Accumulation Pattern of Amygdalin and Prunasin and Its Correlation with Fruit and Kernel Agronomic Characteristics during Apricot (Prunus armeniaca L.) Kernel Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Collection
2.2. Determination of Pomological Characteristics of Apricot Fruit and Kernels
2.3. Extraction and Determination of Apricot Kernel Oil
2.4. Identification and Quantification of Amygdalin and Prunasin in Apricot Kernels
2.5. Evaluation of β-Glucosidase Activity in Apricot Kernels
2.6. Statistical Analysis
3. Results
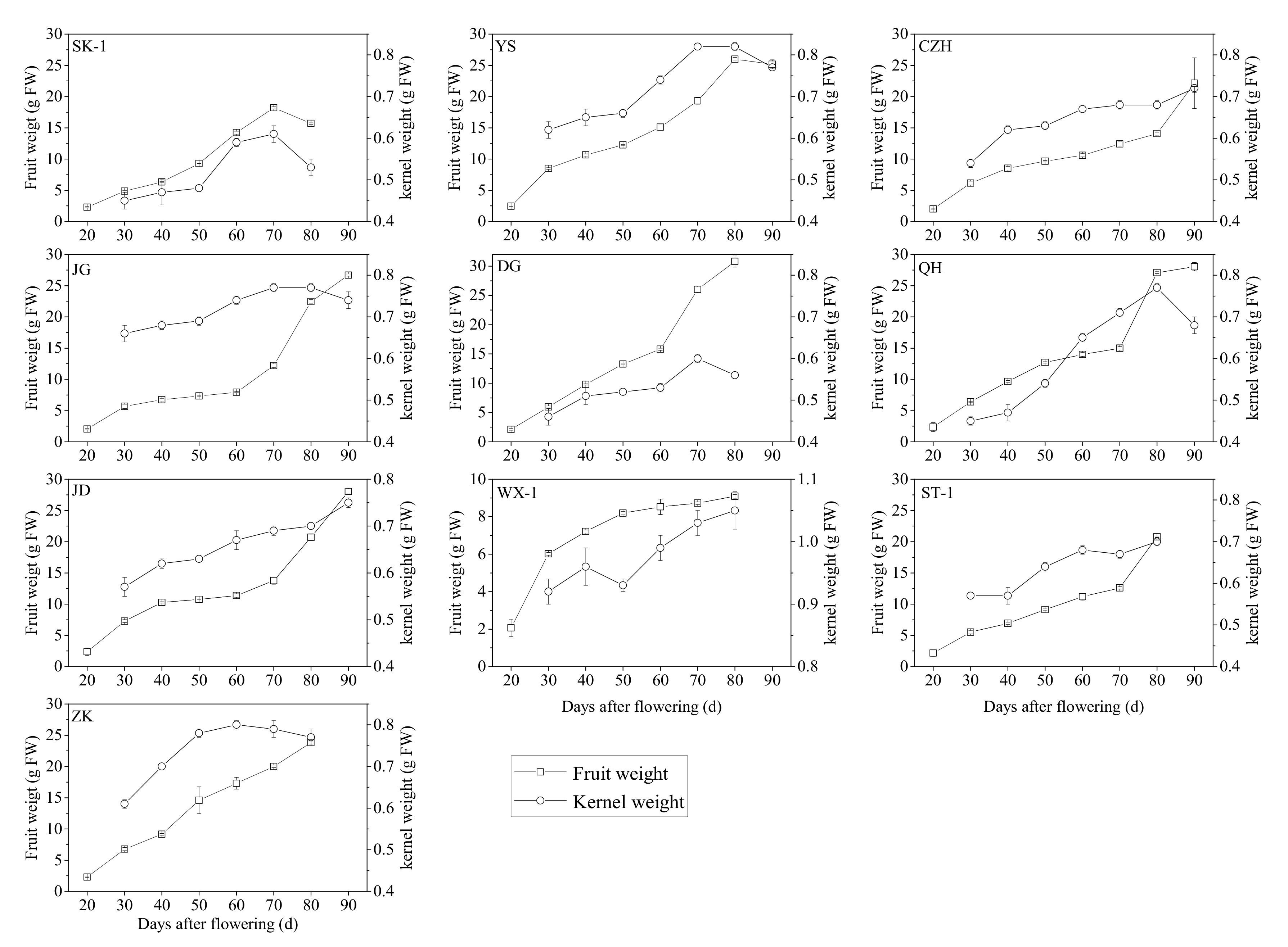
3.1. Changes in the Agronomic Characteristics of Apricot Fruit and Kernels at Different Developmental Stages
3.2. Changes in the Total Lipid Content and Moisture Content during Apricot Kernel Maturation
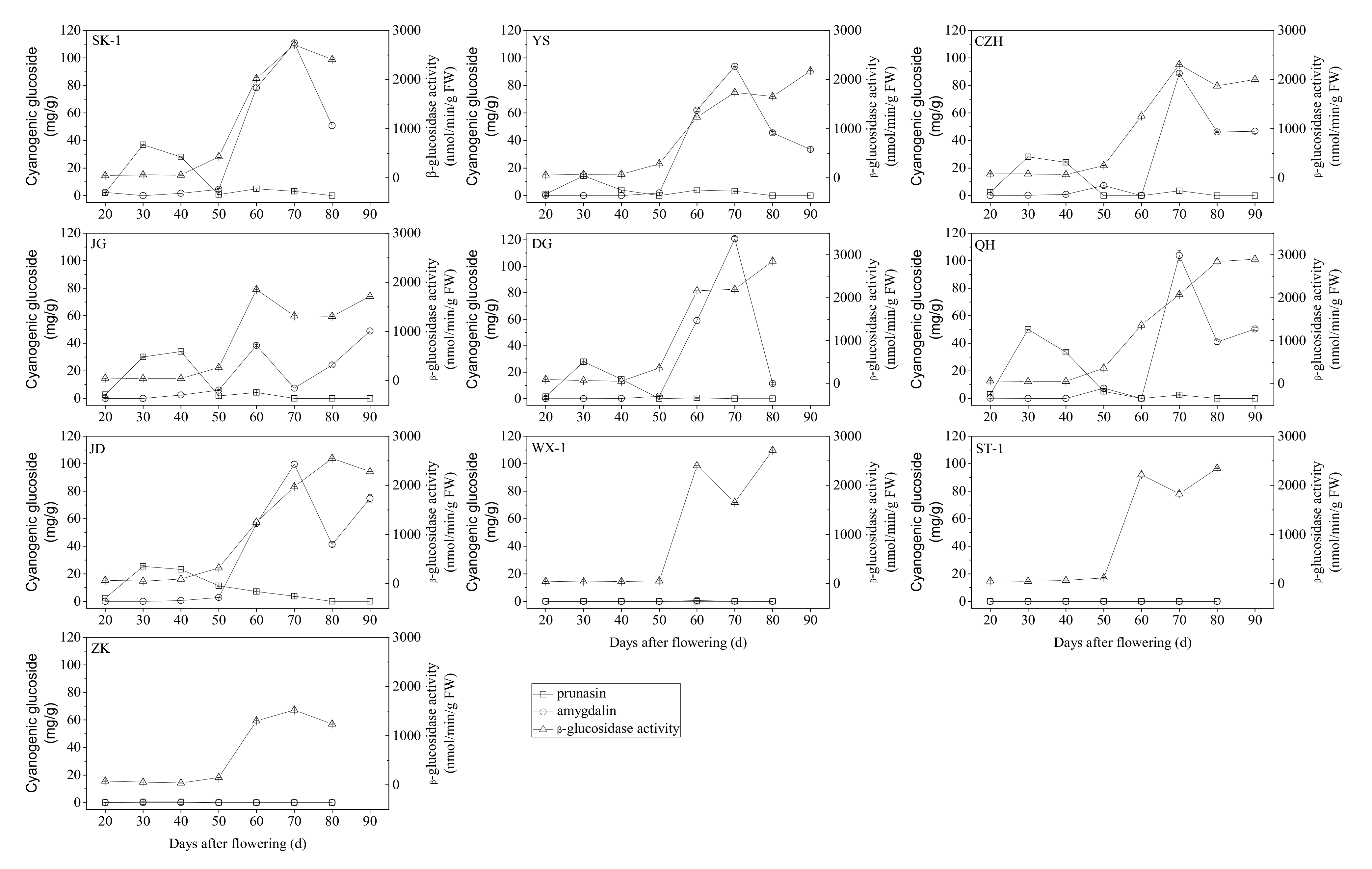
3.3. Accumulation of Cyanogenic Glycosides during Apricot Kernel Development
3.4. β-Glucosidase Activity during Apricot Kernel Development
3.5. Correlation Analysis
3.6. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Gu, H.D.; Zhang, L.; Tian, Z.J.; Zhang, Z.Q.; Shi, X.C.; Ma, W.H. Protective effects of apricot kernel oil on myocardium against ischemia-reperfusion injury in rats. Food Chem. Toxicol. 2011, 49, 3136–3141. [Google Scholar] [CrossRef]
- Alpaslan, M.; Hayta, M. Apricot kernel: Physical and chemical properties. J. Am. Oil Chem. Soc. 2006, 83, 469–471. [Google Scholar] [CrossRef]
- Özcan, M.M.; Özalp, C.; Ünver, A.; Arslan, D.; Dursun, N. Properties of Apricot Kernel and Oils as Fruit Juice Processing Waste. Food Nutr. Sci. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Yildirim, F.A.; Yildirim, A.N.; Askin, M.A.; Kankaya, A. Total oil, fatty acid composition and tocopherol content in kernels of several bitter and sweet apricot (Prunus armeniaca Batsch) cultivars from Turkey. J. Food Agric. Environ. 2010, 8, 196–201. [Google Scholar]
- Korekar, G.; Stobdan, T.; Arora, R.; Yadav, A.; Singh, S.B. Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Foods Hum. Nutr. 2011, 66, 376–383. [Google Scholar] [CrossRef]
- Yigit, D.; Yigit, N.; Mavi, A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, H.; Zhan, P.; Tian, F. Composition and antioxidant and antimicrobial activities of white apricot almond (Amygdalus communis L.) oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1138–1144. [Google Scholar] [CrossRef]
- Gómez, E.; Burgos, L.; Soriano, C.; Marín, J. Amygdalin content in the seeds of several apricot cultivars. J. Sci. Food Agric. 1998, 77, 184–186. [Google Scholar] [CrossRef]
