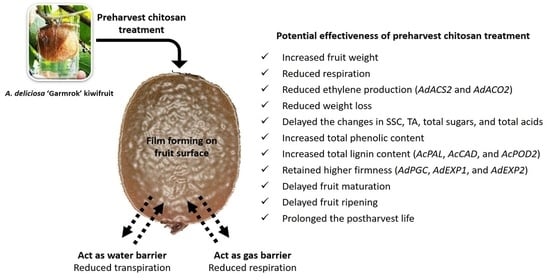
Preharvest Application of Chitosan Improves the Postharvest Life of ‘Garmrok’ Kiwifruit through the Modulation of Genes Related to Ethylene Biosynthesis, Cell Wall Modification and Lignin Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preharvest Chitosan Treatment
2.2. Physicochemical Quality Attributes of Fruit
2.3. RNA Extraction, cDNA Synthesis, and Gene Expression Analysis by Quantitative Real-Time PCR
2.4. Statistical Analysis
3. Results
3.1. Fruit Weight and Size at Harvest
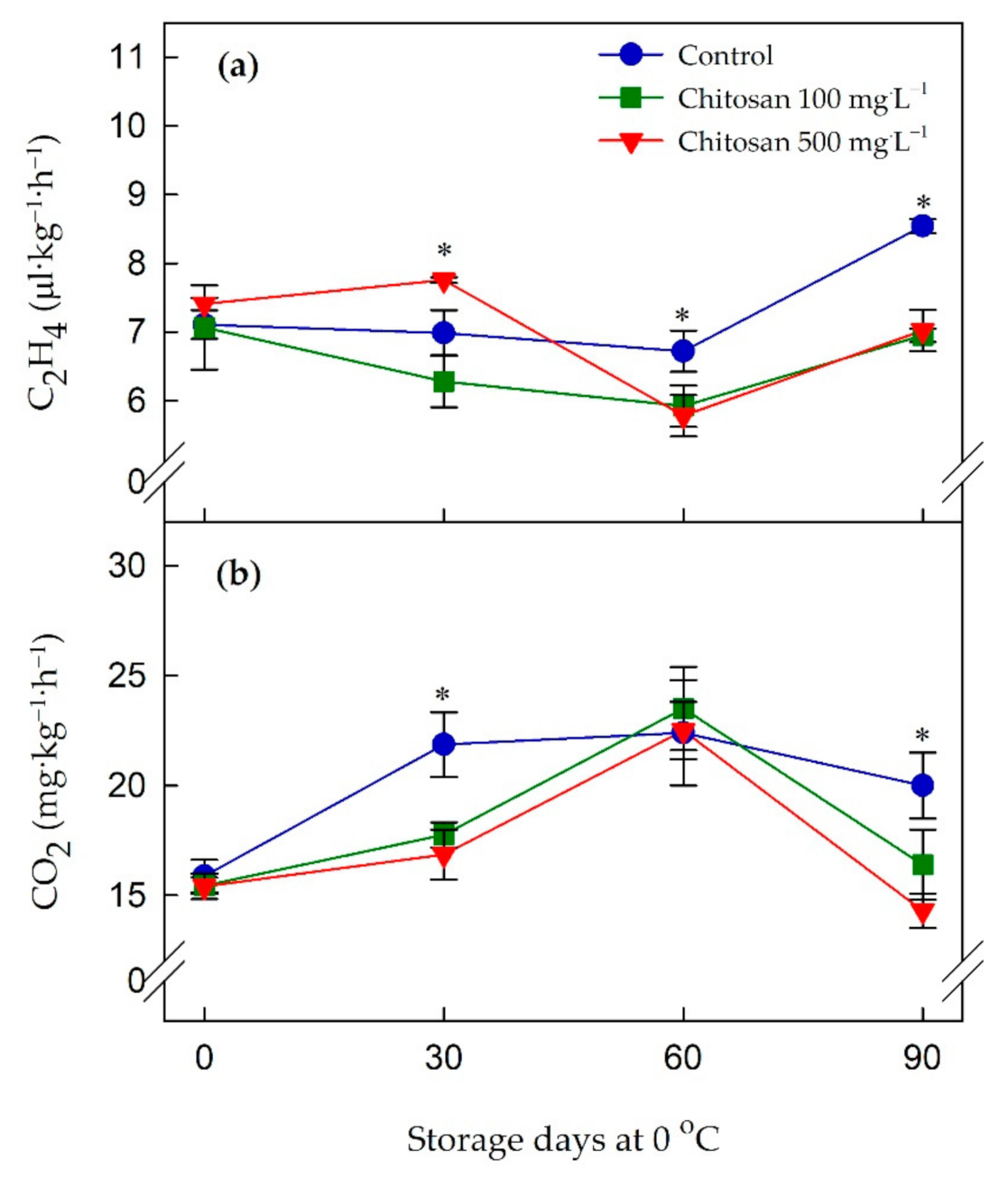
3.2. Ethylene Production (C2H4) and Respiration (CO2) Rates
3.3. Loss of Fruit Weight
3.4. Changes in Soluble Solids Content, Titratable Acidity, Total Sugars, and Total Acids
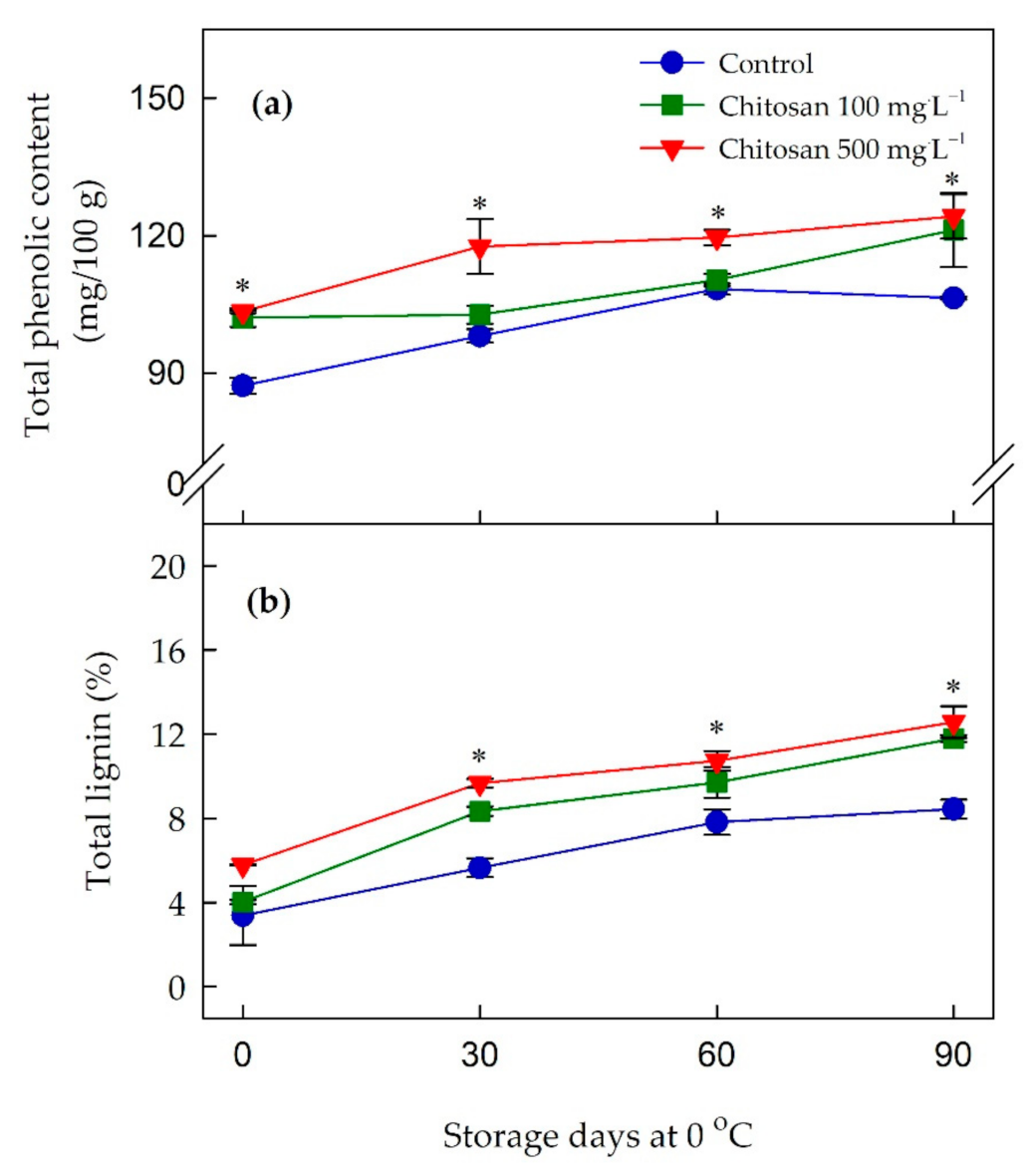
3.5. Changes in Total Phenolic and Lignin Contents
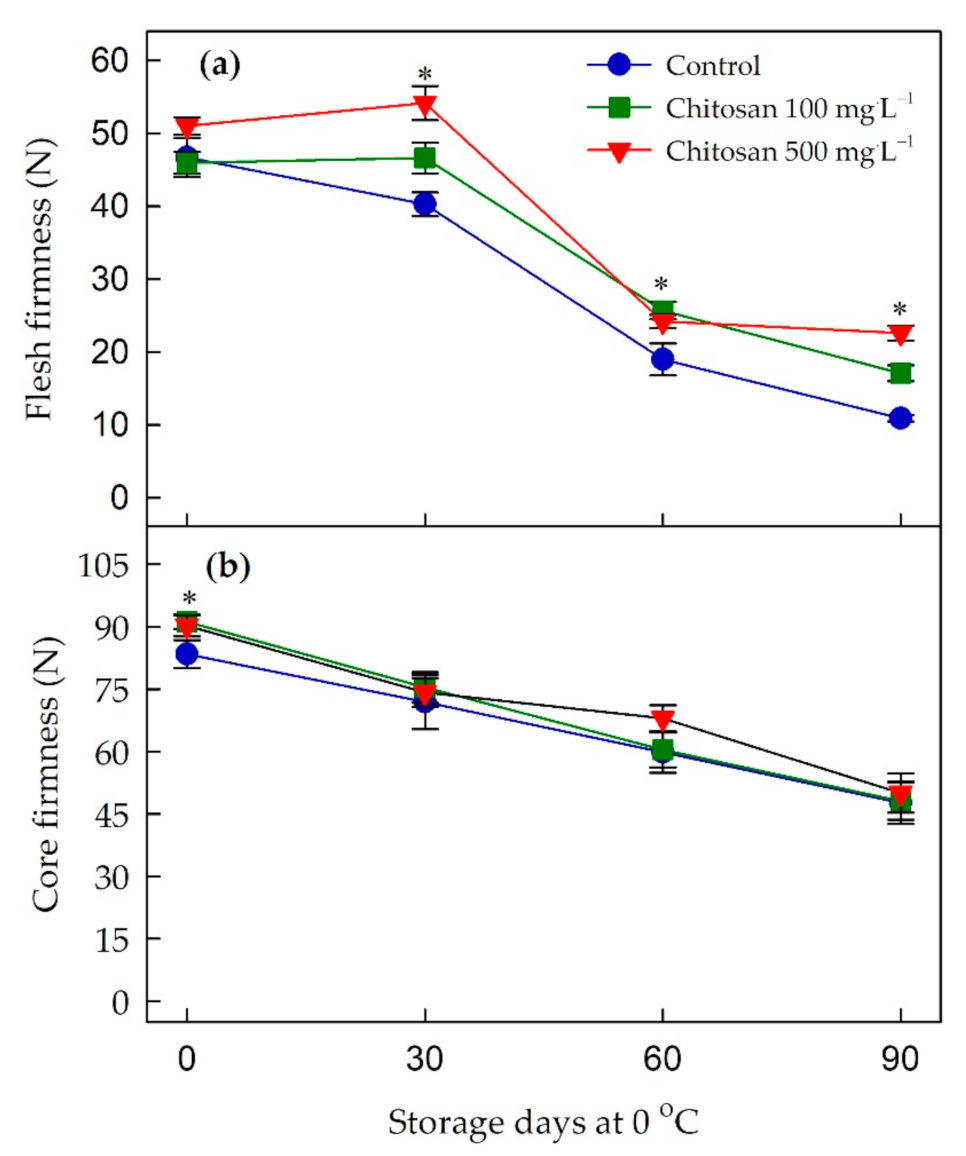
3.6. Changes in Firmness
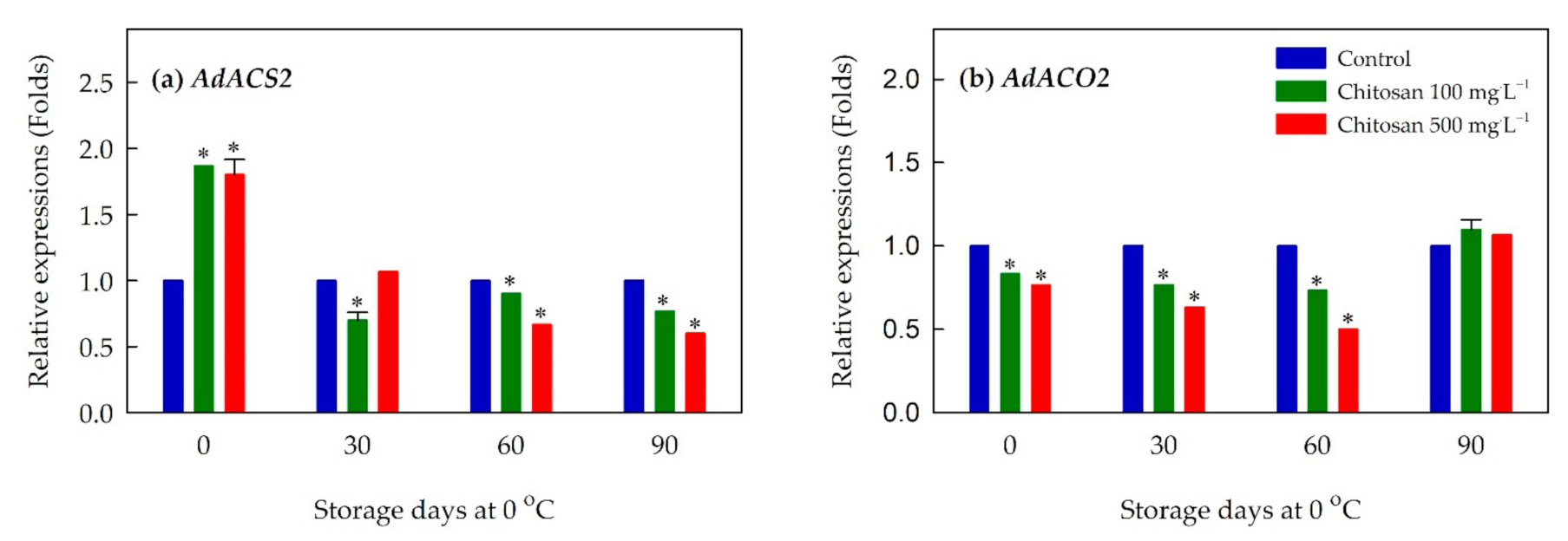
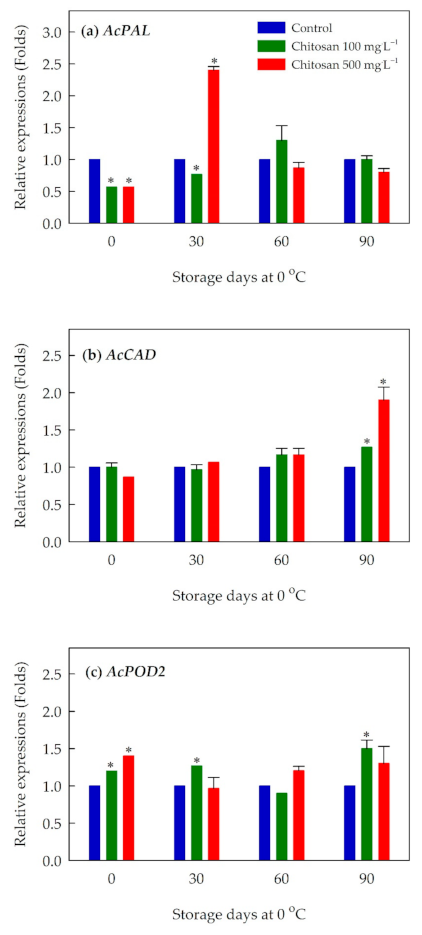
3.7. Expression of Ethylene Biosynthesis-, Cell Wall Modification- and Lignin Metabolism-Related Genes
4. Discussion
4.1. Preharvest Application of Chitosan Increased the Weight of ‘Garmrok’ Kiwifruit at Harvest
4.2. Preharvest Application of Chitosan Is Associated with the Reduced Production of Ethylene and Rate of Respiration during Cold Storage
4.3. Preharvest Application of Chitosan Reduced the Weight Loss of ‘Garmrok’ Kiwifruit during Cold Storage
