Time of Postharvest Ethylene Treatments Affects Phenols, Anthocyanins, and Volatile Compounds of Cesanese Red Wine Grape
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Treatments
- 500 mg L−1 gas ethylene, chamber sealed for 15 h, then opened, air ventilated and sealed again for 21 h to reach the same treatment time as treatment 3;
- 500 mg L−1 ethylene, chamber sealed for 24 h, then opened, air ventilated and sealed again for 12 h to reach the same treatment time as treatment 3;
- 500 mg L−1 ethylene, chamber sealed for 36 h;
- Air treated grapes, chamber sealed for 36 h.
2.3. Quality Analyses
2.3.1. HPLC Detections
2.3.2. Volatiles
2.4. Statistical Analysis
3. Results and Discussion
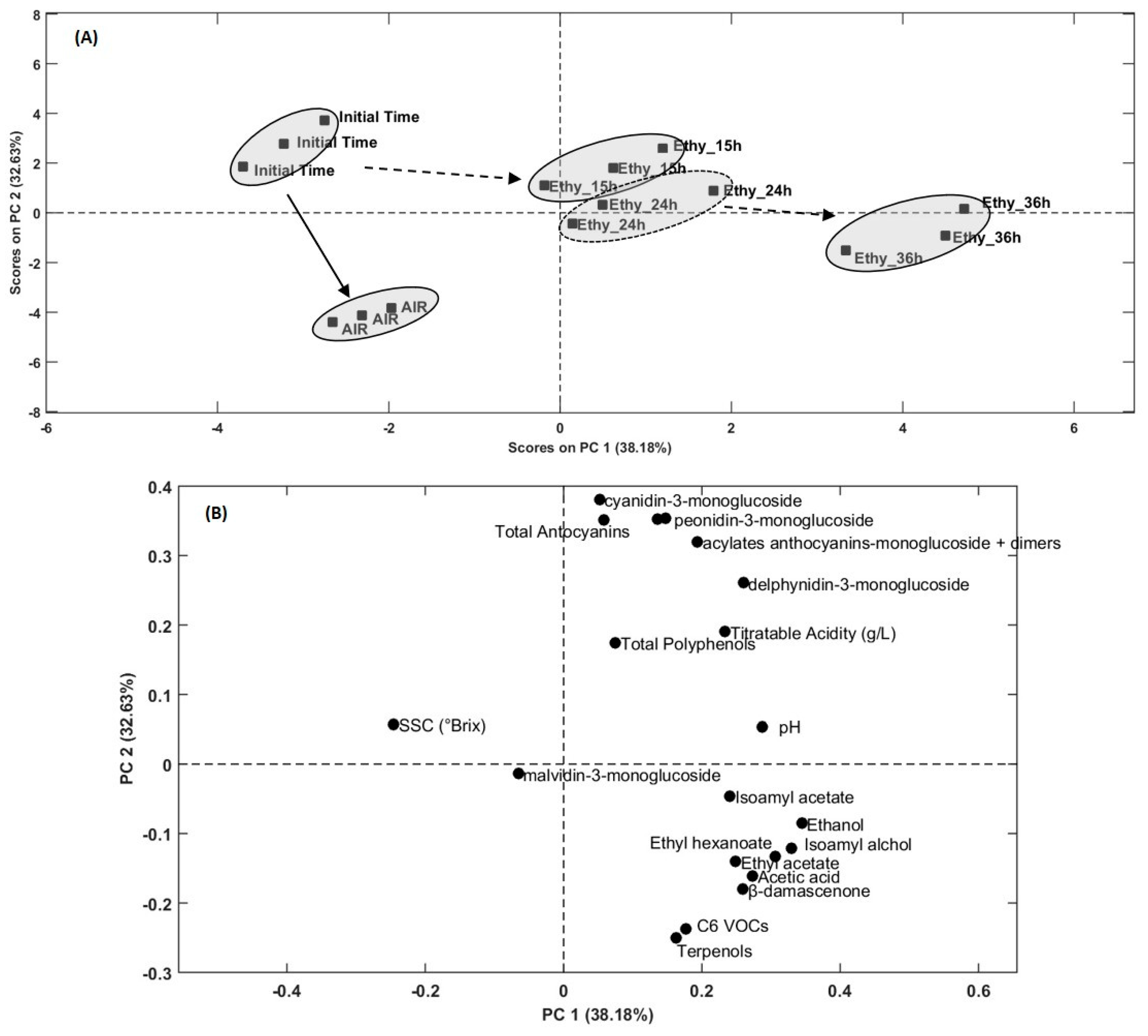
Multivariate Observations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Donnell, P.J.; Calvert, C.; Atzorn, R.; Wasternack, C.; Leyser, H.M.O.; Bowles, D.J. Ethylene as a Signal Mediating the Wound Response of Tomato Plants. Science 1996, 274, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.R.; Davis, R.W. Plant defense genes are regulated by ethylene. Proc. Natl. Acad. Sci. USA 1987, 84, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.R.; Coombe, B.G.; Hawker, J.S. Effects of ethylene and 2-chloroethylphosphonic acid on the ripening of grapes. Plant Physiol. 1970, 45, 620–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weaver, R.J.; Montgomery, R. Effect of ethephon on coloration and maturation of wine grapes. Am. J. Enol. Vitic. 1974, 25, 39–41. [Google Scholar]
- Singh, I.S.; Chundawat, B.S. Effect of ethephon on ripening of’Delight’grapes. HortSci 1978, 13, 251–253. [Google Scholar]
- Powers, J.R.; Shively, E.A.; Nagel, C.W. Effect of ethephon on color of Pinot noir fruit and wine. Am. J. Enol. Vitic. 1980, 31, 203–205. [Google Scholar]
- Cawthon, D.; Morris, J. Uneven ripening of ‘Concord’ grapes: Environmental, cultural and hormonal associations. Hort. Sci. 1981, 102, 147–150. [Google Scholar]
- El-Kereamy, A.; Chervin, C.; Roustan, J.-P.; Cheynier, V.; Souquet, J.-M.; Moutounet, M.; Raynal, J.; Ford, C.; Latché, A.; Pech, J.-C. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003, 119, 175–182. [Google Scholar] [CrossRef]
