Elevated Concentrations of Metal(loids) in Seaweed and the Concomitant Exposure to Humans
Abstract
1. Introduction
2. Cultivation Practices of Seaweed Plants in Malaysia
3. Materials and Methods
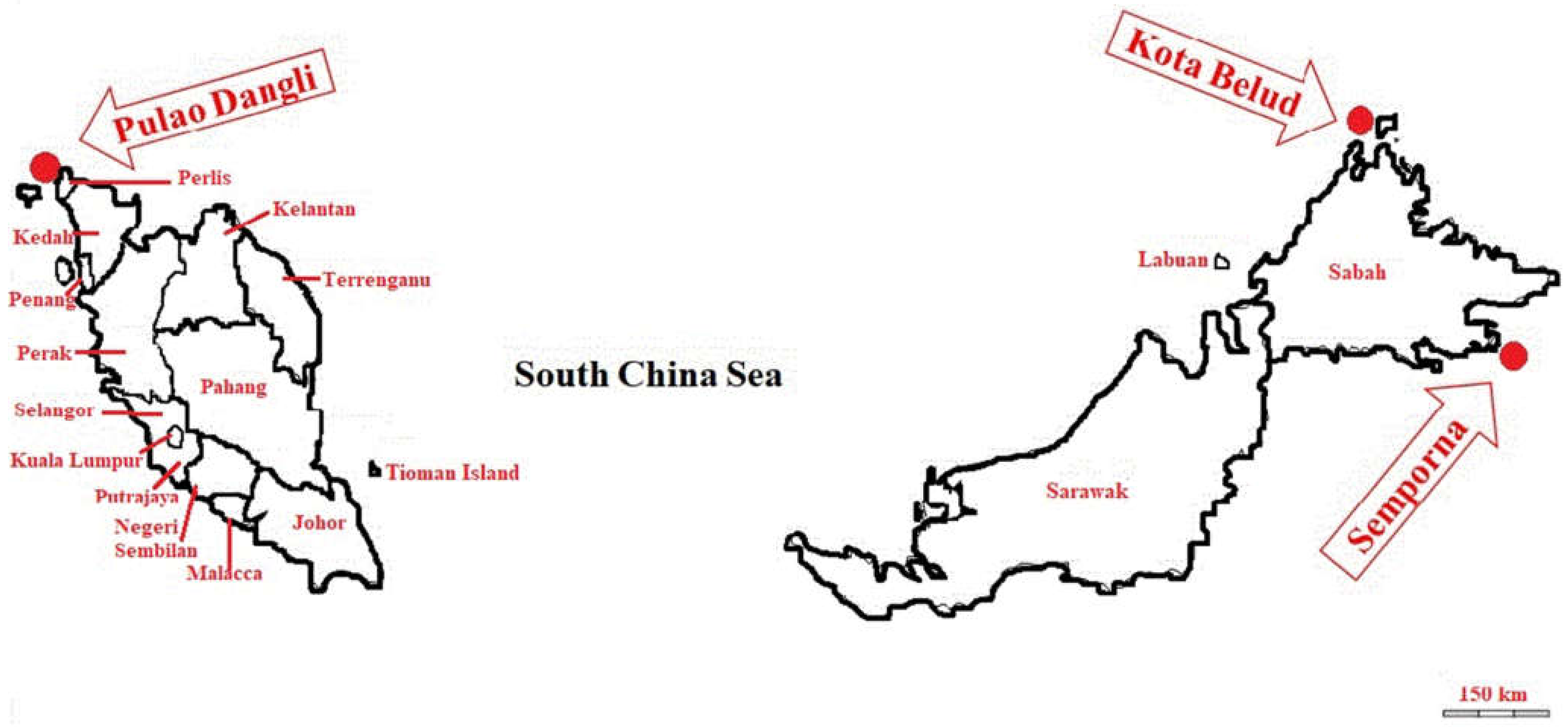
3.1. Sample Collection and Preparation
3.2. Chemical Analyses by ICP-OES
3.3. Assessment of Health Risk
3.4. Daily Intake (DI) of Heavy Metals
3.5. Estimation of Mean Daily Dose (MDD)
3.6. Noncarcinogenic Risk
3.7. Carcinogenic Risk
4. Results and Discussion
4.1. Concentrations of Metals in SEAWEEDS
4.2. Health Risk of Metal Exposures via the Consumption of Seaweeds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khandaker, M.U.; Heffny, N.; Adillah, B.; Amin, Y.M.; Bradley, D.A. Elevated concentration of radioactive potassium in edible algae cultivated in Malaysian seas and estimation of ingestion dose to humans. Algal Res. 2019. [Google Scholar] [CrossRef]
- Pati, M.P.; Sharma, S.D.; Nayak, L.; Panda, C.R. Uses of Seaweed and Its Application to Human Welfare. Int. J. Pharm. Pharm. Sci. 2016. [Google Scholar] [CrossRef]
- Smith, J.; Summers, G.; Wong, R. New Zealand Journal of Crop and Horticultural Science Nutrient and heavy metal content of edible seaweeds in New Zealand. N. Z. J. Crop. Hortic. Sci. 2010, 38, 19–28. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polymers. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Jarvis, T.A.; Bielmyer-Fraser, G.K. Accumulation and effects of metal mixtures in two seaweed species. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2015. [Google Scholar] [CrossRef]
- Chijioke, N.O.; Uddin Khandaker, M.; Tikpangi, K.M.; Bradley, D.A. Metal uptake in chicken giblets and human health implications. J. Food Compos. Anal. 2020. [Google Scholar] [CrossRef]
- Pérez, A.A.; Fajardo, M.A.; Farías, S.S.; Pérez, L.B.; Strobl, A.M.; Roses, O.E. Human dietary exposure to lead and cadmium via the consumption of mussels and seaweeds from San Jorge Gulf, Patagonia Argentina. Int. J. Environ. Health 2011. [Google Scholar] [CrossRef]
- Saraee, K.R.E.; Abdi, M.R.; Naghavi, K.; Saion, E.; Shafaei, M.A.; Soltani, N. Distribution of heavy metals in surface sediments from the South China Sea ecosystem, Malaysia. Environ. Monit. Assess. 2011. [Google Scholar] [CrossRef]
- Wang, S.L.; Xu, X.R.; Sun, Y.X.; Liu, J.L.; Li, H. Bin Heavy metal pollution in coastal areas of South China: A review. Mar. Pollut. Bull. 2013, 72, 6–13. [Google Scholar]
- Santawamaitre, T.; Malain, D.; Al-Sulaiti, H.A.; Matthews, M.; Bradley, D.A.; Regan, P.H. Study of natural radioactivity in riverbank soils along the Chao Phraya river basin in Thailand. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2011, 652, 920–924. [Google Scholar] [CrossRef]
- Malain, D.; Regan, P.H.; Bradley, D.A.; Matthews, M.; Al-Sulaiti, H.A.; Santawamaitre, T. An evaluation of the natural radioactivity in Andaman beach sand samples of Thailand after the 2004 tsunami. Appl. Radiat. Isot. 2012. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M. Environmental toxins and health-The health impact of pesticides. Aust. Fam. Phys. 2007, 36, 1002–1004. [Google Scholar]
