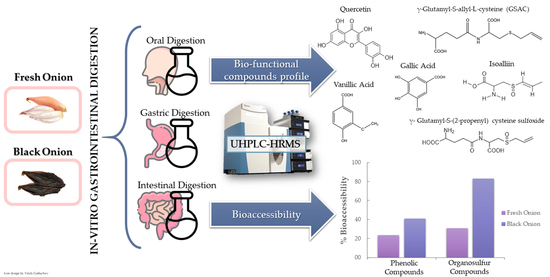
Changes in the Organosulfur and Polyphenol Compound Profiles of Black and Fresh Onion during Simulated Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials and Sample Preparation
2.3. Simulated Gastrointestinal Digestion and Evaluation of Bioaccesibility
2.4. Polyphenol and Organosulfur Compound Extraction and Analysis
2.5. Statistical Analysis
3. Results and Discussion
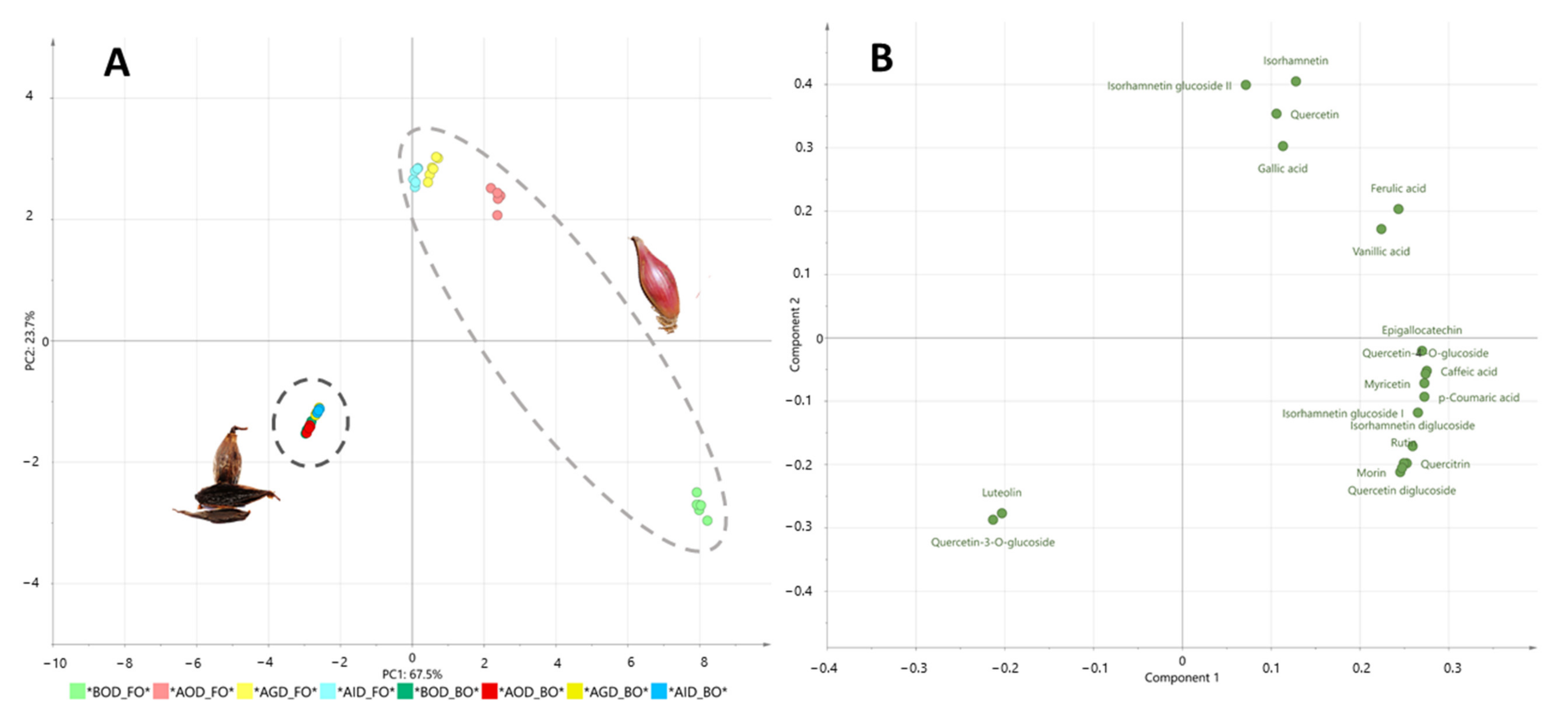
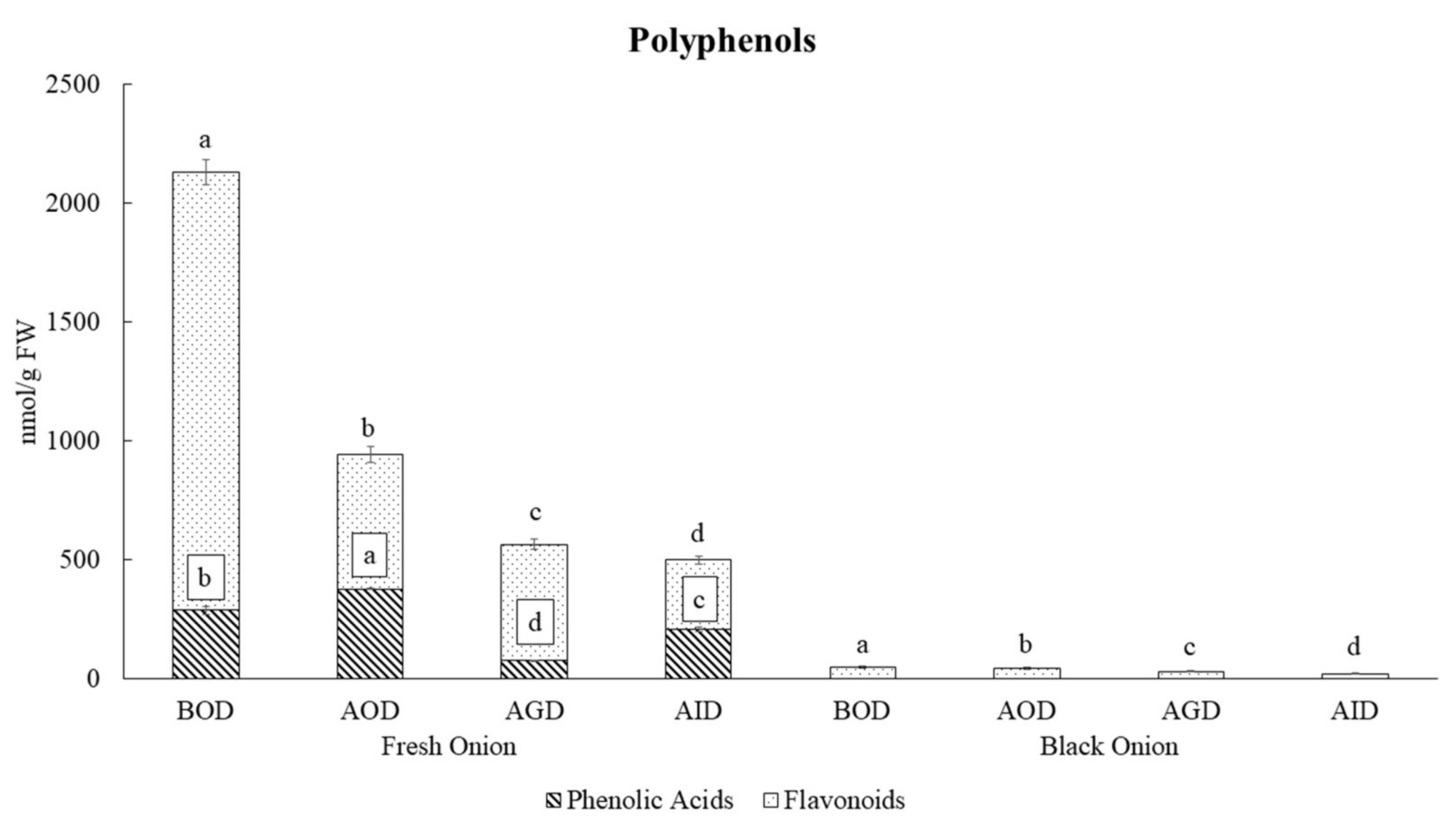
3.1. Changes in Polyphenolic Contents of Fresh and Black Onions after Simulated Gastrointestinal Digestion and Bioaccesibility
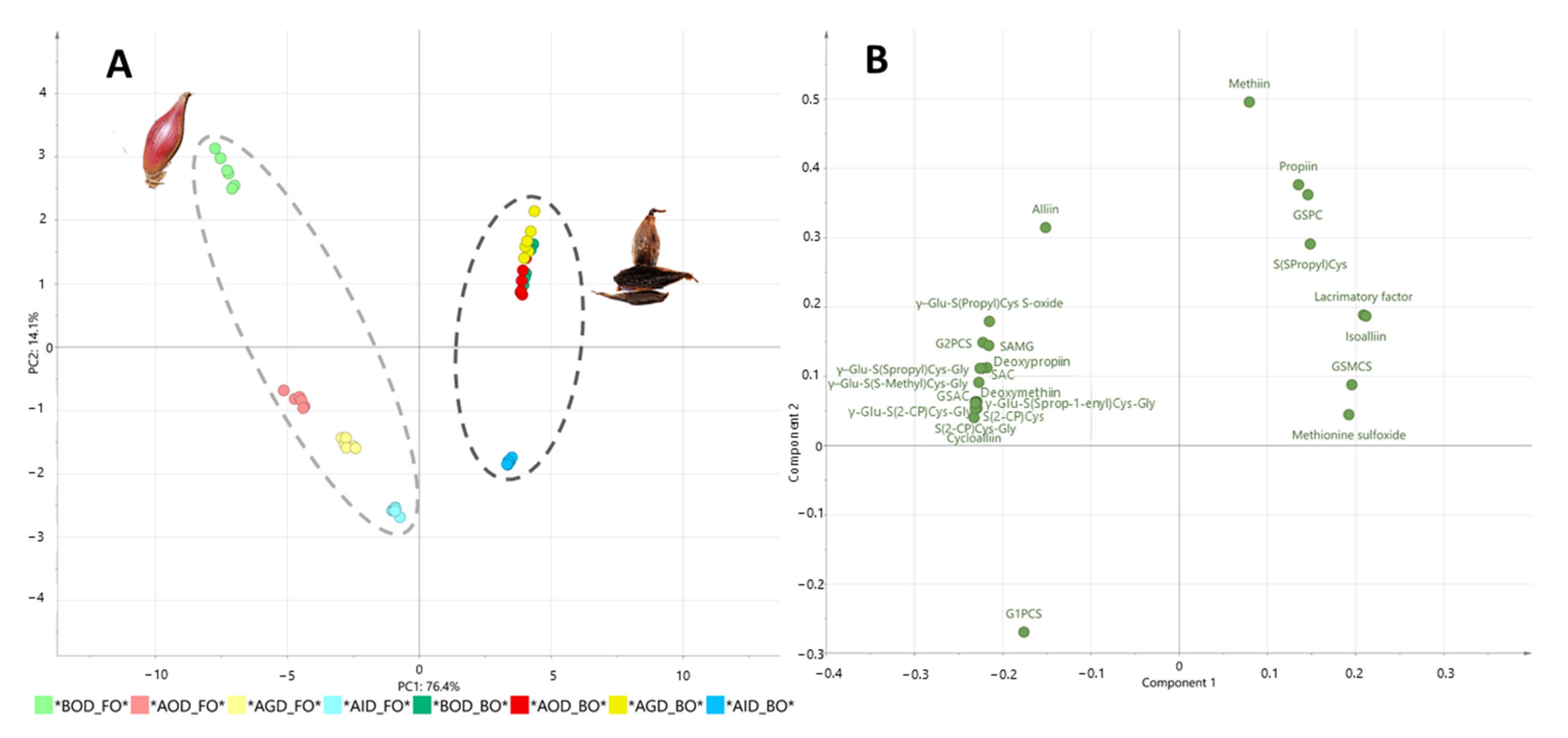
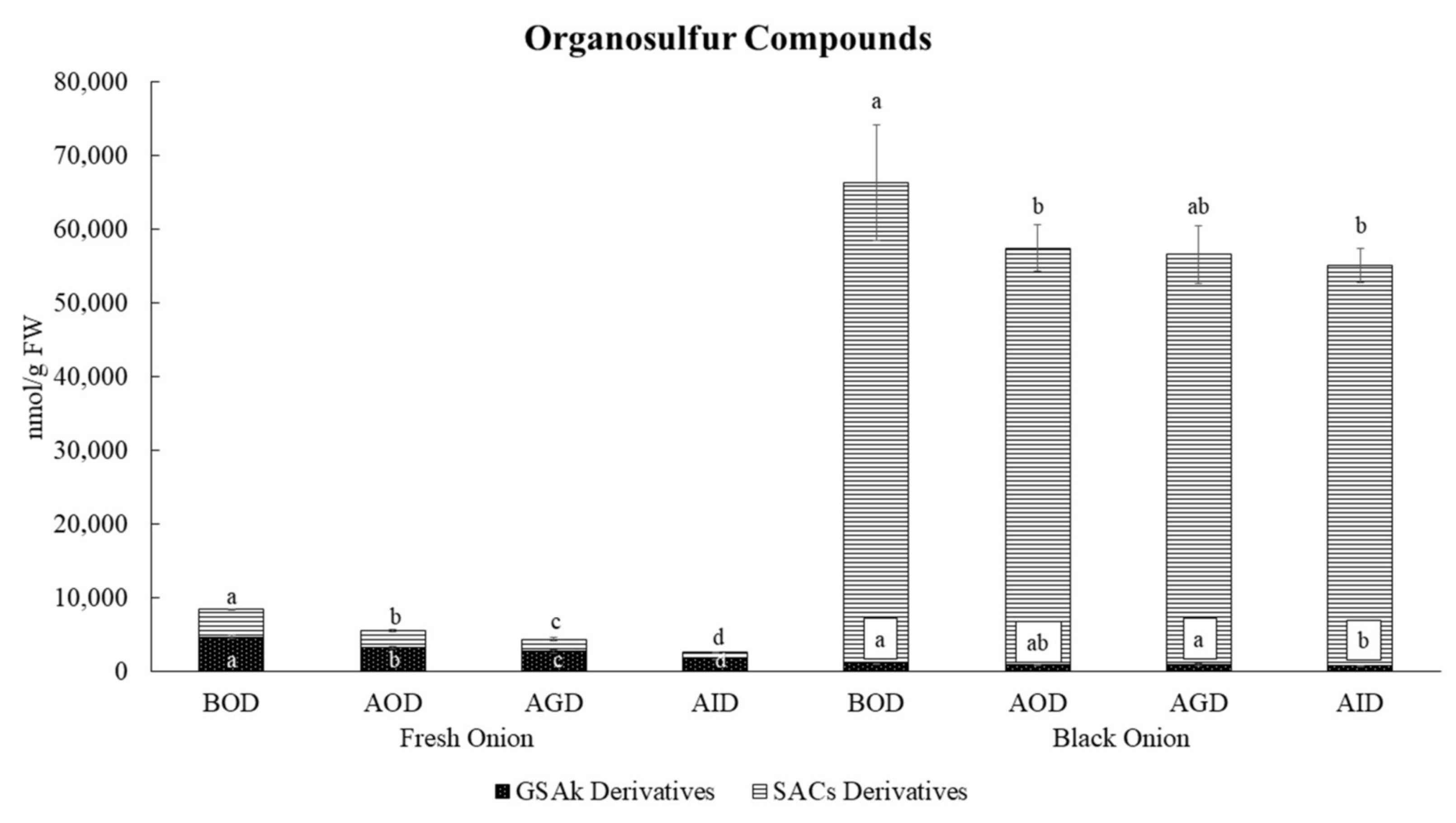
3.2. Changes in Organosulfur Compound Profiles of Fresh and Black Onion after Simulated Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Calixto, F.D.S.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Ortega, R.M. Importance of functional foods in the Mediterranean diet. Public Heal. Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Torija, M.E.; Cruz Matallana, M.; Chalup, N.y. El ajo y la cebolla: De las medicinas antiguas al interés actual. Garlic and onion: From ancient medicine to current interest. Bol. R. Soc. Esp. Hist. Nat. Sec. Biol. 2013, 107, 29–37. [Google Scholar]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and Onions: Their Cancer Prevention Properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid. Based Complement. Altern. Med. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Muñoz-Redondo, J.M.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Moreno-Rojas, J.M. Changes in the antioxidant activity and metabolite profile of three onion varieties during the elaboration of ‘black onion’. Food Chem. 2020, 311, 125958. [Google Scholar] [CrossRef]