- Chervin, C.; Tira-umphon, A.; Terrier, N.; Zouine, M.; Severac, D.; Roustan, J.-P. Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol. Plant. 2008, 134, 534–546. [Google Scholar] [CrossRef]
- Li, L.; Lichter, A.; Chalupowicz, D.; Gamrasni, D.; Goldberg, T.; Nerya, O.; Ben-Arie, R.; Porat, R. Effects of the ethylene-action inhibitor 1-methylcyclopropene on postharvest quality of non-climacteric fruit crops. Postharvest Biol. Technol. 2016, 111, 322–329. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.; Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H 2 O 2, and particularly ethylene. Hortic. Res. 2018, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bellincontro, A.; Fardelli, A.; Santis, D.D.; Botondi, R.; Mencarelli, F. Postharvest ethylene and 1-MCP treatments both affect phenols, anthocyanins, and aromatic quality of Aleatico grapes and wine. Aust. J. Grape Wine Res. 2006, 12, 141–149. [Google Scholar] [CrossRef]
- Becatti, E.; Genova, G.; Ranieri, A.; Tonutti, P. Postharvest treatments with ethylene on Vitis vinifera (cv Sangiovese) grapes affect berry metabolism and wine composition. Food Chem. 2014, 159, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Bonghi, C.; Ranieri, A.M.; Tonutti, P. Physiological responses of wine grape berries to postharvest ethylene treatments. Acta Hortic. 2017, 383–390. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Ren, J.; Qi, J.; Zhang, G.; Leng, P. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Botondi, R.; Lodola, L.; Mencarelli, F. Postharvest ethylene treatment affects berry dehydration, polyphenol and anthocyanin content by increasing the activity of cell wall enzymes in Aleatico wine grape. Eur. Food Res. Technol. 2011, 232, 679–685. [Google Scholar] [CrossRef]
- González, R.; González, M.-R.; Martín, P. Abscisic acid and ethephon treatments applied to ‘Verdejo’white grapes affect the quality of wine in different ways. Sci. Agric. 2018, 75, 381–386. [Google Scholar] [CrossRef]
- Di Stefano, R.; Cravero, M.C. Metodi per lo studio dei polifenoli dell’uva. Riv. Vitic. Enol. 1991, 44, 37–45. [Google Scholar]
- Costantini, V.; Bellincontro, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Metabolic changes of Malvasia grapes for wine production during postharvest drying. J. Agric. Food Chem. 2006, 54, 3334–3340. [Google Scholar] [CrossRef]