- Górnaś, P.; Radziejewska-Kubzdela, E.; Mišina, I.; Biegańska-Marecik, R.; Grygier, A.; Rudzińska, M. Tocopherols, Tocotrienols and Carotenoids in Kernel Oils Recovered from 15 Apricot (Prunus armeniaca L.) Genotypes. J. Am. Oil Chem. Soc. 2017, 94, 693–699. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Profiling triacylglycerols, fatty acids and tocopherols in hazelnut varieties grown in Turkey. J. Food Compos. Anal. 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Girgis, A.Y.; El-Aziz, N.M.A.; El-Salam, S.M.A. Physical and chemical characteristics of toilet soap made from apricot kernel oil and palm stearin. Grasas Y Aceites 1998, 49, 185–192. [Google Scholar] [CrossRef]
- Targais, K.; Stobdan, T.; Yadav, A.; Singh, S.B. Extraction of apricot kernel oil in cold desert Ladakh, India. Indian J. Tradit. Knowl. 2011, 10, 304–306. [Google Scholar]
- Wang, L.; Yu, H. Biodiesel from Siberian apricot (Prunus sibirica L.) seed kernel oil. Bioresour. Technol. 2012, 112, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, H.; Fan, S.; Liang, T.; Yu, H.; Li, J.; He, J.; Li, G.; Wang, L. Genetic variability of biodiesel properties in some Prunus L. (Rosaceae) species collected from Inner Mongolia, China. Ind. Crops Prod. 2015, 76, 244–248. [Google Scholar] [CrossRef]
- Górnaś, P.; Ramos, M.J.; Montano, M.C.; Rudzińska, M.; Grygier, A. Fruit Pits Recovered from 14 Genotypes of Apricot (Prunus armeniaca L.) as Potential Biodiesel Feedstock. Eur. J. Lipid Sci. Technol. 2017, 120, 1700147. [Google Scholar] [CrossRef]
- Morant, A.V.; Jørgensen, K.; Jørgensen, C.; Paquette, S.M.; Sánchezpérez, R.; Møller, B.L.; Bak, S. beta-Glucosidases as detonators of plant chemical defense. Phytochemistry 2008, 69, 1795–1813. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.H.; Dart, R.; Bogdan, G. Sodium Thiosulfate or Hydroxocobalamin for the Empiric Treatment of Cyanide Poisoning? Ann. Emerg. Med. 2007, 49, 806–813. [Google Scholar] [CrossRef]
- Beasley, D.M.G.; Glass, W.I. Cyanide poisoning: Pathophysiology and treatment recommendations. Occup. Med. 1998, 48, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Hamel, J. A Review of Acute Cyanide Poisoning With a Treatment Update. Crit. Care Nurse 2011, 31, 72–81. [Google Scholar] [CrossRef]
- Nelson, L. Acute cyanide toxicity: Mechanisms and manifestations. J. Emerg. Nurs. 2006, 32, S8–S11. [Google Scholar] [CrossRef]
- Akyildiz, B.N.; Kurtoğlu, S.; Kondolot, M.; Tunç, A. Cyanide poisoning caused by ingestion of apricot seeds. Ann. Trop. Paediatr. 2010, 30, 39–43. [Google Scholar] [CrossRef]
