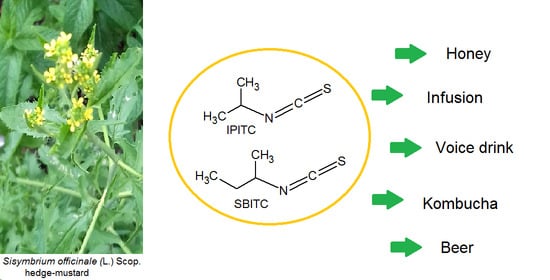
Sisymbrium Officinale (the Singers’ Plant) as an Ingredient: Analysis of Somatosensory Active Volatile Isothiocyanates in Model Food and Drinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Food and Drink Preparations
2.2.1. Honey Enriched with SO Seeds or Flowers
2.2.2. Infusion
2.2.3. Voice Drink
2.2.4. Beer (the “Singers’ Beer”)
2.2.5. Kombucha
2.2.6. Tablet
2.3. Microbiological Analyses
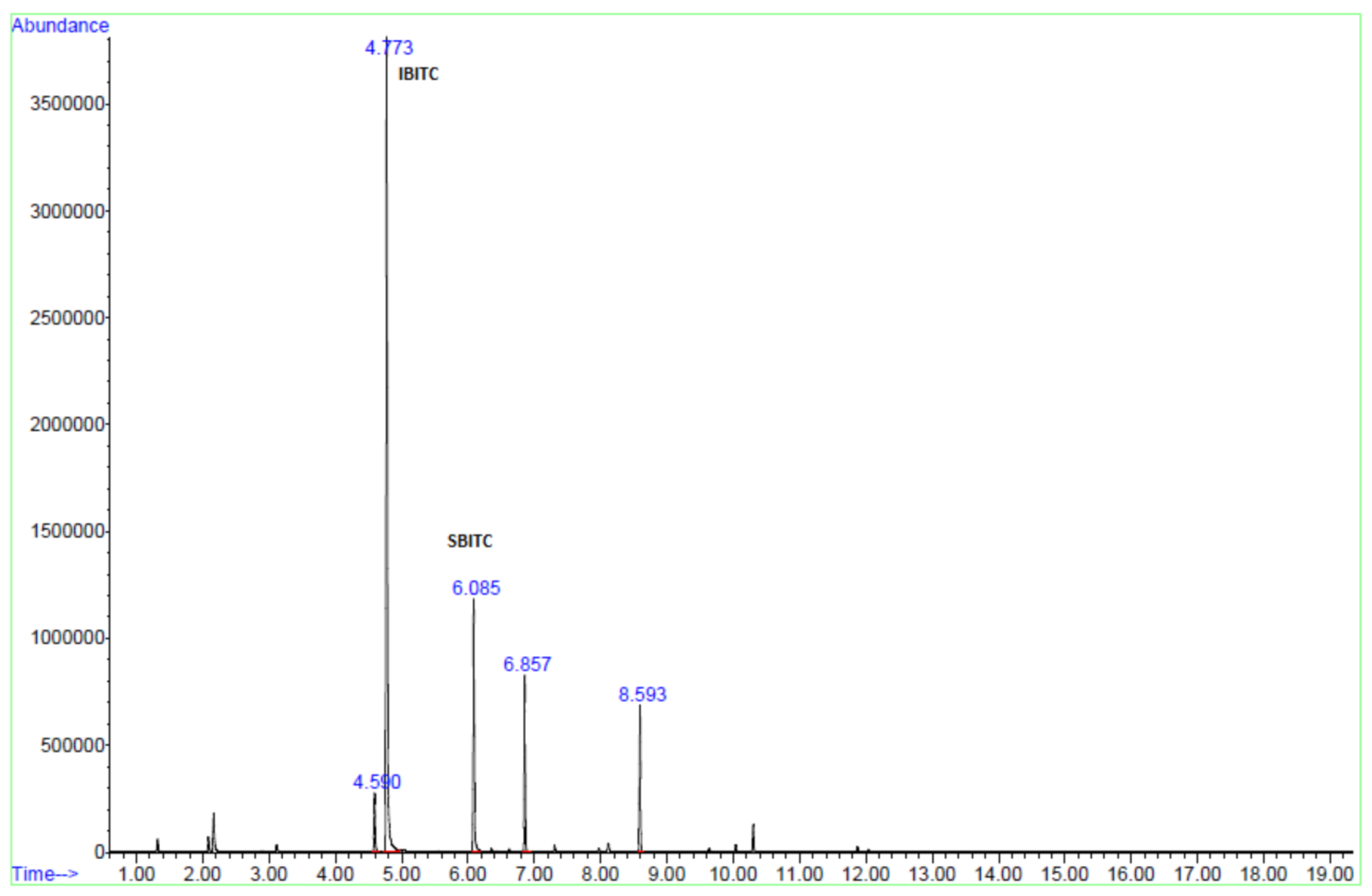
2.4. HS-SPME Coupled with GCMS Analysis
3. Results
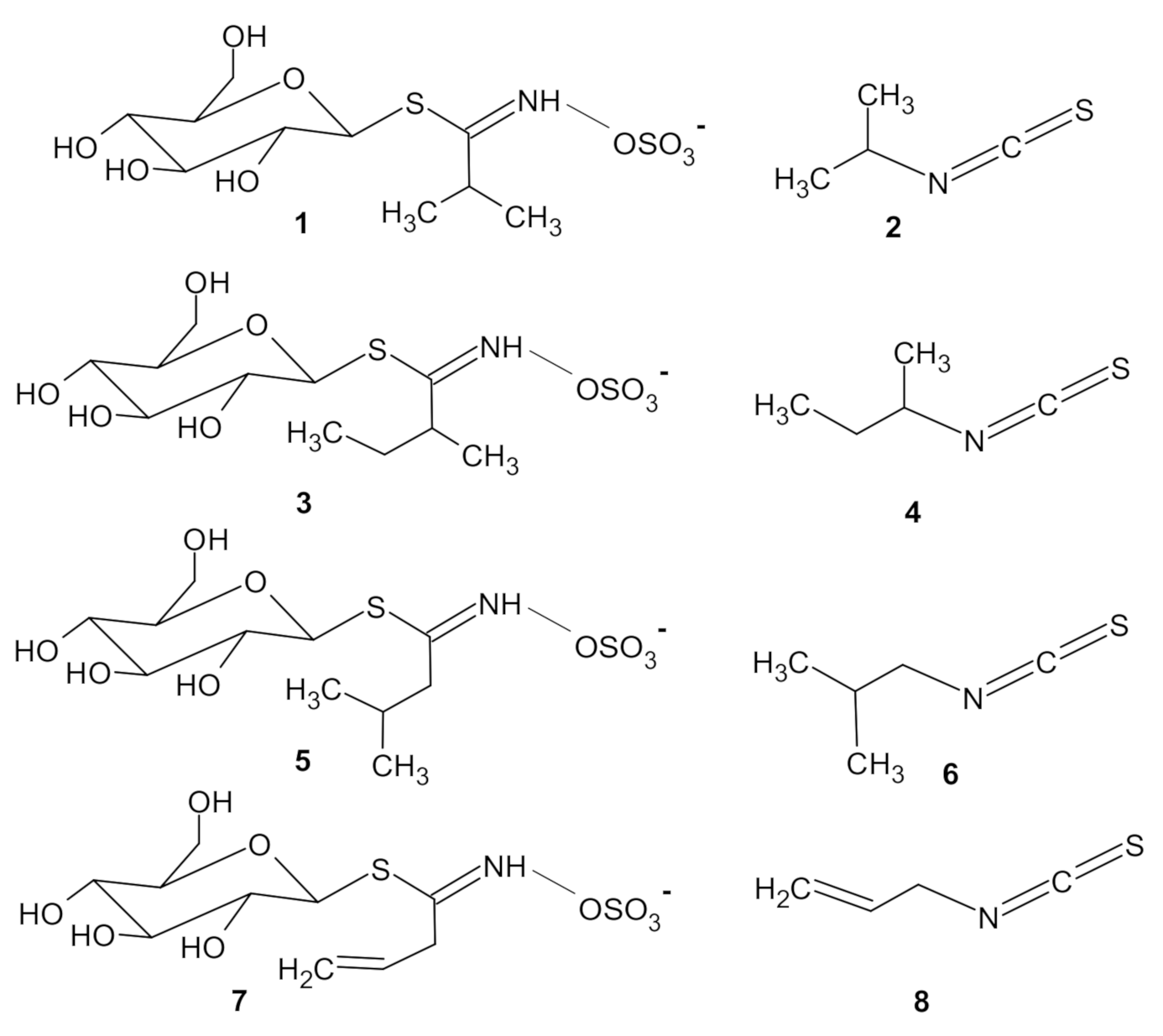
3.1. Identification of ITCs
3.2. Effect of Temperature, Time, and Grinding on ITCs Release
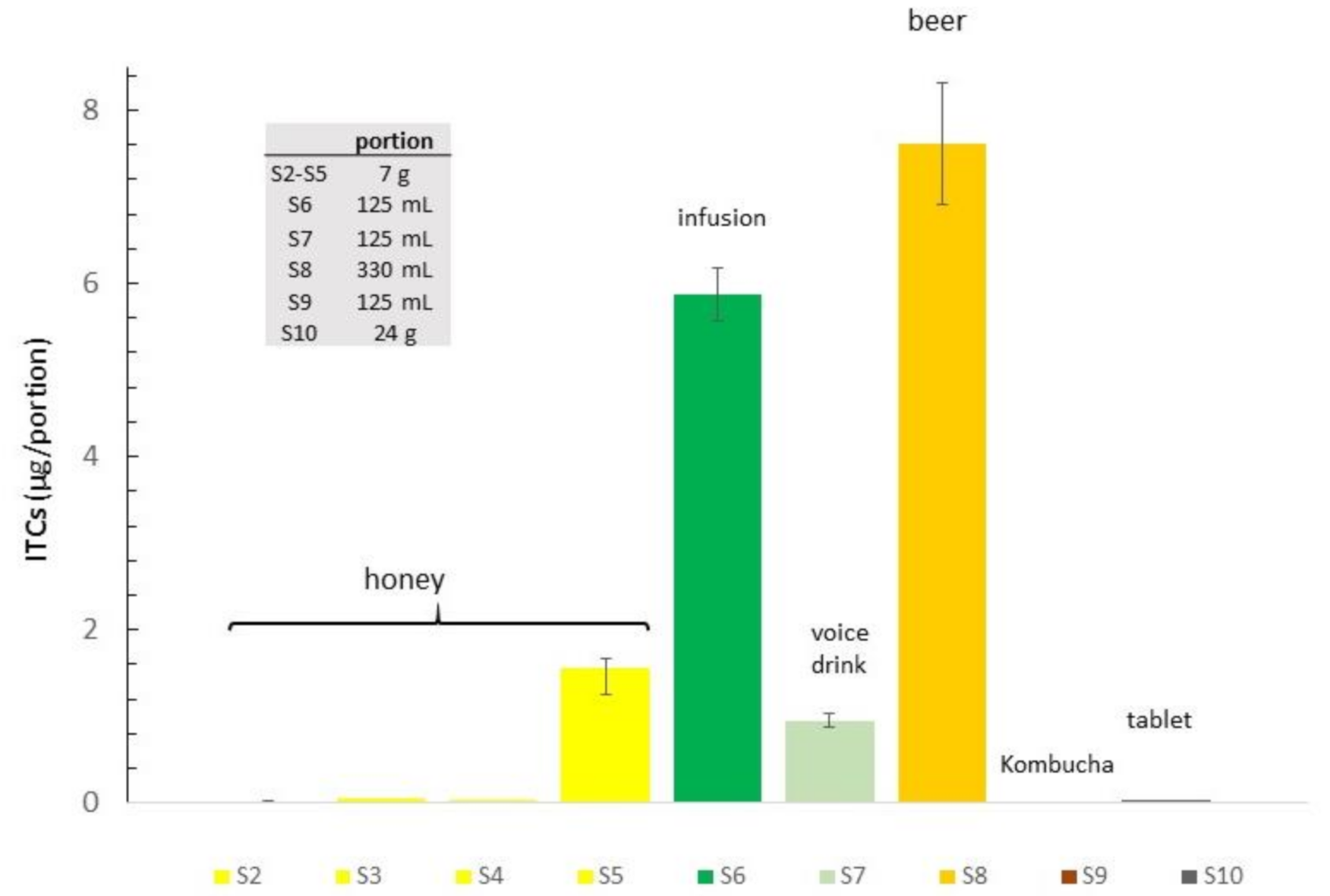
3.3. Food Samples, Choice, and Preparation
3.4. Analysis of Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zorzan, M.; Zucca, P.; Collazuol, D.; Peddio, S.; Rescigno, A.; Pezzani, R. Sisymbrium officinale, the plant of singers: A review