4.4. Preharvest Application of Chitosan Possesses Maturity- and Ripening-Delaying Effects on ‘Garmrok’ Kiwifruit
4.5. Preharvest Application of Chitosan Might Improve the Antioxidant Activity of ‘Garmrok’ Kiwifruit by Positively Affecting Phenolic Metabolism
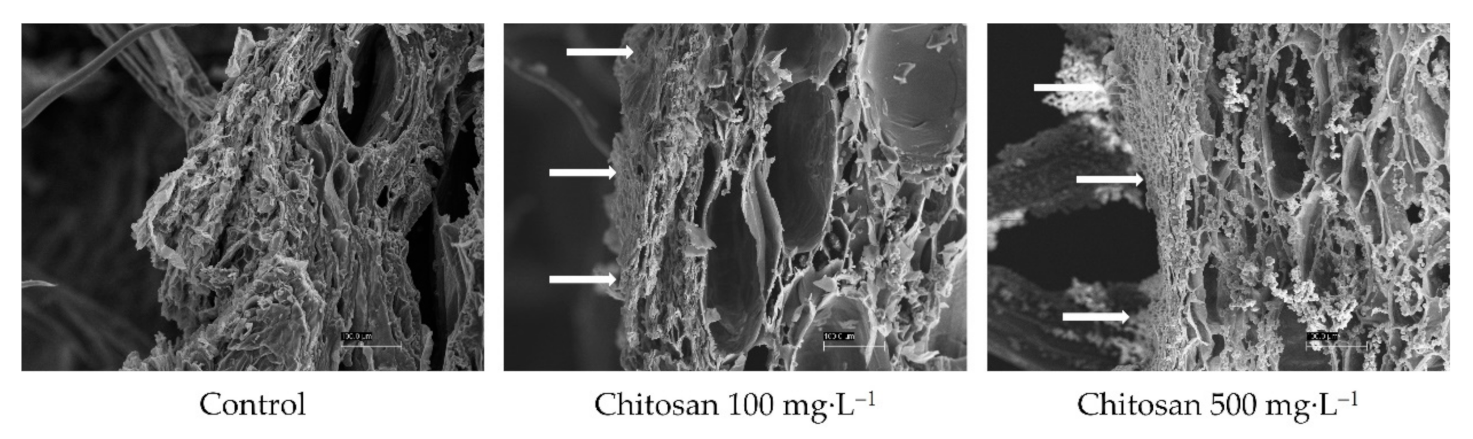
4.6. Preharvest Application of Chitosan Maintained the Firmness of ‘Garmrok’ Kiwifruit during Cold Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed “Hayward” kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Kaya, M.; Česoniene, L.; Daubaras, R.; Leskauskaite, D.; Zabulione, D. Chitosan coating of red kiwifruit (Actinidia melanandra) for extending of the shelf life. Int. J. Biol. Macromol. 2016, 85, 355–360. [Google Scholar] [CrossRef]
- Vivek, K.; Subbarao, K.V. Effect of edible chitosan coating on combined ultrasound and NaOCl treated kiwifruits during refrigerated storage. Int. Food. Res. J. 2018, 25, 101–108. [Google Scholar]
- Kim, J.G.; Beppu, K.; Fukuda, T.; Kataoka, I. Evaluation of fruit characteristics of Shima sarunashi (Actinidia rufa) indigenous to warm regions in Japan. J. Jpn. Soc. Hortic. Sci. 2009, 78, 394–401. [Google Scholar] [CrossRef][Green Version]
- Cha, G.H.; Kumarihami, H.M.P.C.; Kim, H.L.; Kwack, Y.B.; Kim, J.G. Storage temperature influences fruit ripening and changes in organic acids of kiwifruit treated with exogenous ethylene. Hortic. Sci. Technol. 2019, 37, 618–629. [Google Scholar] [CrossRef]