- AkiL, M.; Kaya, A.; Üstyol, L.; Aktar, F.; Akbayram, S. Acute Cyanide Intoxication Due To Apricot Seed Ingestion. J. Emerg. Med. 2013, 44, e285–e286. [Google Scholar] [CrossRef]
- Suchard, J.R.; Wallace, K.L.; Gerkin, R.D. Acute cyanide toxicity caused by apricot kernel ingestion. Ann. Emerg. Med. 1998, 32, 742–744. [Google Scholar] [CrossRef]
- Tatli, M.; Eyüpoğlu, G.; Hocagil, H. Acute cyanide poisoning due to apricot kernel ingestion. J. Acute Dis. 2017, 006, 87–88. [Google Scholar] [CrossRef]
- Vlad, I.A.; Armstrong, J.; Bertilone, C.; Matisons, M. Apricot kernels: A rare case of cyanide toxicity. Emerg. Med. Australas. Ema 2015, 27, 491–492. [Google Scholar] [CrossRef]
- Kupper, J.; Schumann, M.; Wennig, R.; Gorber, U.; Mittelholzer, A.; Artho, R.; Meyer, S.; Kupferschmidt, H.; Naegeli, H. Cyanide poisoning associated with the feeding of apricot kernels to dairy cattle. Vet. Rec. 2008, 162, 488–489. [Google Scholar] [CrossRef]
- Cigolini, D.; Ricci, G.; Zannoni, M.; Codogni, R.; De Luca, M.; Perfetti, P.; Rocca, G. Hydroxocobalamin treatment of acute cyanide poisoning from apricot kernels. Emerg. Med. J. 2011, 28, 804–805. [Google Scholar] [CrossRef][Green Version]
- Kovacikova, E.; Kovacik, A.; Halenar, M.; Tokarova, K.; Chrastinova, L.; Ondruska, L.; Jurcik, R.; Kolesar, E.; Valuch, J.; Kolesarova, A. Potential toxicity of cyanogenic glycoside amygdalin and bitter apricot seed in rabbits-Health status evaluation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Isom, G.E.; Way, J.L. Lethality of cyanide in the absence of inhibition of liver cytochrome oxidase. Biochem. Pharmacol. 1976, 25, 605–608. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic Glycosides: Synthesis, Physiology, and Phenotypic Plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Bassi, D.; Selli, R. Evaluation of fruit quality in peach and apricot. Adv. Hortic. Sci. 1990, 2, 107–111. [Google Scholar]
- Gurrieri, F.; Audergon, J.M.; Albagnac, G.; Reich, M. Soluble sugars and carboxylic acids in ripe apricot fruit as parameters for distinguishing different cultivars. Euphytica 2001, 117, 183–189. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical Characters and Antioxidant, Sugar, and Mineral Nutrient Contents in Fruit from 29 Apricot (Prunus armeniaca L.) Cultivars and Hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef]
- Bianco, R.L.; Farina, V.; Indelicato, S.G.; Filizzola, F.; Agozzino, P. Fruit physical, chemical and aromatic attributes of early, intermediate and late apricot cultivars. J. Sci. Food Agric. 2010, 90, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Mratinić, E.; Popovski, B.; Milošević, T. Evaluation of apricot fruit quality and correlations between physical and chemical attributes. Czech J. Food Sci. 2011, 29, 161–170. [Google Scholar] [CrossRef]
