Biological Effect of Different Spinach Extracts in Comparison with the Individual Components of the Phytocomplex
Abstract
1. Introduction
2. Materials and Methods
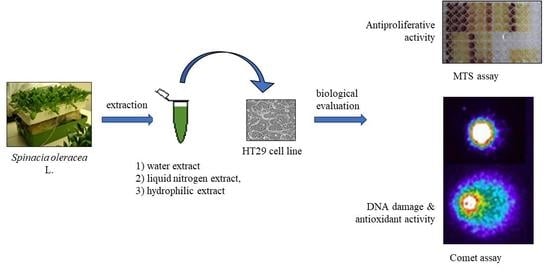
2.1. Spinacia oleracea Extracts
2.2. HT29 Cell Line
2.3. Modulation of the Proliferation
2.3.1. MTS Assay
2.3.2. Trypan Blue Exclusion Method
2.3.3. Comet Assay
2.3.4. Comet Assay—Antioxidant Activity
2.4. Measurement of Reactive Oxygen Species (ROS) Production
2.5. Statistical Analysis
3. Results
3.1. Modulation of the Proliferation
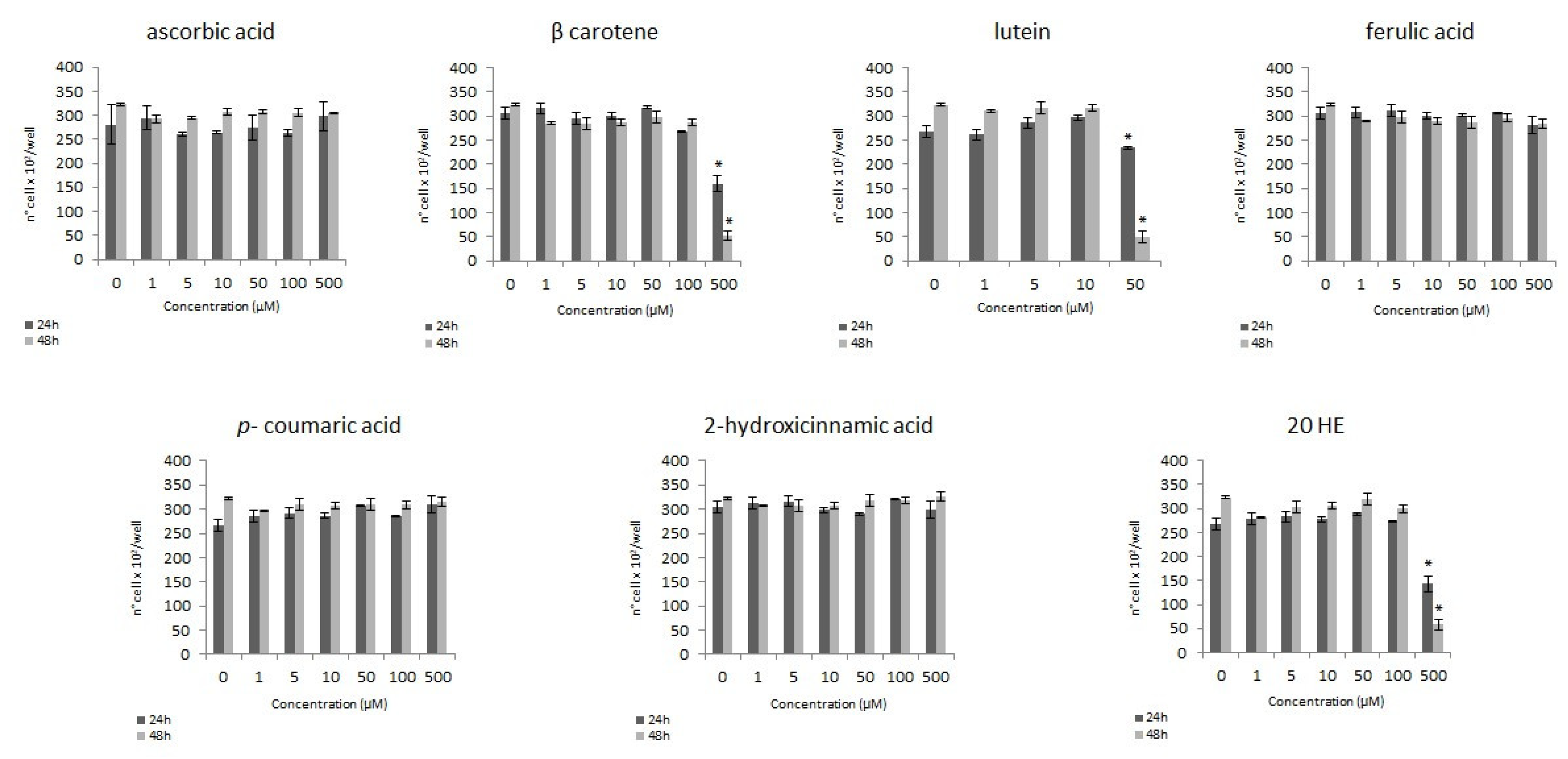
3.1.1. MTS Assay
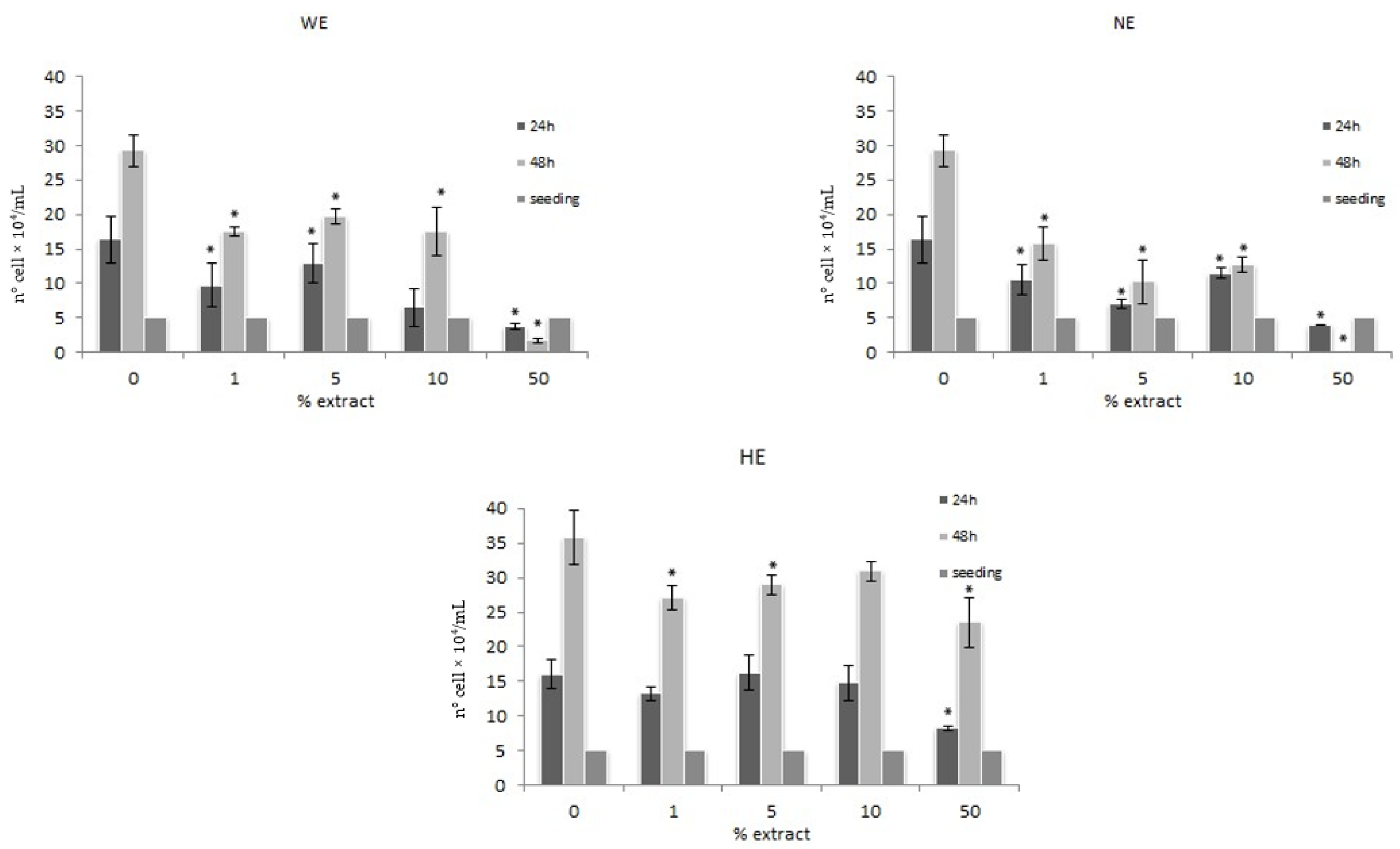
3.1.2. Trypan Blue Exclusion Method
3.2. Genotoxic Activity
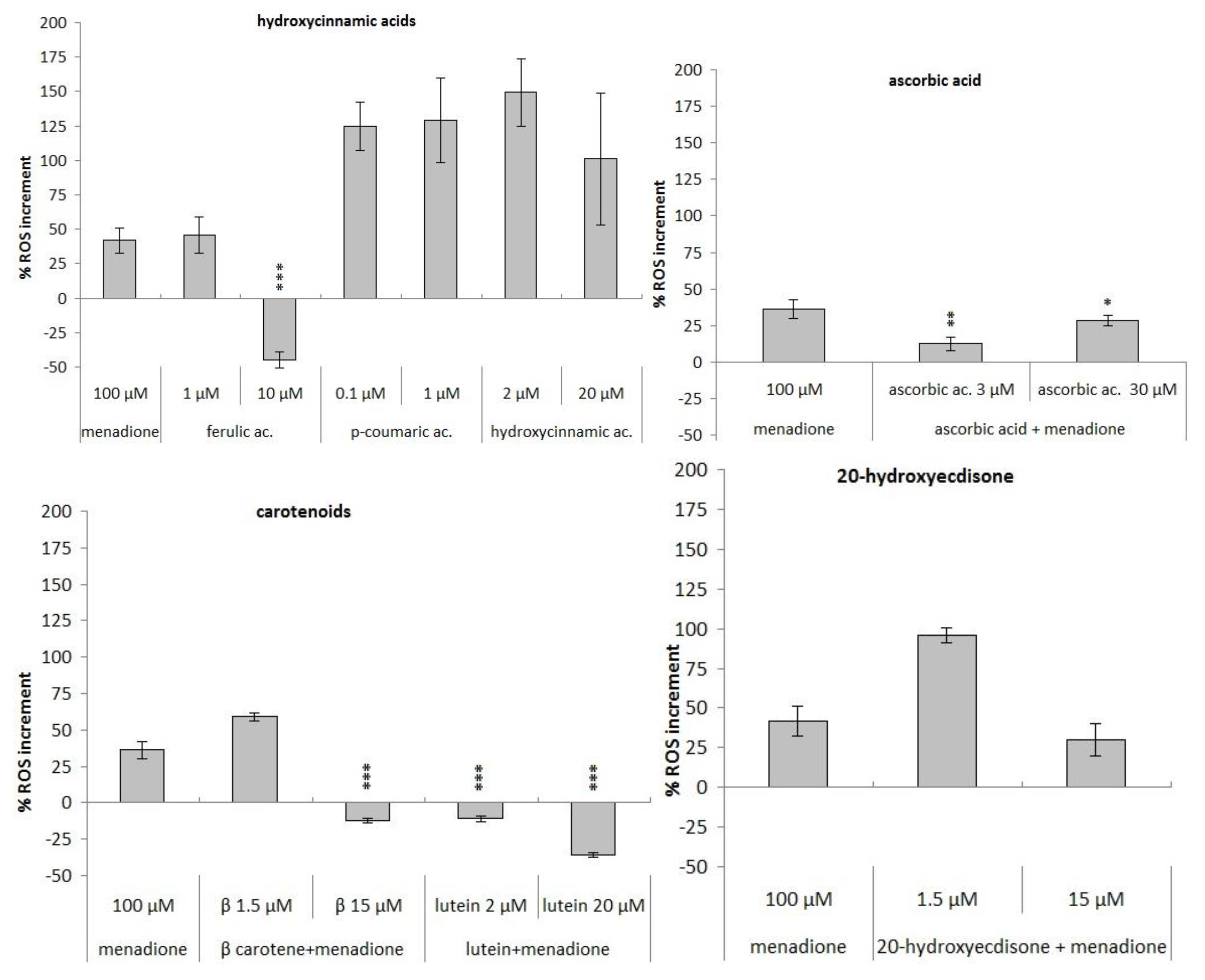
3.3. Antioxidant Activity
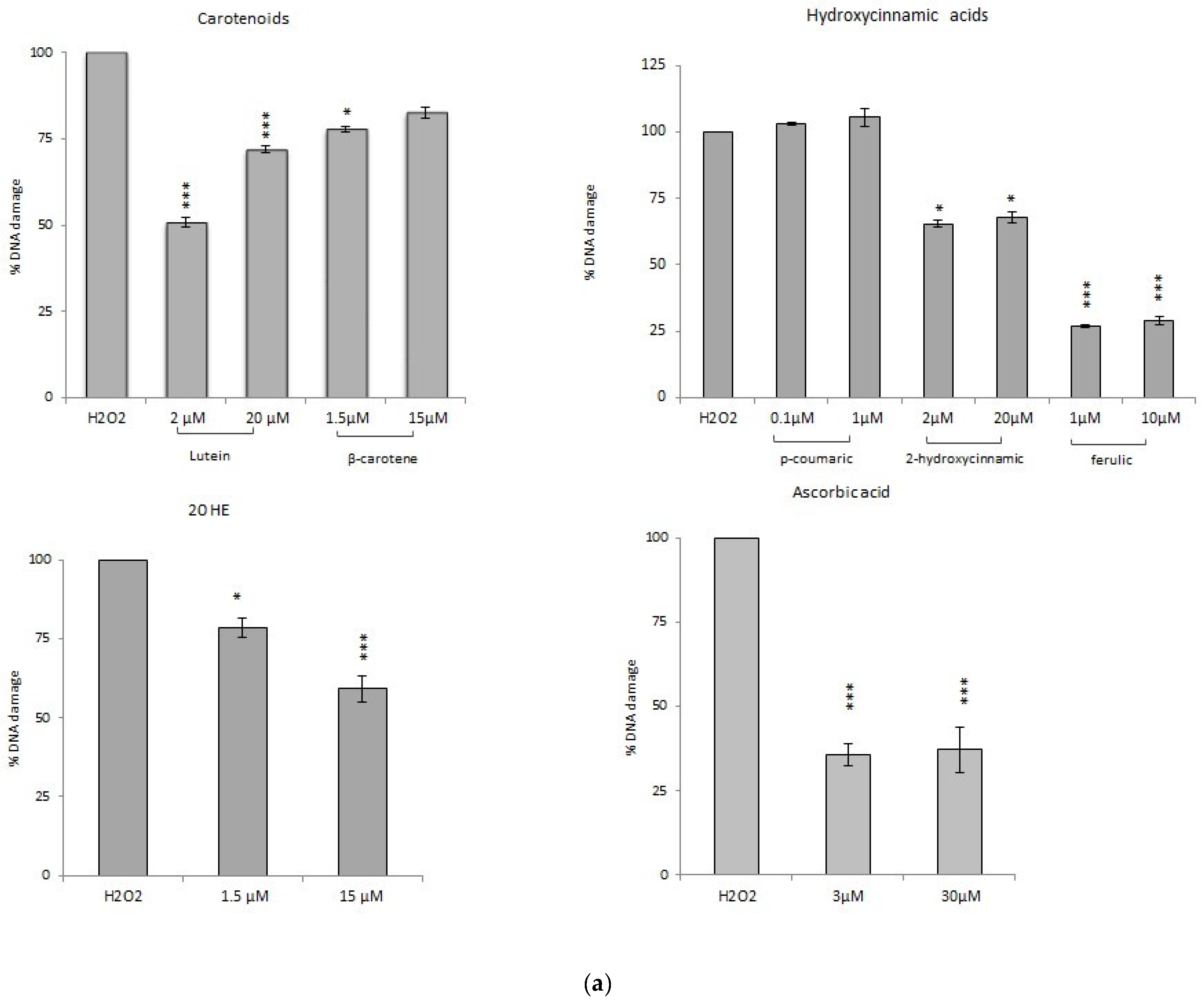
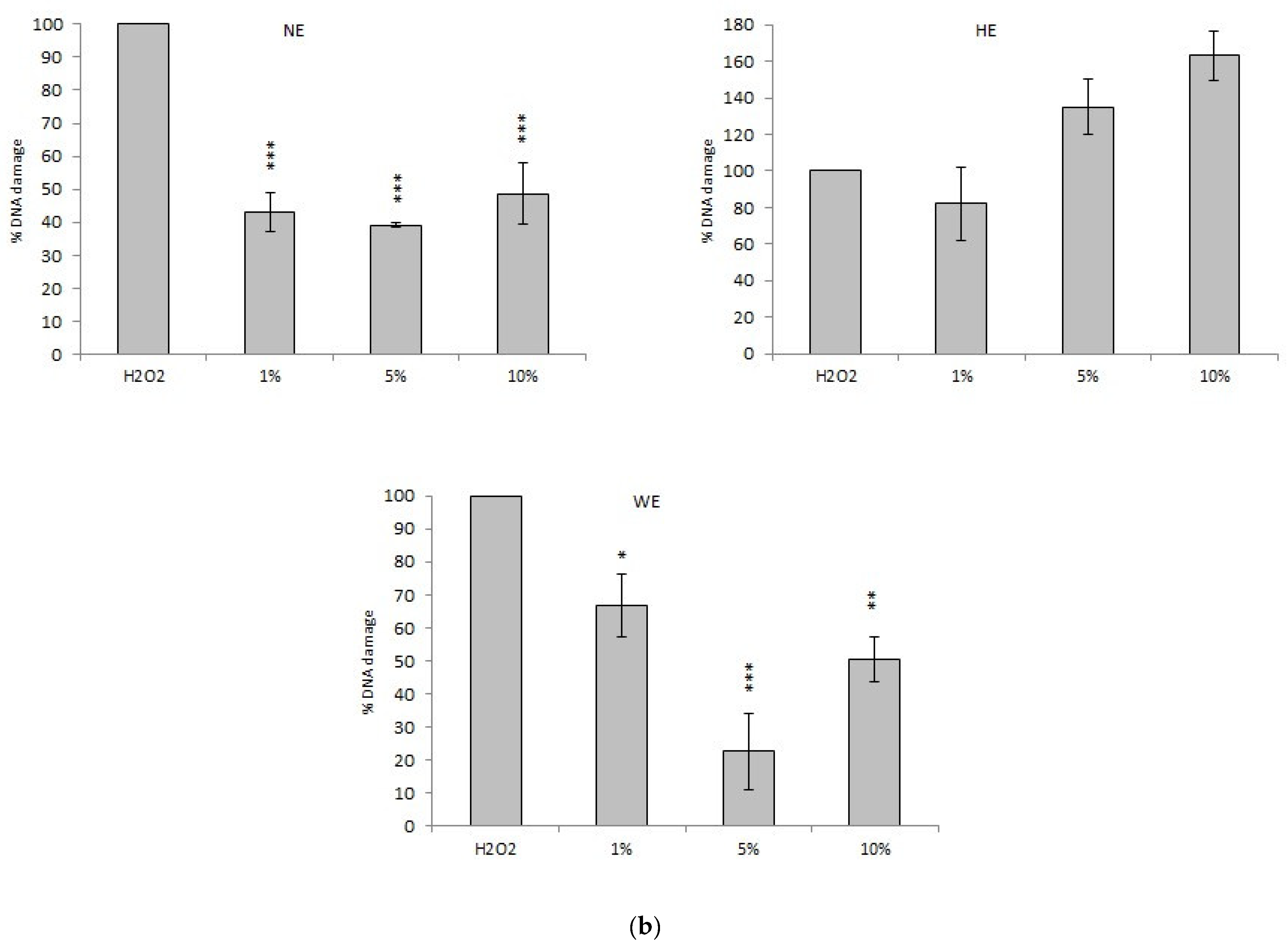
3.3.1. Comet Assay
Comet Assay with Phytochemicals
Comet Assay with Spinach Extracts
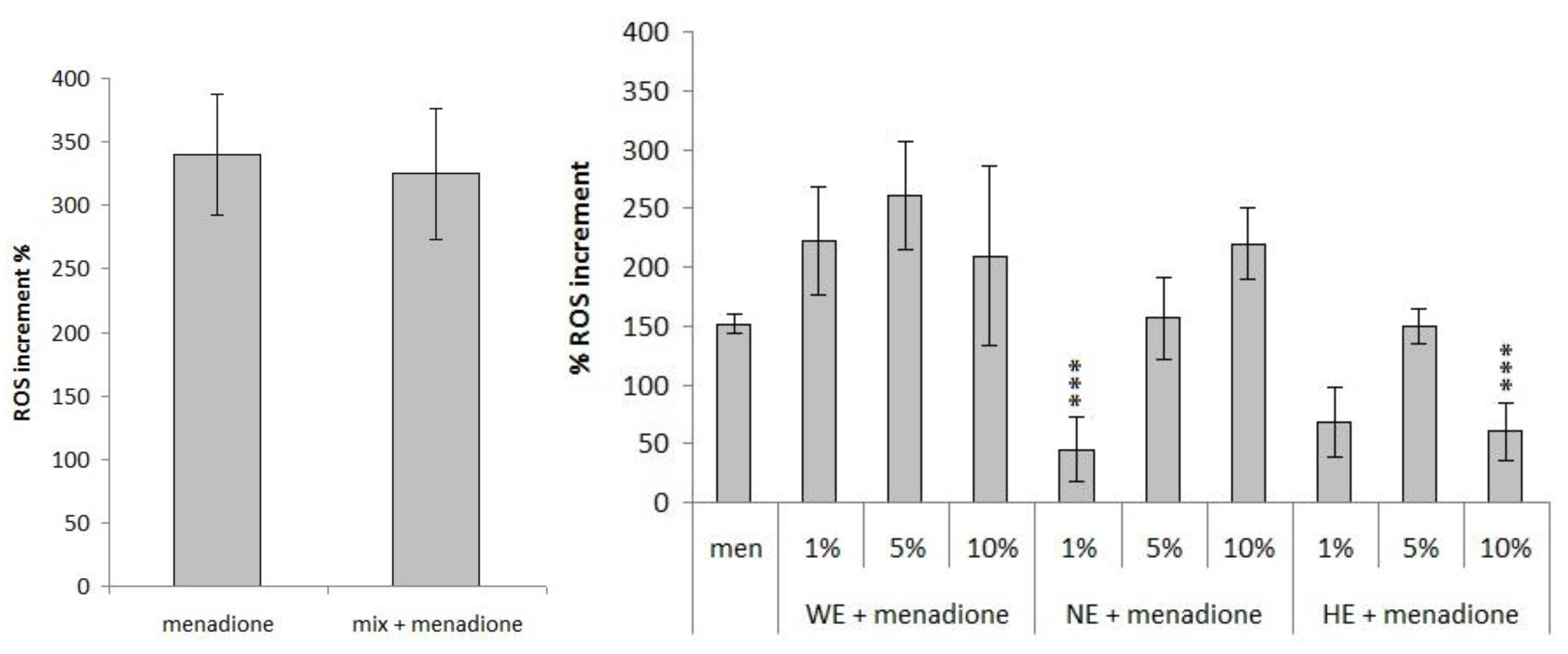
