocyanidins/Prodelphinidins | |||||||
| (Epi)gallocatechin–(epi)catechin or isomer 1 | 2.7 ± 0.1 | 3.1 ± 0.2 | 0.52 ± 0.02 | 1.74 ± 0.03 | 0.80 ± 0.04 | 1.47 ± 0.09 | 2.30 ± 0.02 |
| (Epi)gallocatechin–(epi)catechin or isomer 2 | 4.7 ± 0.5 | 6.2 ± 0.2 | 0.89 ± 0.03 | 4.2 ± 0.2 | 1.56 ± 0.02 | 3.8 ± 0.3 | 4.9 ± 0.1 |
| Procyanidin derivative | 1.7 ± 0.1 | 1.9 ± 0.1 | 0.62 ± 0.02 | 2.5 ± 0.1 | ND | ND | 2.2 ± 0.2 |
| (Epi)catechin–(epi)catechin | 1.85 ± 0.02 | 2.06 ± 0.06 | 0.56 ± 0.01 | 2.60 ± 0.08 | 0.90 ± 0.04 | 1.80 ± 0.03 | 4.4 ± 0.2 |
| Cassanidin A | 3.7 ± 0.1 | 2.10 ± 0.09 | 0.83 ± 0.01 | 4.3 ± 0.1 | 1.34 ± 0.04 | 2.16 ± 0.09 | 6.3 ± 0.2 |
| (Epi)-guibourtinidol-(epi)-catechin or isomer 1 | 42 ± 1 | 29.3 ± 0.3 | 17.1 ± 0.9 | 39.5 ± 0.4 | 25 ± 1 | 28.0 ± 0.4 | 47 ± 1 |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 1 | 41 ± 2 | 28.9 ± 0.4 | 18 ± 1 | 64 ± 2 | 28.1 ± 0.5 | 42 ± 1 | 81.5 ± 0.2 |
| (Epi)-guibourtinidol-(epi)-catechin or isomer 2 | 4.1 ± 0.3 | 2.8 ± 0.2 | ND | 3.9 ± 0.1 | 0.94 ± 0.02 | 2.58 ± 0.04 | 6.6 ± 0.3 |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 2 | ND | ND | 0.47 ± 0.06 | ND | ND | ND | ND |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 3 | 25 ± 1 | 20.9 ± 0.5 | 5.9 ± 0.2 | 23.7 ± 0.4 | 11.8 ± 0.4 | 12.9 ± 0.4 | 29.4 ± 0.9 |
| (Epi)-guibourtinidol-(epi)-catechin or isomer 3 | 5.8 ± 0.3 | 3.3 ± 0.1 | 0.65 ± 0.03 | 5.5 ± 0.2 | 1.30 ± 0.02 | 3.44 ± 0.08 | 9.0 ± 0.5 |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 4 | 2.8 ± 0.3 | 2.1 ± 0.1 | 0.95 ± 0.04 | 3.7 ± 0.2 | 1.4 ± 0.1 | ND | 4.3 ± 0.1 |
| Catechin-guibourtinidol-cassiaflavan | 3.6 ± 0.1 | 3.1 ± 0.1 | 0.75 ± 0.01 | 4.63 ± 0.09 | 1.37 ± 0.02 | 2.9 ± 0.2 | 7.1 ± 0.4 |
| Total proanthocyanidins | 138 ± 5 | 107 ± 1 | 48 ± 3 | 161 ± 2 | 74.2 ± 0.9 | 103 ± 2 | 206 ± 3 |
| Total polyphenols | 262 ± 4 | 236 ± 1 | 87 ± 4 | 266 ± 3 | 136 ± 1 | 200 ± 2 | 327 ± 5 |
| PLE 8 | PLE 9 | PLE 10 | PLE 11 | PLE 12 | PLE 13 | PLE 14 | |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic Acids | |||||||
| Galloyl glucoside | 15.4 ± 0.5 | 14.6 ± 0.9 | 20 ± 1 | 17 ± 1 | 8.6 ± 0.4 | 18 ± 2 | 2.97 ± 0.07 |
| Galloyl glucoside derivative | 2.5 ± 0.2 | ND | ND | 3.1 ± 0.2 | ND | ND | ND |
| Total hydroxybenzoic acids | 17.9 ± 0.4 | 14.6 ± 0.9 | 20 ± 1 | 20 ± 1 | 8.6 ± 0.4 | 18 ± 2 | 2.97 ± 0.07 |
| Flavanols | |||||||
| Theaflavin derivative | 0.74 ± 0.04 | 0.93 ± 0.02 | 1.48 ± 0.01 | 0.69 ± 0.01 | 0.41 ± 0.01 | 0.84 ± 0.03 | 0.08 ± 0.01 |