- Nordberg, G.F. Effects and dose-response relationships of toxic metals. Scand. J. Work Environ. Health. 1976, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.U.; Asaduzzaman, K.; Nawi, S.M.; Usman, A.R.; Amin, Y.M.; Daar, E.; Bradley, D.A.; Ahmed, H.; Okhunov, A.A. Assessment of radiation and heavy metals risk due to the dietary intake of marine fishes (Rastrelliger kanagurta) from the Straits of Malacca. PLoS ONE 2015, 10, e0128790. [Google Scholar] [CrossRef]
- Asaduzzaman, K.; Khandaker, M.U.; Amin, Y.M.; Zainuddin, Z.; Farook, M.S.; Bradley, D.A. Measurement of radioactivity and heavy metal levels in edible vegetables and their impact on Kuala Selangor communities of Peninsular Malaysia. Radiat. Prot. Dosim. 2015. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, K.; Khandaker, M.U.; Binti Baharudin, N.A.; Amin, Y.B.M.; Farook, M.S.; Bradley, D.A.; Mahmoud, O. Heavy metals in human teeth dentine: A bio-indicator of metals exposure and environmental pollution. Chemosphere 2017. [Google Scholar] [CrossRef]
- Shazili, N.A.M.; Yunus, K.; Ahmad, A.S.; Abdullah, N.; Rashid, M.K.A. Heavy metal pollution status in the Malaysian aquatic environment. Aquat. Ecosyst. Health Manag. 2006, 9, 137–145. [Google Scholar] [CrossRef]
- Hamzan, N.A.A.; Zaini, F.F.M.; Ibrahim, M.I.; Ariffin, N.A.N. Assessment of selected heavy metals in seawater and sediment at Klang Coastal area Malaysia. Malays. J. Anal. Sci. 2015, 4, 730–738. [Google Scholar]
- Pan, Y.; Wernberg, T.; de Bettignies, T.; Holmer, M.; Li, K.; Wu, J.; Lin, F.; Yu, Y.; Xu, J.; Zhou, C.; et al. Screening of seaweeds in the East China Sea as potential bio-monitors of heavy metals. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef]
- Karthick, P.; Siva Sankar, R.; Kaviarasan, T.; Mohanraju, R. Ecological implications of trace metals in seaweeds: Bio-indication potential for metal contamination in Wandoor, South Andaman Island. Egypt. J. Aquat. Res. 2012. [Google Scholar] [CrossRef]
- Dadolahi-Sohrab, A.; Seyed, A.N.; Nabavi, M.B.; Safahyeh, A.; Ketal-Mohseni, M. Environmental Monitoring of Heavy Metals in Seaweed and Associated Sediment from the Strait of Hormuz, I.R. Iran. World J. Fish. Mar. Sci. 2011, 3, 576–589. [Google Scholar]
- Malea, P.; Haritonidis, S.; Kevrekidis, T. Metal content of some green and brown seaweeds from Antikyra Gulf (Greece). Hydrobiologia 1995. [Google Scholar] [CrossRef]
- Bryan, G.W.; Hummerstone, L.G. Brown seaweed as an indicator of heavy metals in estuaries in south-west England. J. Mar. Biol. Assoc. UK 1973. [Google Scholar] [CrossRef]
- Mutia, G.M.; Matern, M. Analysis of Bio-Accumulation of Heavy Metals in Seaweeds Ulva rigida and Halimeda opuntia in Validation of Their Safety for Use in Aquaculture Feeds in Kenya. IOSR J. Environ. Sci. Toxicol. Food Technol. 2018, 12, 56–63. [Google Scholar]
- Wilson, S. Nutritional value of detritus and algae in blenny territories on the Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 2002. [Google Scholar] [CrossRef]