- Huang, Z.; Li, J.; Zhang, J.; Gao, Y.; Hui, G. Physicochemical properties enhancement of Chinese kiwifruit (Actinidia chinensis Planch) via chitosan coating enriched with salicylic acid treatment. J. Food. Meas. Charact. 2017, 11, 184–191. [Google Scholar] [CrossRef]
- Xu, S.; Chen, X.; Sun, D.W. Preservation of kiwifruit coated with an edible film at ambient temperature. J. Food. Eng. 2001, 50, 211–216. [Google Scholar] [CrossRef]
- Kumarihami, H.M.P.C.; Cha, G.H.; Kim, J.G.; Kim, H.L.; Lee, M.; Kwack, Y.B.; Cho, J.G.; Kim, J. Effect of preharvest Ca-Chitosan application on postharvest quality of ‘Garmrok’ kiwifruit during cold storage. Hortic. Sci. Technol. 2020, 38, 239–248. [Google Scholar] [CrossRef]
- Shin, M.H.; Kwack, Y.B.; Kim, Y.H.; Kim, J.G. Storage temperature affects the ripening characteristics of ‘Garmrok’, ‘Hayward’, ‘Goldone’, and ‘Jecy Gold’ kiwifruit treated with exogenous ethylene. Hortic. Sci. Technol. 2018, 36, 730–740. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Combined effect of active coating and MAP to prolong the shelf-life of minimally processed kiwifruit (Actinidia deliciosa cv. Hayward). Food Res. Int. 2011, 44, 1224–1230. [Google Scholar] [CrossRef]
- Xu, S.; Da Xu, L.; Chen, X. Determining optimum edible films for kiwifruits using an analytical hierarchy process. Comput. Oper. Res. 2003, 30, 877–886. [Google Scholar] [CrossRef]