of its properties and uses. Planta Med. 2020, 86, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. Hedge mustard- Sisymbrium officinale. In PDR for Herbal Medicines, 3rd ed.; Thompson: Stamford, CT, USA, 2004; pp. 426–427. ISBN 1-56363-512-7. [Google Scholar]
- Leclerc, H. Précis de Phytothérapie; Masson Editions: Paris, France, 1983; pp. 252–253. ISBN 2225858705. [Google Scholar]
- Biagi, M. Phytotherapy in Arts Medicine. In Proceedings of the International Conference CoMeT, Milano, Italy, 4–6 July 2016; pp. 17–19. [Google Scholar]
- Blažević, I.; Radonić, A.; Mastelić, J.; Zekić, M.; Skočibušić, M.; Maravić, A. Hedge mustard (Sisymbrium officinale): Chemical diversity of volatiles and their antimicrobial activity. Chem. Biodivers. 2010, 7, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Vitalone, A.; Nicoletti, M.; Mazzanti, G. Antimutagenic thiocompounds from Sisymbrium officinale. J. Nat. Prod. 2012, 75, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Calcinoni, O. Sisymbrium “Singers’ Plant” Efficacy in Reducing Perceived Vocal Tract Disability. J. Otolaryngol. ENT Res. 2017, 8, 00243. [Google Scholar] [CrossRef]
- Carnat, A.; Fraisse, D.; Carnat, A.P.; Groubert, A.; Lamaison, J.L. Normalisation de l’erysimum, Sisymbrium officinale L. Ann. Pharm. Fr. 1998, 56, 36–39. [Google Scholar]
- Daxenbichler, M.E.; Spencer, G.F.; Carlson, D.G.; Rose, G.B.; Brinker, A.M.; Powell, R.G. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry 1991, 30, 2623–2638. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Deighton, N.; Birch, A.N.E.; Patrian, B.; Baur, R.; Stadler, E. Identification of glucosinolates on the leaf surface of plants from the Cruciferae and other closely related species. Phytochemistry 2001, 57, 693–700. [Google Scholar] [CrossRef]