- Manríquez, D.; El-Sharkawy, I.; Flores, F.B.; El-Yahyaoui, F.; Regad, F.; Bouzayen, M.; Latché, A.; Pech, J.-C. Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics. Plant Mol. Biol. 2006, 61, 675. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, C.; Pradal, M.; El-Kereamy, A.; Torregrosa, L.; Chatelet, P.; Roustan, J.-P.; Chervin, C. Involvement of ethylene signalling in a non-climacteric fruit: New elements regarding the regulation of ADH expression in grapevine. J. Exp. Bot. 2004, 55, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Alpi, A. Plant responses to anaerobiosis. Plant Sci. 1993, 93, 1–17. [Google Scholar] [CrossRef]
- Roufet, M. Etude de la composition lipidique du raisin, Vitis vinifera L: Evolution au cours de la maturation et localisation dans la baie. Vitis 1987, 26, 85–87. [Google Scholar]
- Miele, A.; Bouard, J.; Bertrand, A. Fatty acids from lipid fractions of leaves and different tissues of Cabernet Sauvignon grapes. Am. J. Enol. Vitic. 1993, 44, 180–186. [Google Scholar]
- Mao, L.; Karakurt, Y.; Huber, D.J. Incidence of water-soaking and phospholipid catabolism in ripe watermelon (Citrullus lanatus) fruit: Induction by ethylene and prophylactic effects of 1-methylcyclopropene. Postharvest Biol. Technol. 2004, 33, 1–9. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. The role of ethylene in mango fruit aroma volatiles biosynthesis. J. Hortic. Sci. Biotechnol. 2003, 78, 485–496. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Kader, A.A.; Dandekar, A.M. Apple aroma: Alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci. 2005, 168, 1199–1210. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Manríquez, D.; Flores, F.B.; Regad, F.; Bouzayen, M.; Latche, A.; Pech, J.-C. Functional characterization of a melon alcohol acyl-transferase gene family involved in the biosynthesis of ester volatiles. Identification of the crucial role of a threonine residue for enzyme activity. Plant Mol. Biol. 2005, 59, 345–362. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Waché, Y.; Bosser-DeRatuld, A.; Lhuguenot, J.-C.; Belin, J.-M. Effect of cis/trans isomerism of β-carotene on the ratios of volatile compounds produced during oxidative degradation. J. Agric. Food Chem. 2003, 51, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Mordi, R.C.; Walton, J.C.; Burton, G.W.; Hughes, L.; Keith, I.U.; David, L.A.; Douglas, M.J. Oxidative degradation of β-carotene and β-apo-8′-carotenal. Tetrahedron 1993, 49, 911–928. [Google Scholar] [CrossRef]