- Tran, G.-B.; Nguyen, N.-T.; Nguyen, H.-N.; Pham, H.-H.; Ngo, T.M.T. Chemical composition and antioxidant, anti-inflammatory, and anticancer effects of ethanol extract of black shallot (allium ascalonicum). Pharmacophore 2013, 11, 30–37. [Google Scholar]
- Bisen, P.S.; Emerald, M. Nutritional and Therapeutic Potential of Garlic and Onion (Allium sp.). Curr. Nutr. Food Sci. 2016, 12, 190–199. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Goncharov, N.; Orekhov, A.N.; Voitenko, N.; Ukolov, A.; Jenkins, R.; Avdonin, P. Organosulfur Compounds as Nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 555–568. [Google Scholar]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Lucas-Gonzalez, R.; Fernández-López, J.; Ricci, A.R.; Pérez-Álvarez, J.A.; Sterzo, C.L.; Pérez-Álvarez, J.A. Bioaccessibility of polyphenolic compounds of six quinoa seeds during in vitro gastrointestinal digestion. J. Funct. Foods 2017, 38, 77–88. [Google Scholar] [CrossRef]
- Tsanova-Savova, S.; Ribarova, F.; Petkov, V. Flavonoids content in foods in respect of their bioaccessibility and bioavailability. C. R. Acad. Bulg. Sci. 2016, 69, 1291–1300. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices [Citrus sinensis(L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordoñez-Díaz, J.L.; Moreno-Rojas, R.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M. Bioaccessibility of Bioactive Compounds of ‘Fresh Garlic’ and ‘Black Garlic’ through In Vitro Gastrointestinal Digestion. Foods 2020, 9, 1582. [Google Scholar] [CrossRef]
- Herranz, B.; Fernández-Jalao, I.; Álvarez, M.D.; Quiles, A.; Sánchez-Moreno, C.; Hernando, I.; De Ancos, B. Phenolic compounds, microstructure and viscosity of onion and apple products subjected to in vitro gastrointestinal digestion. Innov. Food Sci. Emerg. Technol. 2019, 51, 114–125. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; De Peña, M.-P. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J. Funct. Foods 2017, 32, 195–207. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 1–1014. [Google Scholar] [CrossRef]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Gomes, T.M.; Toaldo, I.M.; Haas, I.C.D.S.; Burin, V.M.; Caliari, V.; Luna, A.S.; De Gois, J.S.; Bordignon-Luiz, M.T. Differential contribution of grape peel, pulp, and seed to bioaccessibility of micronutrients and major polyphenolic compounds of red and white grapes through simulated human digestion. J. Funct. Foods 2019, 52, 699–708. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordoñez-Díaz, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Böttcher, C.; Krähmer, A.; Stürtz, M.; Widder, S.; Schulz, H. Comprehensive metabolite profiling of onion bulbs (Allium cepa) using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. Metabolomics 2017, 13, 35. [Google Scholar] [CrossRef]
- Friedman, M. Food Browning and Its Prevention: An Overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Kroll, J.; Rawel, H.; Rohn, S. Reactions of Plant Phenolics with Food Proteins and Enzymes under Special Consideration of Covalent Bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Tuszyński, T. Simulation of Phenolic Compounds Transformations and Interactions in an In Vitro Model of the Human Alimentary Tract. Food Sci. Technol. Int. 2009, 15, 235–241. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De Ancos, B. Influence of food matrix and high-pressure processing on onion flavonols and antioxidant activity during gastrointestinal digestion. J. Food Eng. 2017, 213, 60–68. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; Cid, M. Digestibility of (Poly)phenols and Antioxidant Activity in Raw and Cooked Cactus Cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, N.; Chen, M.; Yuan, Y.; He, S.; Wang, Y.; Wu, Q.; Li, L.; Yang, H.; Zeng, Q. Effects ofin vitrodigestion on the composition of flavonoids and antioxidant activities of the lotus leaf at different growth stages. Int. J. Food Sci. Technol. 