- Li, H.; Suo, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. The effect of 1-methylcyclopropene, methyl jasmonate and methyl salicylate on lignin accumulation and gene expression in postharvest ‘Xuxiang’ kiwifruit during cold storage. Postharvest Biol. Technol. 2017, 124, 107–118. [Google Scholar] [CrossRef]
- Fisk, C.L.; Silver, A.M.; Strik, B.C.; Zhao, Y. Postharvest quality of hardy kiwifruit (Actinidia arguta ’Ananasnaya’) associated with packaging and storage conditions. Postharvest Biol. Technol. 2008, 47, 338–345. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of “Hayward” kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Diab, T.; Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- El-Badawy, H.E.M. Effect of chitosan and calcium chloride spraying on fruits quality of Florida Prince. Res. J. Agric. Biol. Sci. 2012, 8, 272–281. [Google Scholar]
- Fortunati, E.; Giovanale, G.; Luzi, F.; Mazzaglia, A.; Kenny, J.M.; Torre, L.; Balestra, G.M. Effective postharvest preservation of kiwifruit and romaine lettuce with a chitosan hydrochloride coating. Coatings 2017, 7, 196. [Google Scholar] [CrossRef]
- Gayed, A.A.N.A.; Shaarawi, S.A.M.A.; Elkhishen, M.A.; Elsherbini, N.R.M. Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of ‘Early Swelling’ peach during cold storage. Ciênc. Agrotecnol. 2017, 41, 220–231. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Drevinskas, T.; Naujokaitytė, G.; Maruška, A.; Kaya, M.; Sargin, I.; Daubaras, R.; Česonienė, L. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of Actinidia kolomikta (kiwifruit). Carbohydr. Polym. 