3.3.2. Measurement of Variation in Reactive Oxygen Species (ROS) Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, S.H.; Park, J.H.; Kim, S.Y.; Lee, S.W.; Chun, S.S.; Park, E. Antioxidant effects of spinach (Spinacia oleracea L.) supplementation in hyperlipidemic rats. Prev. Nutr. Food Sci. 2014, 19, 19–26. [Google Scholar] [CrossRef]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Reza Khatibi, S.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef]
- Rajoria, A.; Kumar, J.; Chauhan, A.K. Anti-oxidative and anti-carcinoginic role of lycopene in human health-A review. J. Dairy Foods Home Sci. 2010, 29, 3–4. [Google Scholar]
- Singh, R.B.; Hristova, K.; Fedacko, J.; Singhal, S.; Khan, S.; Wilson, D.W.; Takahashi, T.; Sharma, Z. Antioxidant vitamins and oxidative stress in chronic heart failure. World Heart J. 2015, 7, 257–264. [Google Scholar]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Panek, D.; Zeitler, H.; Vetter, H.; Wagner, H. Drug development from natural products: Exploiting synergistic effects. Indian J. Exp. Biol. 2010, 48, 208–219. [Google Scholar] [PubMed]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B.J. Synergy-Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis canadensis). Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Ferreres, F.; Tomas-Barberan, F. Effect of postharvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Hait-Darshan, R.; Grossman, S.; Bergman, M.; Deutsch, M.; Zurgil, N. Synergistic activity between a spinach-derived natural antioxidant (NAO) and commercial antioxidants in a variety of oxidation systems. Int. Food Res. 2009, 42, 246–253. [Google Scholar] [CrossRef]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.E.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, efficacy and safety of spinach extracts. Nutr. Cancer 2003, 46, 222–231. [Google Scholar] [CrossRef]
- Lomnitski, L.; Carbonatto, M.; Ben-Shaul, V.; Peano, S.; Conz, A.; Corradin, L.; Maronpot, R.R.; Grossman, S.; Nyska, A. The prophylactic effects of natural water-soluble antioxidant from spinach and apocynin in a rabbit model of lipopolysaccharide-induced endotoxemia. Toxicol. Pathol. 2000, 28, 588–600. [Google Scholar] [CrossRef]