- Mohammad, S.T.; Al-Kayiem, H.H.; Aurybi, M.A.; Khlief, A.K. Measurement of global and direct normal solar energy radiation in Seri Iskandar and comparison with other cities of Malaysia. Case Stud. Therm. Eng. 2020, 18, 100591. [Google Scholar] [CrossRef]
- Intawongse, M.; Kongchouy, N.; Dean, J.R. Bioaccessibility of heavy metals in the seaweed Caulerparacemosa var. corynephora: Human health risk from consumption. Instrum. Sci. Technol. 2018; 46, 628–644. [Google Scholar] [CrossRef]
- Nor, A.M.; Gray, T.S.; Caldwell, G.S.; Stead, S.M. A value chain analysis of Malaysia’s seaweed industry. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Matsumura, Y. Nutrition trends in Japan. Asia Pac. J. Clin. Nutr. 2001. [Google Scholar] [CrossRef]
- Hwang, Y.O.; Park, S.G.; Park, G.Y.; Choi, S.M.; Kim, M.Y. Total arsenic, mercury, lead, and cadmium contents in edible dried seaweed in Korea. Food Addit. Contam. Part. B Surveill. 2010. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef]
- Evaluation of Certain Food Additives and Contaminants. In 32 Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO TRS 960: Geneva, Switzerland, 2011; pp. 28–31.
- California Office of Environmental Health Hazard Assessment, Technical Support. Document for Cancer Potency Factors: Methodologies for Derivation, Listing of Available Values, and Adjustments to Allow for Early-Life Stage Exposures; OEHHA: Sacramento, CA, USA, 2009.
- US EPA. Integrated Risk Information System (IRIS). Chemical Assessment. 1992. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0550_summary.pdf (accessed on 30 November 2020).
- US EPA. Integrated Risk Information System (IRIS) Database; National Centre for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2000.
- US EPA. Regional Screening Levels (RSLs)—Generic Tables. 2017. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 30 November 2020).
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A), EPA/540/1-89/002, December 1989. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 30 November 2020).
- Stara, J.; Patterson, J.; Blackburn, K.; Hertzberg, R.; DeRosa, C. Guidelines for the Health Risk Assessment of Chemical Mixtures; U.S. Environmental Protection Agency: Washington, DC, USA, 1986.
- Resma, N.S.; Meaze, A.K.M.; Hossain, S.; Khandaker, M.U.; Kamal, M.; Deb, N. The presence of toxic metals in popular farmed fish species and estimation of health risks through their consumption. Phys. Open 2020, 5, 100052. [Google Scholar] [CrossRef]
- US EPA. Guidelines for Carcinogen Risk Assessment (epa/630/p-03/001f). 2005. Available online: https://www.epa.gov/sites/production/files/201309/documents/cancer_guidelines_final_3-25-05.pdf (accessed on 2 December 2020).
- US EPA. Reference dose (RfD): Description and Use in Health Risk Assessments, Background Document 1a. 1993. Available online: https://www.epa.gov/iris/reference-doserfd-description-and-usehealth-risk-assessments (accessed on 2 December 2020).