2018, 53, 1631–1639. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, Á.; Pérez-Vicente, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A. Influence of Food Matrix on the Bioaccessibility of Fruit Polyphenolic Compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Keusgen, M.; Schulz, H.; Glodek, J.; Krest, I.; Krüger, H.; Herchert, N.; Keller, J. Characterization of SomeAlliumHybrids by Aroma Precursors, Aroma Profiles, and Alliinase Activity. J. Agric. Food Chem. 2002, 50, 2884–2890. [Google Scholar] [CrossRef]
- Shen, C.; Xiao, H.; Parkin, K.L. In Vitro Stability and Chemical Reactivity of Thiosulfinates. J. Agric. Food Chem. 2002, 50, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Gladyshev, V.N. The biological significance of methionine sulfoxide stereochemistry. Free. Radic. Biol. Med. 2011, 50, 221–227. [Google Scholar] [CrossRef]
- Lagunas, L.L.M.; Castaigne, F. Effect of temperature cycling on allinase activity in garlic. Food Chem. 2008, 111, 56–60. [Google Scholar] [CrossRef]
| Solution | Concentration | SSFs | SGFs | SIFs |
|---|---|---|---|---|
| Molarity | mL | mL | mL | |
| MagCl2(H2O)6 | 0.15 | 0.313 | 0.250 | 0.688 |
| KCl | 0.50 | 9.438 | 4.313 | 4.250 |
| KH2PO4 | 0.50 | 2.313 | 0.563 | 0.500 |
| (NH4)2CO3 | 0.50 | 0.038 | 0.313 | 0.688 |
| NaHCO3 | 1.00 | 4.250 | 7.813 | 26.563 |
| NaCl | 2.00 | - | 7.375 | 6.000 |
| Distilled Water | - | 233.650 | 229.375 | 211.313 |
| Final Volume | 250 | 250 | 250 |
| Compounds | BOD | AOD | % Recovery | AGD | % Recovery | AID | % Recovery-Bioaccessibility | p-Value |
|---|---|---|---|---|---|---|---|---|
| Fresh Onion | ||||||||
| Phenolic Acids | ||||||||
| p-Coumaric acid | 2.48 a | 0.93 b | 37.5 | 0.36 c | 14.5 | 0.32 c | 12.9 | *** |