| Catechin | 1.49 ± 0.08 | 1.69 ± 0.02 | ND | 4.3 ± 0.3 | 2.5 ± 0.3 | ND | 0.82 ± 0.02 |
| (Epi)-catechin | 9.2 ± 0.4 | 8.5 ± 0.3 | 10.0 ± 0.3 | 7.0 ± 0.5 | 2.2 ± 0.2 | 7.1 ± 0.9 | 1.05 ± 0.06 |
| (Epi)-afzelechin or isomer 1 | 3.8 ± 0.2 | 4.4 ± 0.2 | 3.5 ± 0.2 | 4.5 ± 0.3 | 2.31 ± 0.07 | 3.1 ± 0.2 | 0.9 ± 0.1 |
| (Epi)-afzelechin or isomer 2 | ND | ND | ND | 1.14 ± 0.05 | 0.86 ± 0.02 | ND | ND |
| Diflavanoid or isomer 1 | 17.4 ± 0.7 | 16.6 ± 0.3 | 16.9 ± 0.6 | 10.5 ± 0.7 | 4.0 ± 0.1 | 10.0 ± 0.2 | 1.95 ± 0.07 |
| Diflavanoid or isomer 2 | 11.5 ± 0.2 | 10.7 ± 0.2 | 12.1 ± 0.1 | 5.7 ± 0.2 | 2.06 ± 0.08 | 9.2 ± 0.2 | 1.19 ± 0.04 |
| Diflavanoid or isomer 3 | 2.7 ± 0.1 | 2.74 ± 0.09 | 1.61 ± 0.08 | 2.1 ± 0.1 | 1.7 ± 0.1 | 1.00 ± 0.04 | 0.67 ± 0.02 |
| Diflavanoid or isomer 4 | 0.75 ± 0.02 | 0.78 ± 0.03 | 0.49 ± 0.02 | 0.57 ± 0.02 | 0.35 ± 0.02 | ND | 0.14 ± 0.02 |
| Diflavanoid or isomer 5 | 2.9 ± 0.1 | 3.03 ± 0.06 | 3.6 ± 0.1 | 1.48 ± 0.03 | 0.76 ± 0.05 | 1.59 ± 0.08 | 0.22 ± 0.01 |
| Total flavanols | 51 ± 1 | 49.4 ± 0.4 | 49 ± 1 | 38 ± 2 | 17.2 ± 0.8 | 33 ± 1 | 7.05 ± 0.01 |
| Flavonols | |||||||
| Astilbin | 0.79 ± 0.03 | 0.718 ± 0.005 | 0.66 ± 0.03 | 0.54 ± 0.05 | 0.10 ± 0.01 | 0.54 ± 0.06 | 0.040 ± 0.004 |
| Quercentin-3-glucoside | 2.24 ± 0.1 | 2.11 ± 0.02 | 0.81 ± 0.05 | 1.71 ± 0.02 | 0.91 ± 0.02 | 1.8 ± 0.2 | 0.36 ± 0.03 |
| Quercetin-rhamnoside | 2.29 ± 0.04 | 2.24 ± 0.03 | 1.74 ± 0.02 | 1.59 ± 0.08 | 0.63 ± 0.04 | 2.0 ± 0.2 | 0.27 ± 0.01 |
| Kaempferol-rhamnoside or isomer 1 | ND | ND | 3.6 ± 0.2 | 1.35 ± 0.01 | 0.89 ± 0.02 | 1.75 ± 0.06 | 0.32 ± 0.01 |
| Kaempferol-rhamnoside or isomer 2 | 1.58 ± 0.03 | 1.42 ± 0.02 | 4.3 ± 0.2 | 1.52 ± 0.04 | 1.06 ± 0.03 | 2.46 ± 0.03 | 0.37 ± 0.01 |
| Total flavonols | 6.9 ± 0.1 | 6.488 ± 0.005 | 11.1 ± 0.5 | 6.72 ± 0.06 | 3.59 ± 0.09 | 8.6 ± 0.5 | 1.36 ± 0.06 |
| Flavanones | |||||||
| Pinocembrin 7-neohesperidoside | 16.2 ± 0.2 | 16.0 ± 0.4 | 29.7 ± 0.3 | 18.0 ± 0.4 | 13.0 ± 0.3 | 21 ± 2 | 3.40 ± 0.08 |
| Pinocembrin 7-rutinoside | 22.0 ± 0.3 | 21.6 ± 0.2 | 46 ± 3 | 21.5 ± 0.8 | 16.0 ± 0.3 | 26 ± 2 | 4.9 ± 0.2 |
| Total flavanones | 38.2 ± 0.5 | 37.6 ± 0.5 | 75 ± 3 | 40 ± 1 | 29.0 ± 0.7 | 47 ± 5 | 8.3 ± 0.2 |
| Flavones | |||||||
| Hexametoxyflavone | 2.09 ± 0.06 | 2.1 ± 0.1 | 28.1 ± 0.8 | 2.26 ± 0.09 | 2.64 ± 0.04 | 3.0 ± 0.2 | 3.0 ± 0.2 |
| Total flavones | 2.09 ± 0.06 | 2.1 ± 0.1 | 28.1 ± 0.8 | 2.26 ± 0.09 | 2.64 ± 0.04 | 3.0 ± 0.2 | 3.0 ± 0.2 |