- Sollazzo, M.; Baccelloni, S.; D’Onofrio, C.; Bellincontro, A. Combining color chart, colorimetric measurement and chemical compounds for postharvest quality of white wine grapes. J. Sci. Food Agric. 2018, 98, 3532–3541. [Google Scholar] [CrossRef] [PubMed]
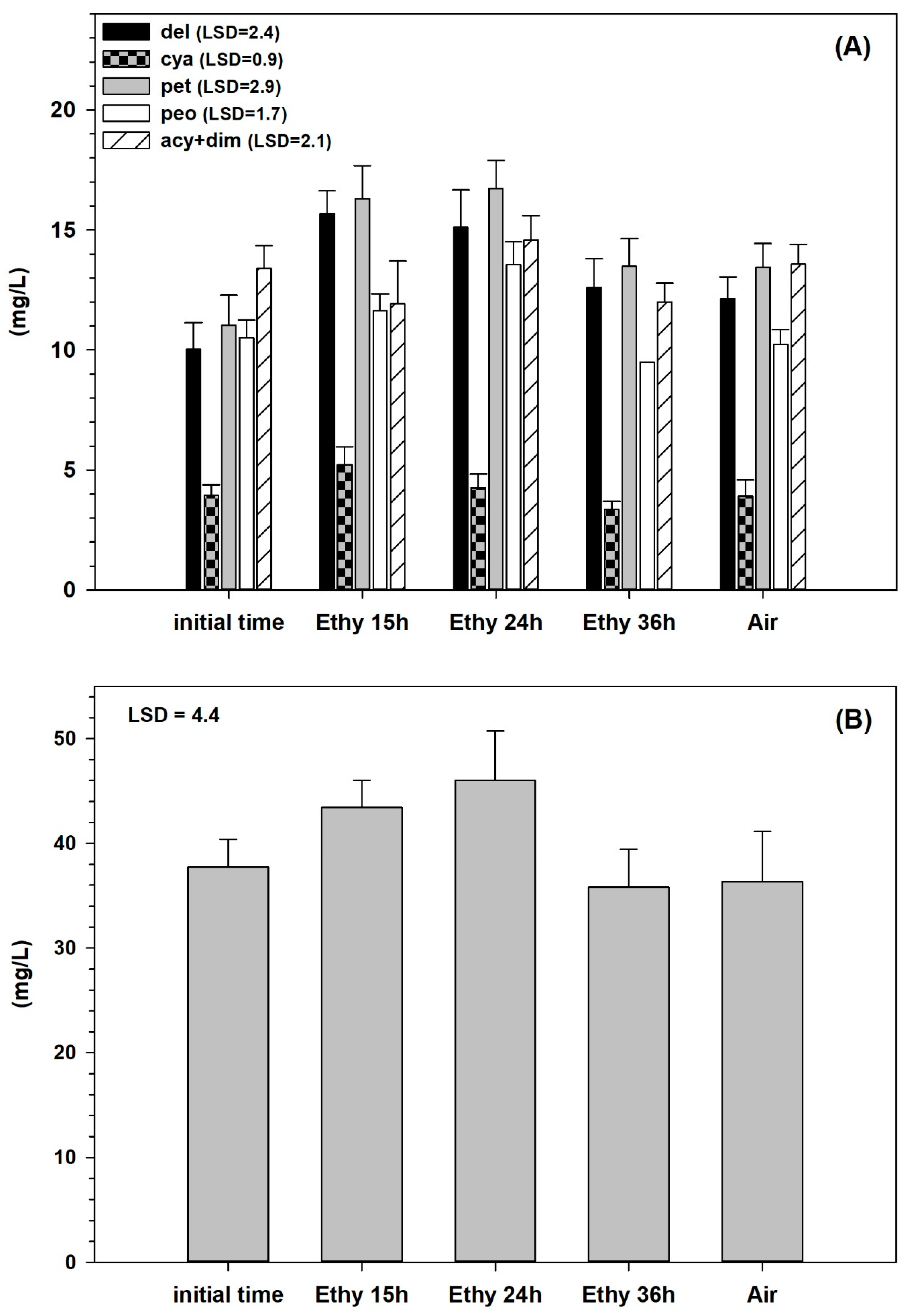
| Air-Treated | Ethylene-Treated (500 mg L−1) | |||
|---|---|---|---|---|
| Time of treatment (h) | 36 | 15 | 24 | 36 |
| Time after treatment (h) | 0 | 21 | 12 | 0 |
| Total time (h) | 36 | 36 | 36 | 36 |
| SSC | pH | TA | Polyphenols | Anthocyanins | A/P Ratio | |
|---|---|---|---|---|---|---|
| (° Brix) | (g L−1) | (mg L−1 of Catechins) | (mg L−1) | (%) | ||
| Initial time | 23.2 ± 0.7 | 3.7 ± 0.04 | 4.5 ± 0.08 | 412 ± 26 | 160 ± 9 | 39 |
| Ethylene 15 h | 21.5 ± 0.5 | 4 ± 0.01 | 4.4 ± 0.03 | 505 ± 33 | 187 ± 12 | 37 |
| Ethylene 24 h | 21.6 ± 0.7 | 4 ± 0.04 | 4.6 ± 0.04 | 488 ± 22 | 150 ± 10 | 31 |
| Ethylene 36 h | 21.4 ± 0.5 | 4.1 ± 0.02 | 4.7 ± 0.03 | 405 ± 31 | 138 ± 8 | 34 |
| Air (control) | 22.2 ± 0.7 | 3.8 ± 0.04 | 4.2 ± 0.06 | 407 ± 28 | 107 ± 9 | 26 |
| LSD (p = 0.05) | 0.9 | 0.3 | 0.4 | 32 | 16 | - |
| Ethylene 15 h | Ethylene 24 h | Ethylene 36 h | |
|---|---|---|---|
| Ethanol | 40 | 107 | 344 |
| Ethyl acetate | 108 | 159 | 1192 |
| Isoamyl acetate | −7 | −17 | 154 |
| Isomyl alcohol | −6 | 0 | 186 |
| Ethyl exanoate | 19 | 190 | 216 |
| Ethyl octanoate | 8 | 13 | 147 |
| Ethyl decanoate | 11 | 26 | 105 |
| C6 volatiles | 7 | 27 | 20 |
| Terpenols | 3 | −12 | −69 |
| β-damascenone | −22 | −11 | −44 |
| PC1 (38.17%) | PC2 (32.63%) | PC3 (12.44%) | PC4 (8.58%) | PC5 (5.59%) | |
|---|---|---|---|---|---|
| Ethanol | 0.95257 | −0.21775 | −0.18708 | 0.051498 | 0.033167 |
| Acetic acid | 0.75478 | −0.41184 | −0.24462 | 0.41223 | 0.14963 |
| Ethyl acetate | 0.68753 | −0.35888 | −0.20985 | 0.26748 | −0.25038 |
| Isoamyl acetate | 0.66541 | −0.11647 | −0.13765 | −0.61637 | 0.032228 |