| Vanillic acid | 274 b | 365 a | 133.2 | 70 d | 25.5 | 193 c | 70.4 | *** |
| Gallic acid | 3.6 c | 4.9 b | 136.1 | 2.6 d | 72.2 | 11.4 a | 316.7 | *** |
| Caffeic acid | 2.05 a | 1.01 b | 49.3 | 0.43 c | 21.0 | 0.27 d | 13.2 | *** |
| Ferulic acid | 4.8 b | 5.3 a | 110.4 | 2.8 c | 58.3 | 3.0 c | 62.5 | *** |
| Total Phenolic Acids | 287 b | 377 a | 131.4 | 76 d | 26.5 | 208 c | 72.5 | *** |
| Flavonoids | ||||||||
| Morin | 56.46 a | 2.99 b | 5.3 | 1.69 b | 3.0 | 0.97 b | 1.7 | *** |
| Quercetin | 80 c | 81 c | 101.3 | 184 a | 230.0 | 132 b | 165.0 | *** |
| Epigallocatechin | 1.56 a | 0.54 c | 34.6 | 0.71 b | 45.5 | 0.20 d | 12.8 | *** |
| Isorhamnetin | 28 c | 60 b | 214.3 | 81 a | 289.3 | 59 b | 210.7 | *** |
| Myricetin | 211.4 a | 86.1 b | 40.7 | 58.1 c | 27.5 | 11.0 d | 5.2 | *** |
| Quercitrin | 9.1 a | 1.3 b | 14.7 | 0.171 c | 1.9 | 0.077 c | 0.8 | *** |
| Quercetin-4-O-glucoside | 503 a | 247 b | 49.1 | 106 c | 21.1 | 55 d | 10.9 | *** |
| Isorhamnetin glucoside I | 4.06 a | 1.81 b | 44.6 | 0.27 c | 6.7 | 0.13 c | 3.2 | *** |
| Isorhamnetin glucoside II | nd | 15.52 a | - | 13.09 b | - | 4.84 c | - | *** |
| Rutin | 12.11 a | 0.96 b | 7.9 | 0.53 b | 4.4 | 0.58 b | 4.8 | *** |
| Quercetin diglucoside | 846 a | 52 b | 6.1 | 36 b | 4.3 | 23 b | 2.7 | *** |
| Isorhamnetin diglucoside | 90.6 a | 16.8 b | 18.5 | 6.5 c | 7.2 | 4.1 c | 4.5 | *** |
| Total Flavonoids | 1842 a | 567 b | 30.8 | 488 c | 26.5 | 292 d | 15.9 | *** |
| Total | 2129 a | 944 b | 44.3 | 564 c | 26.5 | 500 d | 23.5 | *** |
| Black Onion | ||||||||
| Quercetin | 47 a | 39 ab | 83.3 | 28 bc | 59.6 | 18c | 39.3 | *** |
| Isorhamnetin | 1.32 a | 0.89 b | 67.9 | 0.85 b | 64.7 | 1.08 ab | 81.6 | ** |
| Luteolin | 0.23 a | 0.22 a | 98.4 | 0.12 b | 51.3 | 0.14 b | 61.1 | ** |
| Quercetin diglucoside | 0.20 ab | 0.21 a | 106.5 | 0.15 b | 76.7 | 0.19 ab | 95.4 | * |