- Baldicchi, A.; Farinelli, D.; Micheli, M.; Di Vaio, C.; Moscatello, S.; Battistelli, A.; Walker, R.P.; Famiani, F. Analysis of seed growth, fruit growth and composition and phospoenolpyruvate carboxykinase (PEPCK) occurrence in apricot (Prunus armeniaca L.). Sci. Hortic. 2015, 186, 38–46. [Google Scholar] [CrossRef]
- Famiani, F.; Baldicchi, A.; Casulli, V.; Vaio, C.D.; Cruz-Castillo, J.G.; Walker, R.P. The occurrence of phosphoenolpyruvate carboxykinase (PEPCK) and enzymes related to photosynthesis and organic acid/nitrogen metabolism in apricot flowers (Prunus armeniaca L.). Acta Physiol. Plant. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Kostina, K.F. Apricot breeding in the southern zone of the USSR [Ukrainian SSR, Moldavian SSR]. Sadovodstvo 1977, 7, 24–25. (In Russian) [Google Scholar]
- Bassi, D.; Negri, P. Ripening date and fruit traits in apricot progenies. Acta Hortic. 1991, 293, 133–140. [Google Scholar] [CrossRef]
- Dement, W.A.; Mooney, H.A. Seasonal variation in the production of tannins and cyanogenic glucosides in the chaparral shrub, Heteromeles arbutifolia. Oecologia 1974, 15, 65–76. [Google Scholar] [CrossRef]
- Feigl, F.; Anger, V. Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst 1966, 91, 282–284. [Google Scholar] [CrossRef]
- Frehner, M.; Scalet, M.; Conn, E.E. Pattern of the Cyanide-Potential in Developing Fruits: Implications for Plants Accumulating Cyanogenic Monoglucosides (Phaseolus lunatus) or Cyanogenic Diglucosides in Their Seeds (Linum usitatissimum, Prunus amygdalus). Plant Physiol. 1990, 94, 28–34. [Google Scholar] [CrossRef]
- Majak, W.; McDiarmid, R.E.; Hall, J.W. The cyanide potential of saskatoon serviceberry (Amelanchier alnifolia) and chokecherry (Prunus virginiana). Can. J. Anim. Sci. 1981, 61, 681–686. [Google Scholar] [CrossRef]
- Mohsenin, N.N. Physical Properties of Plants and Animals Material; Cordon and Breach Science Publication Inc.: New York, NY, USA, 1980. [Google Scholar]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Baryeh, E.A. Physical properties of bambara groundnuts. J. Food Eng. 2001, 47, 321–326. [Google Scholar] [CrossRef]
- Etienne, C.; Moing, A.; Dirlewanger, E.; Raymond, P.; Rothan, C. Isolation and characterization of six peach cDNAs encoding key proteins in organic acid metabolism and solute accumulation: Involvement in regulating peach fruit acidity. Physiol. Plant. 2010, 114, 259–270. [Google Scholar] [CrossRef]