2017, 173, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Long, Y.H.; Wang, Q.P.; Li, J.H.; Wu, X.M.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Du, J.; Gemma, H.; Iwahori, S. Effects of chitosan coating on the storage of peach, Japanese pear, and kiwifruit. J. Jpn. Soc. Hortic. Sci. 1997, 66, 15–22. [Google Scholar] [CrossRef]
- Zheng, F.; Zheng, W.; Li, L.; Pan, S.; Liu, M.; Zhang, W.; Liu, H.; Zhu, C. Chitosan controls postharvest decay and elicits defense response in kiwifruit. Food Bioprocess Technol. 2017, 10, 1937–1945. [Google Scholar] [CrossRef]
- Kim, J.G.; Park, Y.; Shin, M.H.; Muneer, S.; Lerud, R.; Michelson, C.; Kang, D., II; Min, J.H.; Kumarihami, H.M.P.C. Application of NIR-Spectroscopy to predict the harvesting maturity, fruit ripening and storage ability of Ca-chitosan treated baby kiwifruit. J. Stored Prod. Postharvest Res. 2018, 9, 44–53. [Google Scholar] [CrossRef]
- Kim, J.G.; Beppu, K.; Kataoka, I. Varietal differences in phenolic content and astringency in skin and flesh of hardy kiwifruit resources in Japan. Sci. Hortic. 2009, 120, 551–554. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 analytical procedure—Determination of structural carbohydrates and lignin in Biomass. Lab. Anal. Proced. 2012, 17, 1–15. Available online: http://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 15 December 2019).