- Fornaciari, S.; Milano, F.; Mussi, F.; Pinto-Sanchez, L.; Forti, L.; Buschini, A.; Arru, L. Assessment of antioxidant and antiproliferative properties of spinach plants grown under low oxygen availability. J. Sci. Food Agric. 2015, 95, 490–496. [Google Scholar] [CrossRef]
- Grajek, W.; Olejnik, A. Epithelial cell cultures in vitro as a model to study functional properties of food. Pol. J. Food Nutr. Sci. 2004, 13, 5–24. [Google Scholar]
- Maioli, E.; Greci, L.; Soucek, K.; Hyzdalova, M.; Pecorelli, A.; Fortino, V.; Valacchi, G. Rottlerin inhibits ROS formation and prevents NFkappaB activation in MCF-7 and HT-29 cells. J. Biomed. Biotechnol. 2009, 2009, 742936. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Antiproliferative Activity and Cell Metabolism of Hydroxycinnamic Acids in Human Colon Adenocarcinoma Cell Lines. J. Agric. Food Chem. 2019, 67, 3919–3931. [Google Scholar] [CrossRef]
- Fernandes, G.; Barone, A.W.; Dziak, R. The effect of ascorbic acid on bone cancer cells in vitro. Cogent Biol. 2017, 3, 1288335. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.; Lim, J.W.; Kim, H. β-Carotene-induced apoptosis is mediated with loss of Ku proteins in gastric cancer AGS cells. Genes Nutr. 2015, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, K.R.; Radha, K.S.; Madhyastha, H.K. Cell cycle regulation and induction of apoptosis by beta-carotene in U937 and HL-60 leukemia cells. J. Biochem. Mol. Biol. 2007, 40, 1009–1015. [Google Scholar] [PubMed]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein inhibits proliferation, invasion and migration of hypoxic breast cancer cells via downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Sani, H.A.; Rahmat, A.; Ismail, M.; Rosli, R.; Endrini, S. Potential anticancer effect of red spinach (Amaranthus gangeticus) extract. Asia Pac. J. Clin. Nutr. 2004, 13, 396–400. [Google Scholar]
- Ferguson, L.R.; Zhu, S.T.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Ramana, K.V.; Srivastava, S.K.; Van Kuijk, F.J. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp. Eye Res. 2008, 86, 70–80. [Google Scholar] [CrossRef]
- Griffiths, K.; Aggarwal, B.; Singh, R.; Buttar, H.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Milano, F.; Mussi, F.; Fornaciari, S.; Altunoz, M.; Forti, L.; Arru, L.; Buschini, A. Oxygen Availability during Growth Modulates the Phytochemical Profile and the Chemo-Protective Properties of Spinach Juice. Biomolecules 2019, 9, 53. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Kanimozhi, G.; Prasad, N.R.; Mahalakshmi, R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol. In Vitro 2011, 25, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
| Samples | Content (mg/100 g) | Assayed Concentrations | |
|---|---|---|---|
| Ascorbic acid | 4.0 | 3 µM | 30 µM |
| 20-Hydroxyecdysone | 5.0 | 1.5 µM | 15 µM |
| Lutein | 7.2 | 2 µM | 20 µM |
| β-Carotene | 5.1 | 1.5 µM | 15 µM |
| Ferulic acid | 1.0 | 1 µM | 10 µM |
| p-Coumaric acid | 0.1 | 0.1 µM | 1 µM |
| 2-Hydroxycinnamic acid | 2.8 | 2 µM | 20 µM |
| Samples | Concentration | TI% 1 | Sd |
|---|---|---|---|
| NT 2 | 0.29 | 0.22 | |
| Lutein | 2 μM | 0.33 | 0.01 |
| 20 μM | 52.72 | 0.41 | |
| β-Carotene | 1.5 μM | 0.22 | 0.01 |
| 15 μM | 0.25 | 0.29 | |
| 20HE | 1.5 μM | 0.18 | 0.01 |
| 15 μM | 0.59 | 0.23 | |