- Mirzaee, E.; Rafiee, S.; Keyhani, A.; Djom-Eh, Z.E. Physical properties of apricot to characterize best post harvesting options. Aust. J. Crop Sci. 2009, 3, 139–143. [Google Scholar]
- Milošević, T.; Milošević, N.; Glišić, I.; Krška, B. Characteristics of promising apricot (Prunus armeniaca L,) genetic resources in Central Serbia based on blossoming period and fruit quality. Hortic. Sci. 2010, 37, 46–55. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Glišić, I. Influence of Stock on the Early Tree Growth, Yield and Fruit Quality Traits of Apricot (Prunus armeniaca L.). J. Agric. Sci. Tarim Bilimleri Derg. 2011, 17, 167–176. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Glišić, I.; Mladenović, J. Fruit quality, phenolics content and antioxidant capacity of new apricot cultivars from Serbia. Acta Sci. Pol. Hortorum Cultus Ogrod. 2012, 11, 3–15. [Google Scholar]
- Badenes, M.L.; Martínez-Calvo, J.; Llácer, G. Analysis of apricot germplasm from the European ecogeographical group. Euphytica 1998, 102, 93–99. [Google Scholar] [CrossRef]
- El-Rzek, E.A.; El-Migeed, M.M.M.A.; Abdel-Hamid, N. Effect of spraying garlic extract and olive oil on flowering behavior, yield and fruit quality of ‘Canino’ apricot trees. Am. Eur. J. Agric. Environ. Sci. 2011, 11, 776–781. [Google Scholar]
- Maria, D.; Cristina, P.; Adela, B.; Roman, M.; Andreea, P.; Alina, I. Extension of ripening season of apricot using two new selections to research station Banesa. Sci. Pap. Res. Inst. Fruit Grow. 2011, 27, 325–329. [Google Scholar]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Mratini, E.; Popovski, B.; Miloevi, T.; Popovska, M. Analysis of Morphological and Pomological Characteristics of Apricot Germplasm in FYR Macedonia. J. Agric. Sci. Technol. 2011, 13, 1121–1134. [Google Scholar]
- Kumar, D.; Lal, S.; Ahmed, N. Morphological and pomological diversity among apricot (Prunus armeniaca) genotypes grown in India. Indian J. Agric. Sci. 2015, 85, 1349–1355. [Google Scholar]
- Khadivi-Khub, A.; Khalili, Z. A breeding project: The selection of promising apricot (Prunus armeniaca L.) genotypes with late blooming time and high fruit quality. Sci. Hortic. 2017, 216, 93–102. [Google Scholar] [CrossRef]
- Bouali, I.; Trabelsi, H.; Abdallah, I.B.; Albouchi, A.; Martine, L.; Grégoire, S.; Bouzaien, G.; Gandour, M.; Boukhchina, S.; Berdeaux, O. Changes in Fatty Acid, Tocopherol and Xanthophyll Contents During the Development of Tunisian-Grown Pecan Nuts. J. Am. Oil Chem. Soc. 2013, 90, 1869–1876. [Google Scholar] [CrossRef]