- Nduka, J.K.; Kelle, H.I.; Ogoko, C. Hazards and Risk Assessment of Heavy Metals from Consumption of Locally Manufactured Painkiller Drugs in Nigeria; Toxicology Report; PubMed: Awka, Nigeria, 2020; Volume 7. [Google Scholar] [CrossRef]
- Kolo, M.T.; Khandaker, M.U.; Amin, Y.M.; Abdullah, W.H.B.; Bradley, D.A.; Alzimami, K.S. Assessment of health risk due to the exposure of heavy metals in soil around mega coal-fired cement factory in Nigeria. Results Phys. 2018, 11, 755–762. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wu, J.F.; Shang, D.R.; Ning, J.S.; Ding, H.Y.; Zhai, Y.X. Arsenic Species in Edible Seaweeds Using In Vitro Biomimetic Digestion Determined by High-Performance Liquid Chromatography Inductively Coupled Plasma Mass Spectrometry. Int. J. Food Sci. 2014. [Google Scholar] [CrossRef]
- Zmozinski, A.V.; Llorente-Mirandes, T.; López-Sánchez, J.F.; da Silva, M.M. Establishment of a method for determination of arsenic species in seafood by LC-ICP-MS. Food Chem. 2015, 173, 1073–1082. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002. [Google Scholar] [CrossRef]
- Lorenzo, G. Heavy metal contamination of brown seaweed and sediments from the UK coastline between the Wear river and the Tees river. Environ. Int. 2000, 26, 275–286. [Google Scholar]
- Khaled, A.; Hessein, A.; Abdel-Halim, A.M.; Morsy, F.M. Distribution of heavy metals in seaweeds collected along marsa-matrouh beaches, Egyptian mediterranean sea. Egypt. J. Aquat. Res. 2014. [Google Scholar] [CrossRef]
- Akcali, I.; Kucuksezgin, F. A biomonitoring study: Heavy metals in macroalgae from eastern Aegean coastal areas. Mar. Pollut. Bull. 2011. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Haritonidis, S. Seasonal accumulation of metals by red alga Gracilaria verrucosa (Huds.) Papens. from Thermaikos Gulf, Greece. J. Appl. Phycol. 1999. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Mamish, S.; Budier, Y. Radionuclides and trace metals in eastern Mediterranean Sea algae. J. Environ. Radioact. 2003. [Google Scholar] [CrossRef]
- Schintu, M.; Marras, B.; Durante, L.; Meloni, P.; Contu, A. Macroalgae and DGT as indicators of available trace metals in marine coastal waters near a lead-zinc smelter. Environ. Monit. Assess. 2010. [Google Scholar] [CrossRef]
- US EPA. Department of Health & Human Services-Agency for Toxic Substances and Disease Registry (ATSDR). In Toxicol. Profile Asbestos; 2001. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp61.pdf (accessed on 9 February 2021).
- Dwyer, J.T.; Allison, D.B.; Coates, P.M. Dietary supplements in weight reduction. J. Am. Diet. Assoc. 2005. [Google Scholar] [CrossRef]
- Barua, T.; Bhuian, A.K.M.S.I.; Hossain, S.; Deb, N.; Ahmed, M.; Rashid, M.A.; Khandaker, M.U. The presence of radioactive and metal contaminants in wild mushrooms grown in Chattogram hill tracts, Bangladesh. J. Radioanal. Nucl. Chem. 2019, 322, 173–182. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Tuzen, M.; Mendil, D.; Soylak, M. Assessment of trace element contents of chicken products from turkey. J. Hazard. Mater. 2009. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.U.; Nasir, N.L.M.; Zakirin, N.S.; Kassim, H.A.; Asaduzzaman, K. Radiation dose to the Malaysian populace via the consumption of bottled mineral water. Radiat. Phys. Chem. 2017, 140, 173–179. [Google Scholar] [CrossRef]
- Usman, A.R.; Khandaker, M.U.; Isa, N.F.; Ahmed, Y.A. Elemental Analysis of Nigerian and Nigerien Foods Using Neutron Activation and Estimation of Daily Intake. In International Conference for Innovation in Biomedical Engineering and Life Sciences. ICIBEL 2015. IFMBE Proceedings; Ibrahim, F., Usman, J., Mohktar, M., Ahmad, M., Eds.; Springer: Singapore, 2016; Volume 56. [Google Scholar] [CrossRef]
- Revitt, D.M.; Lundy, L.; Eriksson, E.; Viavattene, C. Comparison of pollutant emission control strategies for cadmium and mercury in urban water systems using substance flow analysis. J. Environ. Manag. 2013. [Google Scholar] [CrossRef]
- Bernard, A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008, 120(4), 557–564. [Google Scholar]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006. [Google Scholar] [CrossRef]
- George, C.M.S.; Laura, A.; Mihalic, M.; Cabrera, J.; Lilia, Z.; Checkley, D.D.; Gilman, W.; Robert, H. Arsenic exposure in drinking water: An unrecognized health threat in Peru. Bull. World Health Organ. 2014, 92, 565–572. [Google Scholar] [CrossRef]
- Sakurai, H.; Tsuchiya, K. A tentative recommendation for the maximum daily intake of selenium. Environ. Physiol. Biochem. 1975, 5, 107–118. [Google Scholar]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014. [Google Scholar] [CrossRef]
- David, M.J. Selenium. Fish Physiol. 2011, 31, 327–374. [Google Scholar] [CrossRef]
- Pennington, J.A.T. Silicon in foods and diets. Food Addit. Contam. 1991. [Google Scholar] [CrossRef]
- US FDA. United States Food and Drug Administration. Code of Federal Regulations Title 21, 21CFR172.480. 2015. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.480 (accessed on 9 February 2021).