| Quercetin-3-O-glucoside | 0.63 a | 0.60 a | 94.6 | 0.57 a | 90.6 | 0.42 b | 66.6 | ** |
| Quercetin-4-O-glucoside | 0.81 a | 0.57 b | 70.2 | 0.81 a | 100.5 | 0.35 c | 43.7 | *** |
| Isorhamnetin-4′-O-glucoside | 0.031 a | 0.031 a | 98.8 | nq | - | nq | - | *** |
| Total | 50 a | 42 b | 83.0 | 30 c | 60.7 | 21 d | 41.1 | *** |
| Compounds | BOD | AOD | % Recovery | AGD | % Recovery | AID | % Recovery-Bioaccessibility | p-Value |
|---|---|---|---|---|---|---|---|---|
| Fresh Onion | ||||||||
| ɣ-Glutamyl-S-alk(en)yl-L-cysteine derivatives (GSAk) | ||||||||
| γ–Glutamyl-S-(2-carboxypropyl) cysteine–glycine | 884 a | 620 b | 70.2 | 499 c | 56.4 | 253 d | 28.6 | *** |
| γ–Glutamyl-S-(S-1-propenyl) cysteine–glycine | 3.09 a | 2.07 b | 67.0 | 1.69 c | 54.7 | 0.94 d | 30.5 | *** |
| γ–Glutamyl-S-(S-methyl) cysteine–glycine | 5.77 a | 4.38 b | 76.0 | 2.39 c | 41.5 | 1.35 d | 23.4 | *** |
| γ–Glutamyl-S-(S-propyl) cysteine–glycine | 17.7 a | 12.9 b | 73.0 | 7.8 c | 43.9 | 2.8 d | 15.9 | *** |
| γ-Glutamyl-S-allyl-L-cysteine (GSAC) | 799 a | 574 b | 71.9 | 437 c | 54.7 | 225 d | 28.1 | *** |
| γ–Glutamyl-S-(propyl) cysteine (GSPC) | 13.78 a | 13.09 a | 95.0 | 0.24 b | 1.8 | nd | 0.0 | *** |
| γ-Glutamyl-S-methyl cysteine sulfoxide (GSMCS) | 15.0 a | 9.3 b | 62.2 | 9.1 b | 60.8 | 7.0 c | 46.4 | *** |
| γ- Glutamyl-S-(2-propenyl) cysteine sulfoxide (G2PCS) | 2615 a | 1908 b | 72.9 | 1566 c | 59.9 | 1102 d | 42.1 | *** |
| γ–Glutamyl-S-(propyl) cysteine sulfoxide | 323 a | 137 b | 42.5 | 94 c | 29.1 | 53 d | 16.5 | *** |
| γ−Glutamyl-S-(1-propenyl)-L-cysteine sulfoxide (G1PCS) | 2.1 c | 4.6 a | 213.7 | 2.9 b | 136.0 | 4.4 a | 206.3 | *** |
| Total GSAk derivatives | 4679 a | 3286b | 70.2 | 2620 c | 56.0 | 1649 d | 35.3 | *** |
| S-Alk(en)-yl-L-cysteine derivatives (SACs) | ||||||||
| S-(2-carboxypropyl) cysteine-glycine | 333 a | 242 b | 72.5 | 198 c | 59.3 | 97 d | 29.1 | *** |