- Turan, S.; Topcu, A.; Karabulut, I.; Vural, H.; Hayaloglu, A.A. Fatty acid, triacylglycerol, phytosterol, and tocopherol variations in kernel oil of Malatya apricots from Turkey. J. Agric. Food Chem. 2007, 55, 10787–10794. [Google Scholar] [CrossRef]
- Mandal, S.; Suneja, P.; Malik, S.K.; Mishra, S.K. Variability in kernel oil, its fatty acid and protein contents of different apricot (Prunus armeniaca) genotypes. Indian J. Agric. Sci. 2007, 77, 464–466. [Google Scholar]
- Manzoor, M.; Anwar, F.; Ashraf, M.; Alkharfy, K.M. Physico-chemical characteristics of seed oils extracted from different apricot (Prunus armeniaca L.) varieties from Pakistan. Grasas Y Actes 2012, 63, 193–201. [Google Scholar]
- Conn, E.E. Cyanogenic Compounds. Annu. Rev. Plant Physiol. 1980, 31, 433–451. [Google Scholar] [CrossRef]
- Dicenta, F.; Martínez-Gómez, P.; Grané, N.; Martín, M.L.; León, A.; Cánovas, J.A.; Berenguer, V. Relationship between Cyanogenic Compounds in Kernels, Leaves, and Roots of Sweet and Bitter Kernelled Almonds. J. Agric. Food Chem. 2002, 50, 2149–2152. [Google Scholar] [CrossRef]
- Arrazola, G. Evolution of the amygdalin and prunasin content during the development of almond (Prunus dulcis Miller). Rev. Fac. Agron. 2015, 32, 63–81. [Google Scholar]
- Sánchez-Pérez, R.; Jørgensen, K.; Olsen, C.E.; Dicenta, F.; Møller, B.L. Bitterness in Almonds. Plant Physiol. 2008, 146, 1040–1052. [Google Scholar] [CrossRef]
- Moradi, B.; Heidari-Soureshjani, S.; Asadi-Samani, M.; Yang, Q. A Systematic Review of Phytochemical and Phytotherapeutic Characteristics of Bitter Almond. Int. J. Pharm. Phytopharm. Res. 2017, 7, 1–9. [Google Scholar]
- Ohtsubo, T.; Ikeda, F. Seasonal Changes of Cyanogenic Glycosides in Mume (Prunus mume Sieb. et Zucc.) Seeds. Engei Gakkai Zasshi 2008, 62, 695–700. [Google Scholar] [CrossRef]
- Del Cueto, J.; Ionescu, I.A.; Pičmanová, M.; Gericke, O.; Motawia, M.S.; Olsen, C.E.; Campoy, J.A.; Dicenta, F.; Møller, B.L.; Sánchez-Pérez, R. Cyanogenic Glucosides and Derivatives in Almond and Sweet Cherry Flower Buds from Dormancy to Flowering. Front. Plant Sci. 2017, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Gebrehiwot, L.; Beuselinck, P.R. Seasonal Variations in Hydrogen Cyanide Concentration of Three Species. Agron. J. 2001, 93, 603–608. [Google Scholar] [CrossRef]
- Femenia, A.; Rossello, C.; Mulet, A.; Canellas, J. Chemical Composition of Bitter and Sweet Apricot Kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Stoewsand, G.S.; Anderson, J.L.; Lamb, R.C. Cyanide content of apricot kernels. J. Food Sci. 1975, 40, 1107. [Google Scholar] [CrossRef]