- Shin, M. Fruit Ripening Characteristics of Kiwifurit by Exogenous Ethylene Tretment and Storage Temperature. Ph.D. Thesis, Gyeongsang National University, Jinju, Korea, 2018. [Google Scholar]
- Shin, M.H.; Kwack, Y.B.; Kim, Y.H.; Kim, J.G. Fruit ripening and related gene expression in ‘Goldone’ and ‘Jecy Gold’ kiwifruit by exogenous ethylene application. Hortic. Sci. Technol. 2019, 37, 54–64. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Macrae, E.A.; Bowen, J.H.; Stec, M.G.H. Maturation of kiwifruit (Actinidia deliciosa cv Hayward) from two orchards: Differences in composition of the tissue zones. J. Sci. Food Agric. 1989, 47, 401–416. [Google Scholar] [CrossRef]
- Whetten, R.; Sederoff, R. Lignin biosynthesis. Plant Cell 1995, 7, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.G.; Muchero, W. Regulation of lignin biosynthesis and its role in growth-defense tradeoffs. Front. Plant. Sci. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Mahmood, N.; Abbasi, N.A.; Hafiz, I.A.; Ali, I.; Zakia, S. Effect of biostimulants on growth, yield and quality of bell pepper cv. Yolo Wonder. Pak. J. Agric. Sci. 2017, 54, 311–317. [Google Scholar] [CrossRef]
- Almunqedi, B.; Kassem, H.; Almunqedhi, B.M.; Kassem, H.A.; Al-Harbi, A.E.A.R. Effect of preharvest chitosan and/or salicylic acid spray on quality and shelf-life of tomato fruits. Artic. J. Sci. Eng. Res. 2017, 4, 114–122. Available online: https://www.researchgate.net/publication/322138527 (accessed on 25 January 2020).