| S-methyl-cysteine (deoxymethiin) | 41.4 a | 33.9 b | 81.9 | 20.7 c | 50.1 | 11.1 d | 26.8 | *** |
| S-Propyl-L-cysteine (deoxypropiin) | 359 a | 165 b | 45.8 | 128 c | 35.6 | 121 c | 33.7 | *** |
| S-Allyl-L-cysteine (SAC) | 343 a | 295 b | 85.9 | 90 c | 26.3 | 68 c | 19.9 | *** |
| S-allylmercaptoglutathione | 0.31 a | 0.19 b | 60.3 | 0.17 b | 55.6 | nd | 0.0 | *** |
| S-(S-propyl) cysteine | 20.9 a | 14.3 b | 68.3 | 8.0 c | 38.5 | 13.9 b | 66.7 | *** |
| S-(2-carboxypropyl) cysteine | 11.2 a | 9.2 b | 82.2 | 5.6 c | 50.4 | 3.3 d | 29.6 | *** |
| Alliin | 330.7 a | nq | - | nq | - | nd | 0.0 | *** |
| Isoalliin | 1348 a | 943 b | 70.0 | 661 c | 49.0 | 379 d | 28.1 | *** |
| Propanethial sulfoxide (lacrimatory factor) | 596a | 418 b | 70.1 | 296 c | 49.6 | 175 d | 29.3 | *** |
| Methyl-L-cysteine sulfoxide (methiin) | 160.2 a | 14.7 b | 9.2 | 15.8 b | 9.9 | 6.3c | 3.9 | *** |
| S-propyl-cysteine sulfoxide (propiin) | 31.5 a | 1.5 b | 4.6 | 0.6b c | 1.9 | nd | 0.0 | *** |
| Cycloalliin | 166 a | 114 b | 68.7 | 93 c | 55.8 | 62 d | 37.6 | *** |
| Methionine sulfoxide | 12.2 a | 6.7 c | 54.6 | 6.3 c | 51.2 | 8.0 b | 65.5 | *** |
| Total SACs derivatives | 3755 a | 2256 b | 60.1 | 1523 c | 40.6 | 945 d | 25.2 | *** |
| Total | 8433 a | 5543 b | 65.8 | 4142 c | 49.2 | 2594 d | 30.8 | *** |
| Black Onion | ||||||||
| ɣ-Glutamyl-S-alk(en)yl-L-cysteine derivatives (GSAk) | ||||||||
| γ-Glutamyl-S-methyl cysteine sulfoxide (GSMCS) | 115 c | 104 c | 90.4 | 138 b | 119.7 | 185 a | 160.3 | *** |
| γ-Glutamyl-S-(2-propenyl) cysteine sulfoxide (G2PCS) | 861 a | 753 a | 87.4 | 800 a | 92.9 | 603 b | 70.0 | *** |
| γ–Glutamyl-S-propyl cysteine sulfoxide (GSPCS) | 62 b | 63 b | 101.8 | 90 a | 144.6 | nd | 0.0 | *** |
| Total GSAk derivatives | 1039 a | 921 ab | 88.6 | 1028 a | 99.0 | 788 b | 75.9 | *** |
| S-Alk(en)-yl-L-cysteine derivatives (SACs) | ||||||||