- Briggs, D.R.; Yuen, D. The determination of cyanide in apricot kernels. Proc. Nutr. Soc. Aust. 1978, 3, 103–104. [Google Scholar]
- Mandenius, C.F.; Bülow, L.; Danielsson, B. Determination of amygdalin and cyanide in industrial food samples using enzymic methods. Acta Chem. Scand. 1983, 37, 739–742. [Google Scholar] [CrossRef][Green Version]
- Stosic, D.; Gorunovic, M.; Popovic, B. Preliminary toxicological study of the kernel and the oil of some Prunus species. Plantes Med. Phytother. 1987, 21, 8–13. (In French) [Google Scholar]
- Negri, P.; Bassi, D.; Magnanini, E.; Rizzo, M.; Bartolozzi, F. Bitterness inheritance in apricot (P. armeniaca L.) seeds. Tree Genet. Genomes 2008, 4, 767–776. [Google Scholar] [CrossRef]
- Sayre, J.W.; Kaymakcalan, S. Cyanide Poisoning from Apricot Seeds among Children in Central Turkey. N. Engl. J. Med. 1964, 270, 1113–1115. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Vecchies, A.C.; Woodrow, I.E. Cyanogenic Eucalyptus nobilis is polymorphic for both prunasin and specific beta-glucosidases. Phytochemistry 2003, 63, 699–704. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Heil, M.; Lieberei, R. Phenotypic Plasticity of Cyanogenesis in Lima Bean Phaseolus lunatus—Activity and Activation of β-Glucosidase. J. Chem. Ecol. 2006, 32, 261–275. [Google Scholar] [CrossRef][Green Version]
- Hughes, M.A.; Sharif, A.L.; Dunn, M.A.; Oxtoby, E.; Pancoro, A. Restriction fragment length polymorphism segregation analysis of the Li locus in Trifolium repens L. Plant Mol. Biol. 1990, 14, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, R.; Belmonte, F.S.; Borch, J.; Dicenta, F.; Moller, B.L.; Jorgensen, K. Prunasin Hydrolases during Fruit Development in Sweet and Bitter Almonds. Plant Physiol. 2012, 158, 1916–1932. [Google Scholar] [CrossRef]
- Dicenta, F.; García, J.E. Inheritance of the kernel flavour in almond. Heredity 1993, 70, 308–312. [Google Scholar] [CrossRef]
- Heppner, M.J. Further Evidence on the Factor for Bitterness in the Sweet Almond. Genetics 1923, 8, 390–391. [Google Scholar] [CrossRef] [PubMed]


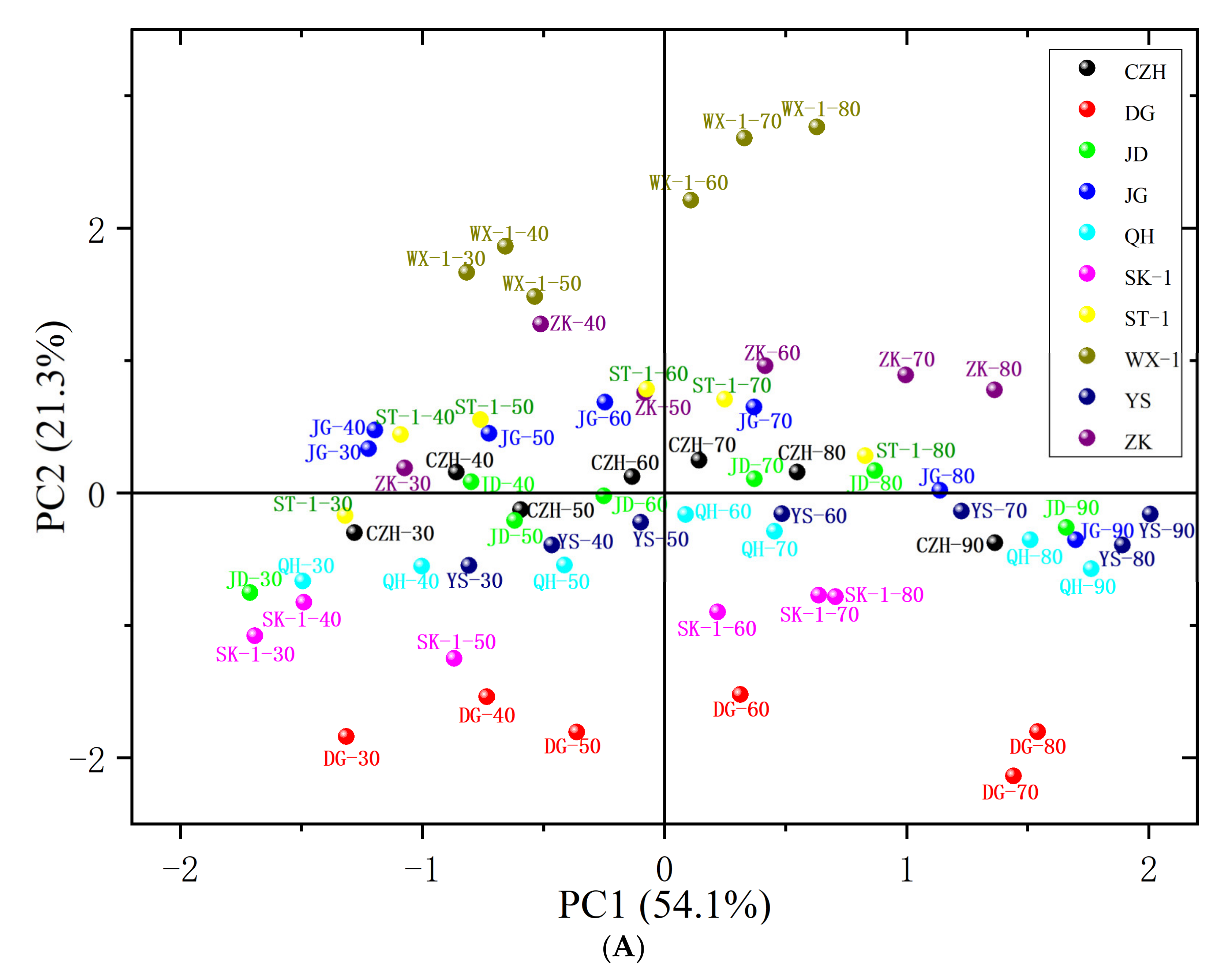
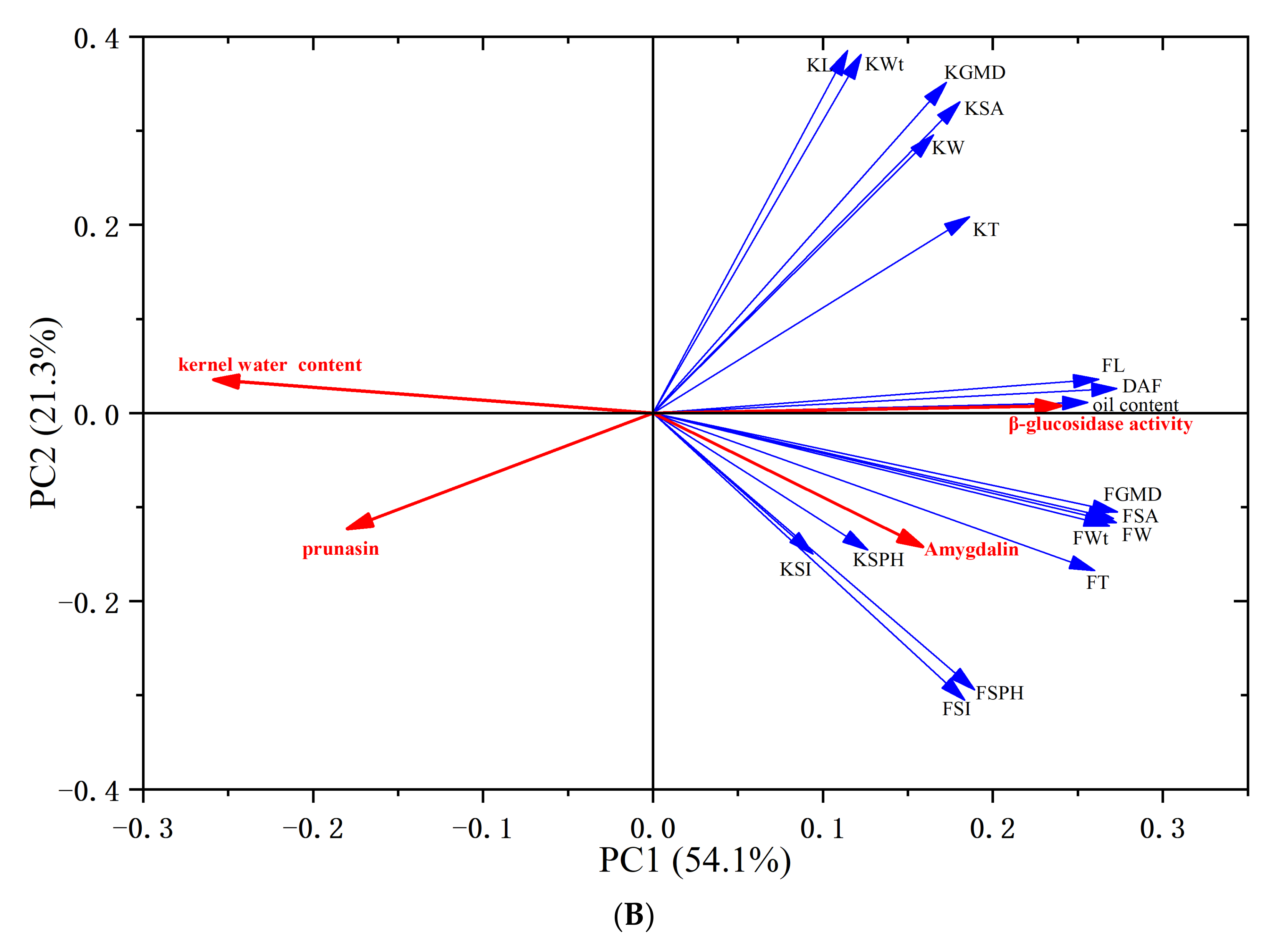
| PC | Eigenvalue | Percentage of Variance (%) | Cumulative Contribution Rate (%) |
|---|---|---|---|
| 1 | 11.90 | 54.07 | 54.07 |
| 2 | 4.69 | 21.32 | 75.39 |
| 3 | 2.02 | 9.17 | 84.56 |
| 4 | 0.93 | 4.24 | 88.80 |
| 5 | 0.62 | 2.81 | 91.62 |
| 6 | 0.58 | 2.62 | 94.24 |
| 7 | 0.39 | 1.77 | 96.01 |
| 8 | 0.32 | 1.45 | 97.46 |
| 9 | 0.14 | 0.62 | 98.09 |
| 10 | 0.11 | 0.51 | 98.59 |
| Variable/Factor | PC1 | PC2 | PC3 |
|---|---|---|---|