| Proanthocyanidins/Prodelphinidins | |||||||
| (Epi)gallocatechin-(epi)catechin or isomer 1 | 1.83 ± 0.08 | 1.85 ± 0.05 | 4.0 ± 0.2 | 2.2 ± 0.1 | 1.20 ± 0.04 | 3.0 ± 0.2 | 0.381 ± 0.007 |
| (Epi)gallocatechin-(epi)catechin or isomer 2 | 4.3 ± 0.2 | 4.49 ± 0.09 | 7.4 ± 0.4 | 4.8 ± 0.3 | 2.63 ± 0.09 | 5.2 ± 0.4 | 0.80 ± 0.02 |
| Procyanidin derivative | 1.63 ± 0.06 | 1.59 ± 0.04 | 2.8 ± 0.2 | ND | 2.8 ± 0.2 | 1.50 ± 0.05 | ND |
| (Epi)catechin-(epi)catechin | 3.2 ± 0.1 | 3.17 ± 0.06 | 3.173 ± 0.06 | 0.86 ± 0.02 | ND | 1.9 ± 0.1 | ND |
| Cassanidin A | 5.0 ± 0.2 | 5.1 ± 0.1 | 2.95 ± 0.09 | 1.85 ± 0.06 | 0.77 ± 0.04 | 3.32 ± 0.02 | 0.45 ± 0.01 |
| (Epi)-guibourtinidol-(epi)-catechin or isomer 1 | 47 ± 1 | 41.8 ± 0.1 | 38.7 ± 0.5 | 29.7 ± 0.8 | 14.7 ± 0.2 | 44 ± 2 | 6.3 ± 0.4 |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 1 | 67.5 ± 0.6 | 71.1 ± 0.6 | 65 ± 1 | 43 ± 3 | 22.1 ± 0.5 | 61 ± 2 | 10.8 ± 0.3 |
| (Epi)-guibourtinidol-(epi)-catechin or isomer 2 | 5.0 ± 0.2 | 4.75 ± 0.07 | 4.4 ± 0.1 | 2.1 ± 0.1 | 0.83 ± 0.04 | 3.6 ± 0.2 | 0.45 ± 0.01 |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 2 | ND | ND | ND | ND | 1.33 ± 0.03 | ND | ND |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 3 | 25.1 ± 0.2 | 18.3 ± 0.3 | 25.1 ± 0.5 | 18.0 ± 0.5 | 5.7 ± 0.3 | 25.9 ± 0.3 | 3.74 ± 0.05 |
| (Epi)-guibourtinidol-(epi)-catechin or isomer 3 | 6.8 ± 0.2 | 6.3 ± 0.2 | 5.3 ± 0.2 | 5.3 ± 0.2 | ND | 5.3 ± 0.3 | ND |
| (Epi)-guibourtinidol-(epi)-afzelechin or isomer 4 | 3.66 ± 0.03 | 3.8 ± 0.2 | ND | 2.9 ± 0.1 | 2.2 ± 0.2 | 2.04 ± 0.09 | 0.77 ± 0.02 |
| Catechin-guibourtinidol-cassiaflavan | 1.14 ± 0.03 | 1.16 ± 0.05 | 1.316 ± 0.009 | ND | 0.46 ± 0.02 | 0.51 ± 0.05 | ND |
| (Epi)gallocatechin-(epi)catechin or isomer 1 | 5.5 ± 0.1 | 5.4 ± 0.2 | 4.8 ± 0.2 | 2.19 ± 0.08 | 0.83 ± 0.03 | 3.27 ± 0.04 | 0.532 ± 0.008 |
| Total proanthocyanidins | 171 ± 3 | 169 ± 1 | 164 ± 2 | 110 ± 4 | 53.5 ± 0.6 | 160 ± 5 | 24.1 ± 0.7 |
| Total pplyphenols | 286 ± 5 | 279 ± 2 | 349 ± 2 | 217 ± 7 | 114 ± 2 | 271 ± 12 | 46.8 ± 0.6 |
| Y1 | |||||
| Variable | Sum of Squares | d.f. | Mean Square | F-Ratio | p-Value |
| X1:Temperature | 252.032 | 1 | 252.032 | 236.47 | 0.0299 |
| X2:EtOH | 4.353 | 1 | 4.353 | 4.08 | 0.2925 |
| X3:Extration time | 10.6513 | 1 | 10.6513 | 9.99 | 0.1950 |
| X1X1 | 22.2878 | 1 | 22.2878 | 20.01 | 0.1371 |
| X1X2 | 4.92866 | 1 | 4.92866 | 4.62 | 0.2771 |
| X1X3 | 6.20363 | 1 | 6.20363 | 5.82 | 0.2502 |