| Isoamyl alchol | 0.91109 | −0.30992 | −0.1482 | 0.20466 | 0.059631 |
| Ethyl hexanoate | 0.84698 | −0.33996 | −0.095672 | −0.38025 | 0.013219 |
| C6 VOCs | 0.48993 | −0.60472 | 0.44466 | −0.43387 | −0.013936 |
| Terpenols | 0.45121 | −0.63937 | 0.44359 | 0.42655 | 0.01025 |
| β−damascenone | 0.71723 | −0.4603 | 0.4576 | 0.23179 | −0.068807 |
| Delphynidin-3-monoglucoside | 0.71817 | 0.66607 | −0.19051 | 0.046359 | −0.02245 |
| Cyanidin-3-monoglucoside | 0.144 | 0.97288 | 0.089111 | 0.13606 | −0.069622 |
| Petunidin-3-monoglucoside | 0.40832 | 0.90318 | −0.0096921 | 0.040272 | −0.12374 |
| Peonidin-3-monoglucoside | 0.37527 | 0.90096 | −0.021239 | 0.20378 | −0.062239 |
| Acylates anthocyanins-monoglucoside + dimers | 0.53409 | 0.81737 | −0.072474 | 0.19128 | −0.05762 |
| Malvidin-3-monoglucoside | −0.17914 | −0.034189 | 0.88987 | 0.35242 | 0.1997 |
| SSC (°Brix) | −0.67895 | 0.14707 | −0.069865 | 0.066328 | 0.69161 |
| pH | 0.79344 | 0.1364 | 0.36137 | −0.10514 | 0.44036 |
| Titratable Acidity | 0.64479 | 0.48599 | −0.22467 | −0.16816 | 0.50142 |
| Total Polyphenols | 0.20811 | 0.44678 | 0.71218 | −0.46353 | −0.16587 |
| Total Antocyanins | 0.16156 | 0.89742 | 0.40293 | 0.02484 | −0.060997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, D.; Bellincontro, A.; Forniti, R.; Botondi, R. Time of Postharvest Ethylene Treatments Affects Phenols, Anthocyanins, and Volatile Compounds of Cesanese Red Wine Grape. Foods 2021, 10, 322. https://doi.org/10.3390/foods10020322
De Santis D, Bellincontro A, Forniti R, Botondi R. Time of Postharvest Ethylene Treatments Affects Phenols, Anthocyanins, and Volatile Compounds of Cesanese Red Wine Grape. Foods. 2021; 10(2):322. https://doi.org/10.3390/foods10020322
Chicago/Turabian StyleDe Santis, Diana, Andrea Bellincontro, Roberto Forniti, and Rinaldo Botondi. 2021. "Time of Postharvest Ethylene Treatments Affects Phenols, Anthocyanins, and Volatile Compounds of Cesanese Red Wine Grape" Foods 10, no. 2: 322. https://doi.org/10.3390/foods10020322
APA StyleDe Santis, D., Bellincontro, A., Forniti, R., & Botondi, R. (2021). Time of Postharvest Ethylene Treatments Affects Phenols, Anthocyanins, and Volatile Compounds of Cesanese Red Wine Grape. Foods, 10(2), 322. https://doi.org/10.3390/foods10020322