| S-(S-propyl) cysteine | 178 a | 175 a | 98.5 | 90 b | 77.9 | nd | 0.0 | *** |
| Isoalliin | 53,117 a | 45,859 ab | 86.3 | 47,859 ab | 90.1 | 44,199 b | 83.2 | * |
| Propanethial sulfoxide (Lacrimatory factor) | 10,663 a | 9299 ab | 87.2 | 8176 b | 76.7 | 8356 b | 78.4 | ** |
| S-methyl-cysteine sulfoxide (methiin) | 181 a | 179 a | 99.0 | 208 a | 114.8 | nd | 0.0 | *** |
| S-propyl-cysteine sulfoxide (propiin) | 83 b | 77 c | 92.6 | 151 a | 181.4 | nd | 0.0 | *** |
| Methionine sulfoxide | 1073 b | 923 b | 86.0 | 1006 b | 93.8 | 1809 a | 168.5 | *** |
| Total SACs derivatives | 65,296 a | 56,511 b | 86.5 | 57,489 ab | 88.0 | 54,364 b | 83.3 | * |
| Total | 66,334 a | 57,432 b | 86.6 | 58,517 ab | 88.1 | 55,153 b | 83.0 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Moreno-Rojas, J.M.; Pereira-Caro, G. Changes in the Organosulfur and Polyphenol Compound Profiles of Black and Fresh Onion during Simulated Gastrointestinal Digestion. Foods 2021, 10, 337. https://doi.org/10.3390/foods10020337
Moreno-Ortega A, Ordóñez JL, Moreno-Rojas R, Moreno-Rojas JM, Pereira-Caro G. Changes in the Organosulfur and Polyphenol Compound Profiles of Black and Fresh Onion during Simulated Gastrointestinal Digestion. Foods. 2021; 10(2):337. https://doi.org/10.3390/foods10020337
Chicago/Turabian StyleMoreno-Ortega, Alicia, José Luis Ordóñez, Rafael Moreno-Rojas, José Manuel Moreno-Rojas, and Gema Pereira-Caro. 2021. "Changes in the Organosulfur and Polyphenol Compound Profiles of Black and Fresh Onion during Simulated Gastrointestinal Digestion" Foods 10, no. 2: 337. https://doi.org/10.3390/foods10020337
APA StyleMoreno-Ortega, A., Ordóñez, J. L., Moreno-Rojas, R., Moreno-Rojas, J. M., & Pereira-Caro, G. (2021). Changes in the Organosulfur and Polyphenol Compound Profiles of Black and Fresh Onion during Simulated Gastrointestinal Digestion. Foods, 10(2), 337. https://doi.org/10.3390/foods10020337