- European Union Commission regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, 295, 1–177.
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health Part. B Crit. Rev. 2007, 10 (Suppl. 1), 1–269. [Google Scholar] [CrossRef]
- US EPA. Office of Pesticides and Toxic Substances (7508W), EPA-738-F-93-005. June 1993. Available online: archive.epa.gov/pesticides/reregistration/web/pdf/4082fact.pdf (accessed on 4 December 2020).
- Greene, R.M.; Su, W.P.D. Argyria. Am. Fam. Phys. 1987. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Silver; U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (TP-90-24): Atlanta, GA, USA, 1990.
- WHO. Guideline: Potassium Intake for Adults and Children; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2012; ISBN 978-92-4-150482-9. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Novkovic, M. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997; ISBN 9958-9881-4-3. [Google Scholar]
- Australia New Zealand Food Authority. The 19th Australian Total Diet Survey; Appendix 1; ANZFA: Majura Park, Austrialia, 2001; pp. 35–40.
- Cuciureanu, R.R.; Urzică, A.; Voitcu, M.; Antoniu, A. Assessment of Daily Aluminum Intake by Food Consumption. Rev. Med. Chir. Soc. Med. Nat. Iasi 2000, 104, 107–112. [Google Scholar]
- Australia New Zealand Food Authority. Nutrient and Metals Reference Values for Australia and New Zealand for Men and Women; National Health and Medical Research Council: Bethesda, MD, USA, 2006; ISBN 1864962437.
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2000; ISBN 0-309-51199-2. [Google Scholar] [CrossRef]
- EPA (U.S. Environmental Protection Agency). EPA’s Integrated Risk Information Program, Progress Report and Report to Congress. Office of Research and Development, U.S. Environmental Protection Agency; 2012. Available online: http://www.epa.gov/iris/pdfs/irisprogressreport2012.pdf (accessed on 3 December 2020).
- Abduljaleel, S.A.; Shuhaiml-Othman, M.; Babji, A. Assessment of trace metals contents in chicken (Gallus gallus domesticus) and quail (Coturnix coturnix japonica) tissues from selangor (Malaysia). J. Environ. Sci. Technol. 2012. [Google Scholar] [CrossRef]
- Holmes, P.; James, K.A.F.; Levy, L.S. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009, 408, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Jessica Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Assembly Resolution, IARC Statement on the Adoption of the New Cancer Resolution by the World Health Assembly; Global Cancer Control: Geneva, Switzerland, 2017. [Google Scholar]
- Nazal, K.M. Marine Algae Bioadsorbents for Adsorptive Removal of Heavy Metals. In Advanced Sorption Process Applications; Intech Open: London, UK, 2019. [Google Scholar]
- Nkpaa, K.W.; Patrick-Iwuanyanwu, K.C.; Wegwu, M.O.; Essien, E.B. Health risk assessment of hazardous metals for population via consumption of seafood from Ogoniland, Rivers State, Nigeria; a case study of Kaa, B-Dere, and Bodo City. Environ. Monit. Assess. 2016. [Google Scholar] [CrossRef]
- EPA (U.S. Environmental Protection Agency). Integrated Risk Information System Program: Summary Report from November 2012 Public Stakeholder Meeting; 2012. Available online: https://www.epa.gov/iris/iris-public-stakeholder-meeting-november-2012 (accessed on 9 February 2021).
- US EPA. Ehrlich A. Risk Assessment Guidelines Update; EPA/600/D-88/264 NTIS PB89133417; U.S. Environmental Protection Agency: Washington, DC, USA, 1988.