- Dauvergne, X.; Cérantola, S.; Salaüna, S.; Magné, C.; Kervarec, N.; Bessières, M.; Deslandes, E. General occurrence of the glucosinolate glucocochlearin within the Cochlearia genus. Carbohydr. Res. 2006, 341, 2166–2169. [Google Scholar] [CrossRef]
- Toledo-Martín, E.M.; Font, R.; Obregón-Cano, S.; De Haro-Bailón, A.; Villatoro-Pulido, M.; Del Río-Celestino, M. Rapid and cost-effective quantification of glucosinolates and total phenolic content in rocket leaves by visible/near-infrared spectroscopy. Molecules 2017, 22, 851. [Google Scholar] [CrossRef]
- Borgonovo, G.; Zimbaldi, N.; Guarise, M.; Bedussi, F.; Winninng, M.; Vennegeerts, T.; Bassoli, A. Glucosinolates in Sisymbrium officinale (L.) Scop.: Comparative analysis in cultivated and wild plants and in vitro assays with T2Rs bitter taste receptors. Molecules 2019, 24, 4572. [Google Scholar] [CrossRef]
- Bassoli, A.; Borgonovo, G.; Scaglioni, L. Cucina con L’erisimo. 2018. Available online: http://www.erisimo-a-milano.it/2018/11/in-cucina-con-lerisimo/ (accessed on 21 December 2020).