| X2X2 | 668.137 | 1 | 668.137 | 626.89 | 0.0254 |
| X2X3 | 0.946107 | 1 | 0.946107 | 0.89 | 0.5189 |
| X3X3 | 28.7276 | 1 | 28.7276 | 26.95 | 0.1211 |
| Lack-of-fit | 72.3088 | 6 | 24.1029 | 22.61 | 0.1938 |
| Pure error | 1.0658 | 1 | 1.0658 | ||
| Total (corr.) | 1469.87 | 13 | |||
| R2 | 0.95008 | ||||
| Y2 | |||||
| Variable | Sum of Squares | d.f. | Mean Square | F-Ratio | p-Value |
| X1:Temperature | 31,277.3 | 1 | 31,277.3 | 1177.03 | 0.0173 |
| X2:EtOH | 24,795.9 | 1 | 24,795.9 | 933.12 | 0.0196 |
| X3:Extration time | 399.097 | 1 | 399.097 | 15.02 | 0.1704 |
| X1X1 | 31,802.3 | 1 | 31,802.3 | 1196.79 | 0.0185 |
| X1X2 | 984.21 | 1 | 984.21 | 37.04 | 0.1037 |
| X1X3 | 16,413.9 | 1 | 16,413.9 | 617.69 | 0.0236 |
| X2X2 | 11,195.3 | 1 | 11,195.3 | 421.30 | 0.0324 |
| X2X3 | 47.0042 | 1 | 47.0042 | 1.77 | 0.4104 |
| X3X3 | 2415.8 | 1 | 2415.8 | 90.91 | 0.0533 |
| Lack-of-fit | 3833.34 | 6 | 1277.78 | 48.09 | 0.1231 |
| Pure error | 26.5731 | 1 | 26.5731 | ||
| Total (corr.) | 109,034 | 13 | |||
| R2 | 0.96569 | ||||
| Factors | Temperature X1 (°C) | EtOH X2 (%) | Time X3 (min) | Theoretical Optimum |
|---|---|---|---|---|
| Variable Response | ||||
| Yield | 200 | 49.8 | 22 | 34.4% |
| TPC | 46.3 | 73.8 | 22 | 363 mg/g |
| Multiple response | 146.5 | 54.8 | 3 | Yield = 25.7% TPC = 281 mg/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, J.A.M.; López-Salas, L.; Borrás-Linares, I.; Navarro-Alarcón, M.; Segura-Carretero, A.; Lozano-Sánchez, J. Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods 2021, 10, 398. https://doi.org/10.3390/foods10020398
Fuentes JAM, López-Salas L, Borrás-Linares I, Navarro-Alarcón M, Segura-Carretero A, Lozano-Sánchez J. Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods. 2021; 10(2):398. https://doi.org/10.3390/foods10020398
Chicago/Turabian StyleFuentes, Jhunior Abrahan Marcía, Lucía López-Salas, Isabel Borrás-Linares, Miguel Navarro-Alarcón, Antonio Segura-Carretero, and Jesús Lozano-Sánchez. 2021. "Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds" Foods 10, no. 2: 398. https://doi.org/10.3390/foods10020398
APA StyleFuentes, J. A. M., López-Salas, L., Borrás-Linares, I., Navarro-Alarcón, M., Segura-Carretero, A., & Lozano-Sánchez, J. (2021). Development of an Innovative Pressurized Liquid Extraction Procedure by Response Surface Methodology to Recover Bioactive Compounds from Carao Tree Seeds. Foods, 10(2), 398. https://doi.org/10.3390/foods10020398