- Kubo, Y. Ethylene, oxygen, carbon dioxide, and temperature in postharvest physiology. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 14–45. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R. Role of internal atmosphere on fruit ripening and storability—A review. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef]
- Rothan, C.; Nicolas, J. High CO2 levels reduce ethylene production in kiwifruit. Physiol. Plant. 1994, 92, 1–8. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Sfakiotakis, E.M. Ethylene biosynthesis and ripening behaviour of “Hayward” kiwifruit subjected to some controlled atmospheres. Postharvest Biol. Technol. 2002, 26, 167–179. [Google Scholar] [CrossRef]
- De Wild, H.P.J.; Otma, E.C.; Peppelenbos, H.W. Carbon dioxide action on ethylene biosynthesis of preclimacteric and climacteric pear fruit. J. Exp. Bot. 2003, 54, 1537–1544. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef]
- El-wahab, S.M.A.; El-aziz, A.A.F.A.; El-hafeez, A.E.A.A.; Emam, I.A.I. Effect of pre-harvest treatments and different cold storage temperatures on fruit quality of “ Wonderful “ pomegranate. Middle East. J. Agric. 2017, 6, 1057–1077. [Google Scholar]
- Mohamed, M.A.A.; El-khalek, A.F.A.; Elmehrat, H.G. Preharvest applications of potassium silicate, chitosan and calcium chloride to improve mango cv. ‘Zibda’ fruit quality and storability. Egypt J. Hortic. 2017, 44, 17–32. [Google Scholar] [CrossRef]
- Mazaro, S.M.; Deschamps, C.; De Mio, L.L.M.; Biasi, L.A.; De Gouvea, A.; Sautter, C.K. Post harvest behavior of strawberry fruits after preharvest treatment with chitosan and acibenzolar-s-methyl. Rev. Bras. Frutic. 2008, 30, 185–190. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; Fargoni, G.P.; Geerdink, G.M.; Kluge, R.A. Chitosan applications pre- or postharvest prolong raspberry shelf-life quality. Postharvest Biol. Technol. 2014, 91, 72–77. [Google Scholar] [CrossRef]
- Meng, X.; Tian, S. Effects of preharvest application of antagonistic yeast combined with chitosan on decay and quality of harvested table grape fruit. J. Sci. Food. Agric. 2009, 89, 1838–1842. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea quality of strawberry fruit. Postharvest Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Fullerton, C.G.; Prakash, R.; Ninan, A.S.; Atkinson, R.G.; Schaffer, R.J.; Hallett, I.C.; Schröder, R. Fruit from two kiwifruit genotypes with contrasting softening rates show differences in the xyloglucan and pectin domains of the cell wall. Front. Plant Sci. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Schröder, R.; Atkinson, R.G. Kiwifruit cell walls: Towards an understanding of softening? N. Z. J. For. Sci. 2006, 36, 112–129. [Google Scholar]
- Payasi, A.; Mishra, N.N.; Chaves, A.L.S.; Singh, R. Biochemistry of fruit softening: An overview. Physiol. Mol. Biol. Plants 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Wang, Z.Y.; MacRae, E.A.; Wright, M.A.; Bolitho, K.M.; Ross, G.S.; Atkinson, R.G. Polygalacturonase gene expression in kiwifruit: Relationship to fruit softening and ethylene production. Plant Mol. Biol. 2000, 42, 317–328. [Google Scholar] [CrossRef]
- Yang, S.; Xu, C.; Zhang, B.; Li, X.; Chen, K. Involvement of both subgroups A and B of expansin genes in kiwifruit fruit ripening. HortScience 2007, 42, 315–319. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.; Chen, K.S. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]

| Treatment | Fruit Weight (g) | Fruit Size (mm) | ||
|---|---|---|---|---|
| Length | Longitudinal Width | Transverse Width | ||
| Control | 81.49 ± 1.1 | 55.17 ± 0.7 | 55.85 ± 0.7 | 52.06 ± 0.6 * |