- Isa, B.K.; Amina, S.B.; Aminu, U.; Sabo, Y. Health risk assessment of heavy metals in water, air, soil and fish. Afr. J. Pure Appl. Chem. 2015. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy metal contamination in rice-producing soils of Hunan province, China and potential health risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef]
| Analyte | Total Concentration of Metals in CRM 7405-a (mg/kg) | % Recovery | |
|---|---|---|---|
| Certified Value | Recovered | ||
| K | 47,500 ± 700 | 46,550 ± 686 | 98.0 |
| Ca | 15,200 ± 300 | 15,048 ±297 | 99.0 |
| Mg | 6790 ± 100 | 6620 ± 97.5 | 97.5 |
| Pb | 0.43 ± 0.03 | 0.45 ± 0.04 | 104.7 |
| Cd | 0.79 ± 0.02 | 0.75 ± 0.03 | 94.9 |
| Al | 147 ± 7 | 141 ± 6 | 95.9 |
| Mn | 14.1 ± 0.7 | 14.5 ± 0.6 | 102.8 |
| Cu | 1.55 ± 0.07 | 1.52 ± 0.06 | 98.1 |
| Zn | 13.4 ± 0.5 | 13.1 ± 0.4 | 97.7 |
| Fe | 311 ± 11 | 317 ± 8 | 101.9 |
| As | 35.8 ± 0.9 | 35.26 ± 0.88 | 98.5 |
| Na | 16,200 ± 200 | 16,038 ± 198 | 99.0 |
| Ni | 2.2 ± 0.1 | 2.3 ± 0.08 | 104.6 |
| Cr | 3.4 ± 0.1 | 3.29 ± 0.09 | 96.8 |
| Analyte | Spectral Line Wavelength (nm) | SD (Blank) | Number of Calibration Standards | Slope of Calibration Curve, m | LoD = 3 × SD(B)/M (µg/kg) |
|---|---|---|---|---|---|
| K | 766.49 | 33.65 | 7 | 881.4 | 114.53 |
| Ca | 317.933 | 29.53 | 7 | 10,700 | 8.28 |
| Mg | 285.213 | 38.01 | 7 | 25,990 | 4.39 |
| Pb | 220.353 | 10.76 | 3 | 2853 | 11.31 |
| Cd | 228.802 | 15.22 | 3 | 21,210 | 2.15 |
| Se | 196.026 | 5.25 | 3 | 1077 | 14.62 |
| Al | 396.153 | 14.36 | 7 | 13,990 | 3.08 |
| Mn | 257.61 | 9.394 | 3 | 23,490 | 1.20 |
| Cu | 327.393 | 58.6 | 3 | 93,150 | 1.89 |
| Zn | 206.2 | 3.9 | 3 | 13,140 | 0.89 |
| Fe | 238.204 | 2.51 | 7 | 7492 | 1.01 |
| As | 188.979 | 4.67 | 3 | 965.5 | 14.51 |
| Na | 589.592 | 13.6 | 7 | 4427 | 9.22 |
| Ni | 231.604 | 7.95 | 3 | 12,110 | 1.97 |
| Cr | 267.716 | 10.21 | 3 | 6573 | 4.66 |
| Ag | 328.068 | 9.48 | 3 | 82,420 | 0.35 |
| Si | 251.611 | 81.21 | 7 | 6421 | 37.94 |
| Metal | Measured Concentrations of Metals in Microgram Per Gram in the Seaweed Samples under This Study | Literature Data in Microgram Per Gram, Together with the Country of Study | |||
|---|---|---|---|---|---|
| LKW (n = 3) | SPN (n = 3) | KBL (n = 2) | Overall Mean Value | ||
| Mean ± RSD (Range) | Mean ± RSD (Range) | Mean ± RSD (Range) | |||
| K | 95,450 ± 2.10 (93,400–97,950) | 79,575 ± 1.95 (52,950–106,200) | 6815 ± 0.94 (6133.5–7496.5) | 60,613 | 7900 ± 3900–71,200 ± 20,200 [3], New Zealand; 31,840–115,790 ± 1280 [47], Spain |
| Ca | 1097 ± 1.59 (267–2731) | 1929 ± 0.96 (1481–2378) | 2357 ± 1.01 (2121.3–2592.7) | 1794 | 8500 ± 4500–15,300 ± 800 [3], New Zealand 3900 ± 170–10,050 ± 50 [47], Spain |
| Mg | 772 ± 2.19 (765–779) | 2280 ± 1.34 (742–3818) | 549 ± 0.99 (439.2–658.8) | 1200 | 5650 ± 110–11,810 ± 340 [47], Spain |