| days after flowering (DAF) | 0.27301 | 0.02615 | −0.12214 |
| fresh fruit weight (FWt) | 0.26851 | −0.1198 | −0.066 |
| fruit length (FL) | 0.26229 | 0.03593 | −0.14301 |
| fruit width (FW) | 0.27265 | −0.1164 | −0.01781 |
| fruit thickness (FT) | 0.26007 | −0.16754 | −0.05229 |
| fruit geometric mean diameter (FGMD) | 0.27327 | −0.10509 | −0.07017 |
| fruit surface area (FSA) | 0.27087 | −0.11215 | −0.06472 |
| fruit sphericity (FSPH) | 0.18928 | −0.29423 | 0.05414 |
| fruit shape index (FSI) | 0.18351 | −0.30525 | 0.07131 |
| fresh kernel weight (KWt) | 0.12257 | 0.38121 | 0.11151 |
| kernel length (KL) | 0.11462 | 0.38554 | −0.18601 |
| kernel width (KW) | 0.16517 | 0.29583 | 0.1716 |
| kernel thickness (KT) | 0.18619 | 0.20886 | 0.2568 |
| kernel geometric mean diameter (KGMD) | 0.17266 | 0.35145 | 0.12211 |
| kernel surface area (KSA) | 0.18064 | 0.33091 | 0.14784 |
| kernel sphericity (KSPH) | 0.12641 | −0.14565 | 0.56668 |
| kernel shape index (KSI) | 0.09433 | −0.14983 | 0.60095 |
| kernel water content | −0.25867 | 0.03558 | 0.15471 |
| oil content | 0.25584 | 0.01133 | −0.15608 |
| β-glucosidase activity | 0.24009 | 0.0078 | −0.14923 |
| prunasin | −0.17993 | −0.12324 | −0.02838 |
| Amygdalin | 0.15883 | −0.1423 | −0.08524 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, P.; Cui, B.; Zhu, H.; Phommakoun, B.; Zhang, D.; Li, Y.; Zhao, F.; Zhao, Z. Accumulation Pattern of Amygdalin and Prunasin and Its Correlation with Fruit and Kernel Agronomic Characteristics during Apricot (Prunus armeniaca L.) Kernel Development. Foods 2021, 10, 397. https://doi.org/10.3390/foods10020397
Deng P, Cui B, Zhu H, Phommakoun B, Zhang D, Li Y, Zhao F, Zhao Z. Accumulation Pattern of Amygdalin and Prunasin and Its Correlation with Fruit and Kernel Agronomic Characteristics during Apricot (Prunus armeniaca L.) Kernel Development. Foods. 2021; 10(2):397. https://doi.org/10.3390/foods10020397
Chicago/Turabian StyleDeng, Ping, Bei Cui, Hailan Zhu, Buangurn Phommakoun, Dan Zhang, Yiming Li, Fei Zhao, and Zhong Zhao. 2021. "Accumulation Pattern of Amygdalin and Prunasin and Its Correlation with Fruit and Kernel Agronomic Characteristics during Apricot (Prunus armeniaca L.) Kernel Development" Foods 10, no. 2: 397. https://doi.org/10.3390/foods10020397
APA StyleDeng, P., Cui, B., Zhu, H., Phommakoun, B., Zhang, D., Li, Y., Zhao, F., & Zhao, Z. (2021). Accumulation Pattern of Amygdalin and Prunasin and Its Correlation with Fruit and Kernel Agronomic Characteristics during Apricot (Prunus armeniaca L.) Kernel Development. Foods, 10(2), 397. https://doi.org/10.3390/foods10020397