| Chitosan 100 mg·L−1 | 86.27 ± 2.4 * | 54.72 ± 1.2 | 54.25 ± 0.5 | 50.28 ± 0.4 |
| Chitosan 500 mg·L−1 | 91.79 ± 2.9 * | 55.17 ± 1.1 | 55.45 ± 0.5 | 51.86 ± 0.6 |
| Treatment | Storage Days at 0 °C | |||
|---|---|---|---|---|
| 0 | 30 | 60 | 90 | |
| Fruit weight loss (%) | ||||
| Control | 0.0±0.0 | 0.9 ± 0.0 | 1.5 ± 0.1 | 2.3 ± 0.1 |
| Chitosan 100 mg·L−1 | 0.0±0.0 | 0.5 ± 0.1 * | 1.0 ± 0.1 * | 1.8 ± 0.1 * |
| Chitosan 500 mg·L−1 | 0.0±0.0 | 0.6 ± 0.1 * | 1.2 ± 0.1 * | 2.2 ± 0.2 |
| Treatment | Storage Days at 0 °C | |||
|---|---|---|---|---|
| 0 | 30 | 60 | 90 | |
| Soluble solids content (SSC, %) | ||||
| Control | 6.2 ± 0.2 | 9.6 ± 0.1 | 11.8 ± 0.2 | 12.7 ± 0.1 |
| Chitosan 100 mg·L−1 | 6.0 ± 0.2 | 9.3 ± 0.2 * | 11.7 ± 0.2 | 11.9 ± 0.1 * |
| Chitosan 500 mg·L−1 | 6.0 ± 0.2 | 9.9 ± 0.2 | 11.4 ± 0.1 | 11.6 ± 0.3 * |
| Titratable acidity (TA, %) | ||||
| Control | 1.7 ± 0.0 | 1.5 ± 0.0 | 1.2 ± 0.1 | 1.1 ± 0.0 |
| Chitosan 100 mg·L−1 | 1.9 ± 0.1 * | 1.5 ± 0.0 | 1.3 ± 0.0 * | 1.0 ± 0.0 |
| Chitosan 500 mg·L−1 | 1.8 ± 0.0 * | 1.5 ± 0.0 | 1.2 ± 0.0 | 0.9 ± 0.0 * |
| Total sugar content (g/100 g) | ||||
| Control | 6.9 ± 0.2 | 9.7 ± 0.4 | 9.2 ± 0.5 | 9.4 ± 0.5 |
| Chitosan 100 mg·L−1 | 6.8 ± 0.2 | 9.2 ± 0.3 | 8.9 ± 0.2 | 9.2 ± 0.3 |
| Chitosan 500 mg·L−1 | 6.7 ± 0.2 | 8.4 ± 0.2 * | 8.5 ± 0.4 | 8.1 ± 0.2 * |
| Total acid content (g/100 g) | ||||
| Control | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.0 |
| Chitosan 100 mg·L−1 | 1.4 ± 0.0* | 1.4 ± 0.0 * | 1.3 ± 0.0 * | 1.4 ± 0.0 * |
| Chitosan 500 mg·L−1 | 1.4 ± 0.0* | 1.3 ± 0.0 | 1.3 ± 0.0 * | 1.3 ± 0.0 |
| Fructose + glucose/sucrose ratio | ||||
| Control | 4.3 ± 0.3 | 2.3 ± 0.2 | 2.5 ± 0.0 | 2.6 ± 0.1 |
| Chitosan 100 mg·L−1 | 3.6 ± 0.2* | 2.1 ± 0.1 | 2.3 ± 0.3 | 3.1 ± 0.7 |
| Chitosan 500 mg·L−1 | 3.4 ± 0.1* | 2.1 ± 0.2 | 2.4 ± 0.0 | 2.6 ± 0.2 |
| Citric acid/quinic acid ratio | ||||
| Control | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.0 |
| Chitosan 100 mg·L−1 | 1.3 ± 0.0 * | 1.2 ± 0.0 | 1.1 ± 0.0 * | 1.1 ± 0.0 |
| Chitosan 500 mg·L−1 | 1.4 ± 0.0 * | 1.1 ± 0.1 | 1.2 ± 0.0 | 0.9 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumarihami, H.M.P.C.; Kim, J.G.; Kim, Y.-H.; Lee, M.; Lee, Y.-S.; Kwack, Y.-B.; Kim, J. Preharvest Application of Chitosan Improves the Postharvest Life of ‘Garmrok’ Kiwifruit through the Modulation of Genes Related to Ethylene Biosynthesis, Cell Wall Modification and Lignin Metabolism. Foods 2021, 10, 373. https://doi.org/10.3390/foods10020373
Kumarihami HMPC, Kim JG, Kim Y-H, Lee M, Lee Y-S, Kwack Y-B, Kim J. Preharvest Application of Chitosan Improves the Postharvest Life of ‘Garmrok’ Kiwifruit through the Modulation of Genes Related to Ethylene Biosynthesis, Cell Wall Modification and Lignin Metabolism. Foods. 2021; 10(2):373. https://doi.org/10.3390/foods10020373
Chicago/Turabian StyleKumarihami, H. M. Prathibhani C., Jin Gook Kim, Yun-Hee Kim, Mockhee Lee, Young-Suk Lee, Yong-Bum Kwack, and Joonyup Kim. 2021. "Preharvest Application of Chitosan Improves the Postharvest Life of ‘Garmrok’ Kiwifruit through the Modulation of Genes Related to Ethylene Biosynthesis, Cell Wall Modification and Lignin Metabolism" Foods 10, no. 2: 373. https://doi.org/10.3390/foods10020373
APA StyleKumarihami, H. M. P. C., Kim, J. G., Kim, Y.-H., Lee, M., Lee, Y.-S., Kwack, Y.-B., & Kim, J. (2021). Preharvest Application of Chitosan Improves the Postharvest Life of ‘Garmrok’ Kiwifruit through the Modulation of Genes Related to Ethylene Biosynthesis, Cell Wall Modification and Lignin Metabolism. Foods, 10(2), 373. https://doi.org/10.3390/foods10020373