| Pb | 8.47 ± 5.49 (6.75–11) | 3.75 ± 4.56 (2.75–4.75) | 10.85 ± 1.16 (9.76–11.94) | 7.69 | 0.1–12.1 + 4.0 [48], UK; <LOD–6.96 [31], China; 0.14 ± 0.02–1.83 ± 0.99 [3], New Zealand; 8.38–159.39 [49], Egypt 0.003 [50], Turkey 9.5–19.0 [51], Greece 1.31–2.33 [52], Syria 108.54–333.60 [53], Italy |
| Cd | 1.73 ± 1.66 (1.6–1.95) | 1.7 ± 3.29 (1.4–2) | 1.45 ± 0.46 (1.23–1.67) | 1.63 | 0.23–1.34 [49], Egypt; 0.002–6.4 [31], China; 0.02–10.03 + 4.12 [48], UK 0.011–0.18 [50], Turkey 0.8–3.1 [51], Greece <0.1–<0.5 [52], Syria 5.22–9.58 [53], Italy |
| Se | 4.9 ± 7.36 (4.3–5.5) | 33.95 ± 8.89 (6–61.9) | 36.85 ± 2.04 (33.18–40.55) | 25.2 | 0.07 ± 0.03–0.17 ± 0.02 [3], New Zealand <LOD‒12 [31], China |
| Al | 69.2 ± 4.13 (47.6–98.6) | 18.55 ± 3.28 (16.7–20.4) | 36 ± 0.76 (32.4–39.6) | 41.3 | 0.173–4505 [31], China |
| Mn | 13 ± 4.13 (6.5–25.6) | 3.35 ± 2.68 (2.5–4.2) | 4.7 ± 0.85 (3.53–5.88) | 7.02 | 3.7 ± 0.2–192.3 ± 142.1 [3], New Zealand 0.404–407 [31], China 18.8 + 2.2–778.4 + 38.8 [43], UK <5.0–55.0 ± 1.1 [47], Spain |
| Cu | 2.38 ± 1.86 (1.8–2.9) | 1.225 ± 1.16 (0.85–1.6) | 1.3 ± 0.83 (0.91–1.70) | 1.64 | 0.47–65.72 [49], Egypt <LOD–39.2 [31], China 4.8+2.0–50.6+10.6 [48], UK 5.09 ± 3.83–23.62 ± 16.03 [3], New Zealand <5.0 [47], Spain |
| Zn | 18.2 ± 2.29 (15.7–20.75) | 13.875 ± 1.14 (12–15.75) | 22.3 ± 0.62 (13.4–31.2) | 18.1 | 4.95–111.70 [49], Egypt 12.9 + 0.8–1015.5 + 54.4 [48], UK 9.9 ± 0.2–61.0 ± 22.7 [3], New Zealand 17.4 ± 0–71.4 ± 1.3 [47], Spain |
| Fe | 283 ± 1.94 (232.5–324.2) | 160 ± 2.77 (133–187) | 239 ± 1.74 (215.1–262.9) | 227 | 65.0 + 4.1–1208 + 98.2 [48], UK 13.7 ± 1.8–1227 ± 522 [3], New Zealand 32.9 ± 5.4–103 ± 4.1 [47], Spain 72.38–3865.96 [49], Egypt |
| As (III) | 1.91 ± 6.47 (1.35–2.25) | 6.3 ± 2.86 (4.7–7.9) | 5 ± 7.75 (4.3–5.7) | 4.40 | 1.88 ± 0.63–51.32 ± 6.49 [3], New Zealand 0.185–71 [31], China 0.10–1.47 [45], China |
| Na | 4286 ± 1.74 (4227–4396) | 2375 ± 2.22 (1576–3174) | 31.18 ± 1.03 (28.06–34.3) | 2231 | 36,270 ± 1150–17,640 ± 1660 [47], Spain 1700 ± 600–56,700 ± 18,100 [3], New Zealand |
| Ni | 12.97 ± 1.80 (12.3–13.4) | 10 ± 1.07 (8–12.75) | 10.6 ± 0.25 (6.9–14.3) | 10.0 | 0.3 + 0.1–70.5 + 8.9 ([48]), UK <LOD–8.82 [31], China 3.15–52.56 [49], Egypt |
| Cr-VI | 40.57 ± 1.50 (37–45.8) | 30.3 ± 1.75 (23.8–36.8) | 40.15 ± 2.27 (32.9–47.37) | 26.0 | 0.8 + 0.1–5.0 + 0.6 [48], UK 0.089–35.7 [31], China 0.44 ± 0.23–2.61 ± 3.08 [3], New Zealand |
| Ag | 0.2 ± 3.96 (0.15–0.25) | 0.125 ± 4.88 (0.1–0.15) | 0.2 ± 2.84 (0.15–0.25) | 0.18 | 0.9 + 0.2–4.2 + 0.6 [48], UK |
| Si | 391 ± 2.28 (237.7–489.7) | 122 ± 5.85 (74–170) | 209 ± 1.05 (177.7–240.4) | 241 | NA |
| Metal | Daily Intake of Metals (Microgram Per Kilogram/kg/Day) | Recommended Level of Daily Intake of Metals (Microgram Per Gram/kg/Day) | |||
|---|---|---|---|---|---|
| LKW | SPN | KBL | Mean | ||
| K | 14,685 | 12,242 | 1048 | 9325 | 3510 [74] |
| Ca | 169 | 297 | 363 | 276 | 800 [75] |
| Mg | 119 | 351 | 85 | 185 | 350 [76] |
| Pb | 1.30 | 0.58 | 1.67 | 1.2 | 3.5 [77] |
| Cd | 0.27 | 0.26 | 0.22 | 0.3 | 1 [77] |
| Se | 0.75 | 5.22 | 5.67 | 3.9 | 12.5 [77] |