| Ascorbic acid | 3 μM | 0.32 | 0.35 |
| 30 μM | 0.25 | 0.29 | |
| p-Coumaric acid | 0.1 μM | 0.31 | 0.58 |
| 1 μM | 0.51 | 0.45 | |
| 2-Hydroxicinnamic acid | 2 μM | 0.20 | 0.34 |
| 20 μM | 0.39 | 0.31 | |
| Ferulic acid | 1 μM | 0.21 | 0.14 |
| 10 μM | 0.20 | 0.07 | |
| Mix | 0.23 | 0.11 | |
| WE | 1% | 0.69 | 0.17 |
| 5% | 1.03 | 0.01 | |
| 10% | 0.77 | 0.06 | |
| 50% | 4.33 | 0.70 | |
| NE | 1% | 0.86 | 0.08 |
| 5% | 0.38 | 0.08 | |
| 10% | 1.33 | 0.46 | |
| 50% | toxic | ||
| HE | 1% | 0.87 | 0.17 |
| 5% | 1.85 | 0.86 | |
| 10% | 1.90 | 0.6 | |
| 50% | toxic | ||
| Phytochemical (Physiological Dose) | Antioxidant Activity | ||
|---|---|---|---|
| No Stress | Menadione | H2O2 | |
| p-Coumaric acid | ⇿ | ↑ | ⇿ |
| 2-Hydroxycinnamic acid | ⇿ | ↑ | ↓ (40%) |
| Ferulic acid | ⇿ | ↑ | ↓ (75%) |
| 20 HE | ⇿ | ↑ | ↓ (20%) |
| Ascorbic acid | ⇿ | ↓ | ↓ (60%) |
| Lutein | ⇿ | ↓ | ↓ (50%) |
| β-Carotene | ⇿ | ⇿ | ↓ (20%) |
| Mix | ⇿ | ⇿ | ↓ (32%) |
| Sample | Antioxidant Activity | ||
|---|---|---|---|
| No Stress | Menadione | H2O2 | |
| Phytochemical mix | ⇿ | ⇿ | ↓ (32%) |
| HE 1% | ⇿ | ⇿ | ⇿ |
| WE 1% | ⇿ | ↑ | ↓ (35%) |
| NE 1% | ⇿ | ↓ | ↓ (60%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arru, L.; Mussi, F.; Forti, L.; Buschini, A. Biological Effect of Different Spinach Extracts in Comparison with the Individual Components of the Phytocomplex. Foods 2021, 10, 382. https://doi.org/10.3390/foods10020382
Arru L, Mussi F, Forti L, Buschini A. Biological Effect of Different Spinach Extracts in Comparison with the Individual Components of the Phytocomplex. Foods. 2021; 10(2):382. https://doi.org/10.3390/foods10020382
Chicago/Turabian StyleArru, Laura, Francesca Mussi, Luca Forti, and Annamaria Buschini. 2021. "Biological Effect of Different Spinach Extracts in Comparison with the Individual Components of the Phytocomplex" Foods 10, no. 2: 382. https://doi.org/10.3390/foods10020382
APA StyleArru, L., Mussi, F., Forti, L., & Buschini, A. (2021). Biological Effect of Different Spinach Extracts in Comparison with the Individual Components of the Phytocomplex. Foods, 10(2), 382. https://doi.org/10.3390/foods10020382