- Calcinoni, O.; Borgonovo, G.; Cassanelli, A.; Banfi, E.; Bassoli, A. Herbs for Voice Database: Developing a rational approach to the study of herbal remedies used in voice care. J. Voice 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; Zimbaldi, N.; Guarise, M.; De Nisi, P.; De Petrocellis, L.; Schiano-Moriello, A.; Bassoli, A. Isothiocyanates and glucosinolates from Sisymbrium officinale (L.) Scop. (the “singers’plant”): Isolation and in vitro assays on the somatosensory and pain receptor TRPA1 channel. Molecules 2019, 24, 949. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of cooking methods on glucosinolates and isothiocyanates content in novel cruciferous foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Guarise, M.; Borgonovo, G.; Bassoli, A.; Ferrante, A. Evaluation of two ecotypes of hedge mustard (Sisymbrium officinale (L.) Scop.) as a potential leafy vegetable. Horticulturae 2019, 5, 13. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Vigentini, I.; De Lorenzis, G.; Picozzi, C.; Imazio, S.; Merico, A.; Galafassi, S.; Piškur, J.; Foschino, R. Intraspecific variations of the Intron Splice Site in Dekkera/Brettanomyces bruxellensis genome studied by capillary electrophoresis separation. Int. J. Food Microbiol. 2012, 157, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wilmotte, A.; Van der Auwera, G.; De Wachter, R. Structure of the 16S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (Mastigocladus laminosus HTF) strain PCC7518 and phylogenetic analysis. FEBS Lett. 1993, 317, 96–100. [Google Scholar] [CrossRef]
- Spirek, M.; Yang, J.; Groth, C.; Petersen, R.F.; Langkiær, R.B.; Naumova, E.S.; Sulo, P.; Naumov, G.I.; Piškur, J. High-rate evolution of Saccharomyces sensu lato chromosomes. FEMS Yeast Res. 2003, 3, 363–373. [Google Scholar] [PubMed]
- Jirovetz, L.; Smith, L.; Buchbauer, L. Aroma compound Analysis of Eruca sativa (Brassicaceae) SPME Headspace leaf samples using GC, GC−MS, and olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef]
- Rohloff, J.; Bones, A.M. Volatile profiling of Arabidopsis thaliana—Putative olfactory compounds in plant communications. Phytochemistry 2005, 66, 1941–1955. [Google Scholar] [CrossRef]
- Raffo, A.; Masci, M.; Moneta, E.; Nicoli, S.; Sánchez del Pulgar, J.; Paoletti, F. Characterization of volatiles and identification of odor-active compounds of rocket leaves. Food Chem. 2018, 240, 1161–1170. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Van Boekel, M.A.J.S.; Dekker, M. Effect of water content and temperature on inactivation kinetics of myrosinase in broccoli (Brassica oleracea var. italica). Food Chem. 2014, 163, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and health: A review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Microorganisms in Foods 2. In Sampling for Microbiological Analysis: Principles and Specific Applications. Available online: https://seafood.oregonstate.edu/sites/agscid7/files/snic/sampling-for-microbiological-analysis-principles-and-specific-applications-icmsf.pdf (accessed on 21 December 2020).
- European Commission. Commission Recommendation (EC) No 24/2004 of 19 December 2003 Concerning a Coordinated Programme for the Official Control of Food Stuffs for 2004. 2004. Available online: https://eur-lex.europa.eu/eli/reco/2004/24/oj (accessed on 21 December 2020).