| Al | 10.65 | 2.85 | 5.54 | 6.3 | 10 [78] |
| Mn | 2.00 | 0.52 | 0.72 | 1.1 | 5.5 [3] |
| Cu | 0.37 | 0.19 | 0.20 | 0.3 | 10 [3] |
| Zn | 2.80 | 2.13 | 3.43 | 2.8 | 45 [77] |
| Fe | 43.5 | 24.6 | 36.8 | 35 | 8 [79] |
| As | 0.29 | 0.97 | 0.77 | 0.7 | 10 [80] |
| Na | 659 | 365 | 4.8 | 343 | 1500 [76] |
| Ni | 2.00 | 1.54 | 1.08 | 1.5 | 1.0 [80] |
| Cr-VI | 6.24 | 4.66 | 1.08 | 4.0 | 3 [81] |
| Ag | 0.03 | 0.02 | 0.03 | 0.03 | 5 [71] |
| Si | 60.2 | 18.8 | 32.2 | 37 | NA |
| Metal | Oral Toxicity Reference Dose, RfD | Cancer Slope Factor | Estimated Mean Daily Dose (MDD) | Noncarcinogenic Risk | Carcinogenic Risk (LTCR) | ||
|---|---|---|---|---|---|---|---|
| (mg/kg/day) | References | (mg/kg/day)−1 | References | (mg/kg/day) | Hazard quotient (HQ) | ||
| K | NA | NA | 6.39 | ||||
| Ca | NA | NA | 0.189 | ||||
| Mg | 0.140 | [36] | 0.126 | 0.903 | |||
| Pb | 0.004 | [33] | 0.01 | [33] | 0.001 | 0.203 | 6.08 × 10−4 |
| Cd | 0.001 | [36] | 0.38 | [87] | 2 × 10−4 | 0.171 | 4.89 × 10−3 |
| Se | 0.005 | [36] | 0.003 | 0.532 | |||
| Al | 7.00 | [32] | 0.004 | 0.001 | |||
| Mn | 0.046 | [88] | 0.001 | 0.016 | |||
| Cu | 0.040 | [36] | 2 × 10−4 | 0.004 | |||
| Zn | 0.3 | [41] | 0.002 | 0.006 | |||
| Fe | 0.700 | [88] | 0.024 | 0.034 | |||
| As | 0.0003 | [36] | 1.5 | [89] | 0.001 | 1.547 | 5.22 × 10−2 |
| Na | NA | NA | 0.235 | ||||
| Ni | 0.020 | [36] | 1.7 | [90] | 0.001 | 0.053 | 1.34 × 10−1 |
| Cr-VI | 0.003 | [36] | 0.50 | [91] | 0.003 | 0.912 | 1.03 × 10−1 |
| Ag | NA | NA | 1.84 × 10−5 | ||||
| Si | NA | NA | 0.0254 | ||||
| Hazard index (HI) due to all metals in seaweeds, HI = ΣHQc = 4.38 | ΣLCR = 2.94 × 10−1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandaker, M.U.; Chijioke, N.O.; Heffny, N.A.B.; Bradley, D.A.; Alsubaie, A.; Sulieman, A.; Faruque, M.R.I.; Sayyed, M.I.; Al-mugren, K.S. Elevated Concentrations of Metal(loids) in Seaweed and the Concomitant Exposure to Humans. Foods 2021, 10, 381. https://doi.org/10.3390/foods10020381
Khandaker MU, Chijioke NO, Heffny NAB, Bradley DA, Alsubaie A, Sulieman A, Faruque MRI, Sayyed MI, Al-mugren KS. Elevated Concentrations of Metal(loids) in Seaweed and the Concomitant Exposure to Humans. Foods. 2021; 10(2):381. https://doi.org/10.3390/foods10020381
Chicago/Turabian StyleKhandaker, Mayeen Uddin, Nwokoma Oliver Chijioke, Nurul’ Adillah Binti Heffny, David A. Bradley, Abdullah Alsubaie, Abdelmoneim Sulieman, Mohammad Rashed I. Faruque, M. I. Sayyed, and K. S. Al-mugren. 2021. "Elevated Concentrations of Metal(loids) in Seaweed and the Concomitant Exposure to Humans" Foods 10, no. 2: 381. https://doi.org/10.3390/foods10020381
APA StyleKhandaker, M. U., Chijioke, N. O., Heffny, N. A. B., Bradley, D. A., Alsubaie, A., Sulieman, A., Faruque, M. R. I., Sayyed, M. I., & Al-mugren, K. S. (2021). Elevated Concentrations of Metal(loids) in Seaweed and the Concomitant Exposure to Humans. Foods, 10(2), 381. https://doi.org/10.3390/foods10020381