- Gil, V.; MacLeod, A.J. The effects of pH on glucosinolate degradation by a thioglucoside glucohydrolase preparation. Phytochemistry 1980, 19, 2547–2551. [Google Scholar] [CrossRef]
- Reva, O.N.; Zaets, I.E.; Ovcharenko, L.P.; Kukharenko, O.E.; Shpylova, S.P.; Podolich, O.V.; Kozyrovska, N.O. Metabarcoding of the Kombucha microbial community grown in different microenvironments. AMB Express 2015, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Nilius, B.; Appendino, G.; Owsianik, G. The Transient Receptor Potential Channel TRPA1: From gene to pathophysiology. Pflugers Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef]
- González-Ramírez, R.; Chen, Y.; Liedtke, W.B.; Morales-Lázaro, S.L. TRP channels and pain. In Neurobiology of TRP Channels, 2nd ed.; Emir, T.L.R., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Nociceptive TRP channels: Sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals 2016, 9, 72. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in Food. In Glucosinolates; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2016; pp. 1–46. [Google Scholar] [CrossRef]
| Sample | Grinding (Yes/No) | T (°C) | Time (min) | Total ITCs (µg/g) |
|---|---|---|---|---|
| S1/1 | no | 37 | 10 | 0.002 |
| S1/2 | yes | 37 | 10 | 0.804 |
| S1/3 | yes | 80 | 10 | 0.126 |
| S1/4 | no | 80 | 5 | 0.140 |
| S1/5 | no | 80 | 10 | 0.150 |
| Entry | Samples 1 | 2 (µg/kg) | 4 (µg/kg) | Total ITCs (2 + 4) (µg/kg) | Portion | Total ITCs (2 + 4) (µg/Portion) |
|---|---|---|---|---|---|---|
| S2 | Honey FW | 3.10 ± 0.63 | 0.25 ± 0.01 | 3.51 | 7 g | 0.02 |
| S3 | Honey FG | 6.11 ± 0.65 | 0.70 ± 0.09 | 6.71 | 7 g | 0.05 |
| S4 | Honey SW | 3.98 ± 1.13 | 0.30 ± 0.18 | 4.01 | 7 g | 0.03 |
| S5 | Honey SG | 204.06 ± 17.90 | 18.28 ± 3.50 | 222.34 | 7 g | 1.55 |
| S6 | Infusion 2 | 38.56 ± 8.51 | 8.29 ± 1.00 | 46.84 | 125 mL | 5.85 |
| S7 | Voice drink | 4.55 ± 1.13 | 3.06 ± 1.60 | 7.61 | 125 mL | 0.95 |
| S8 | Beer | 19.64 ± 0.44 | 3.46 ± 0.76 | 23.11 | 330 mL | 7.62 |
| S9 | Kombucha | nd | nd | - | - | - |
| S10 | Tablet 3 | 1.71 ± 0.11 | 0.20 ± 0.02 | 1.91 | 1 (24 g) | 0.04 |
| Leaves | Flowers | Seeds | Reference | |
|---|---|---|---|---|
| Total bacterial count | 4.1 × 103 CFU/g | 3.4 × 103 CFU/g | 2.5 × 103 CFU/g | [28] |
| Yeasts and moulds | <102 CFU/g | <102 CFU/g | <102 CFU/g | [28] |
| Escherichia coli | <10 CFU/g | <10 CFU/g | <10 CFU/g | [29] |
| Bacillus cereus | <102 CFU/g | <102 CFU/g | <102 CFU/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nisi, P.; Borgonovo, G.; Tramontana, S.; Grassi, S.; Picozzi, C.; Scaglioni, L.; Mazzini, S.; Mangieri, N.; Bassoli, A. Sisymbrium Officinale (the Singers’ Plant) as an Ingredient: Analysis of Somatosensory Active Volatile Isothiocyanates in Model Food and Drinks. Foods 2021, 10, 308. https://doi.org/10.3390/foods10020308
De Nisi P, Borgonovo G, Tramontana S, Grassi S, Picozzi C, Scaglioni L, Mazzini S, Mangieri N, Bassoli A. Sisymbrium Officinale (the Singers’ Plant) as an Ingredient: Analysis of Somatosensory Active Volatile Isothiocyanates in Model Food and Drinks. Foods. 2021; 10(2):308. https://doi.org/10.3390/foods10020308
Chicago/Turabian StyleDe Nisi, Patrizia, Gigliola Borgonovo, Samuele Tramontana, Silvia Grassi, Claudia Picozzi, Leonardo Scaglioni, Stefania Mazzini, Nicola Mangieri, and Angela Bassoli. 2021. "Sisymbrium Officinale (the Singers’ Plant) as an Ingredient: Analysis of Somatosensory Active Volatile Isothiocyanates in Model Food and Drinks" Foods 10, no. 2: 308. https://doi.org/10.3390/foods10020308
APA StyleDe Nisi, P., Borgonovo, G., Tramontana, S., Grassi, S., Picozzi, C., Scaglioni, L., Mazzini, S., Mangieri, N., & Bassoli, A. (2021). Sisymbrium Officinale (the Singers’ Plant) as an Ingredient: Analysis of Somatosensory Active Volatile Isothiocyanates in Model Food and Drinks. Foods, 10(2), 308. https://doi.org/10.3390/foods10020308