Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Samples
2.2. Chemicals
2.3. Extraction Procedures
2.4. Analytical Investigations
2.4.1. Chromatographic Determinations
2.4.2. Quantitative UV-Vis Spectrophotometric Determinations
2.5. Statistical Data Processing Methods
3. Results and Discussions
3.1. Polyphenolic Composition
3.2. Triterpene Acids Composition
3.3. Apple Cultivar Discrimination Based on Polyphenolic and Triterpene Contents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Evaluation of the Presence of Phenolic Compounds in Different Varieties of Apple by Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Anal. Methods 2015, 8, 696–709. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-inflammatory procyanidins and triterpenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef] [PubMed]
- Babic, D.; Babic, T.; Missoni, S. Apples and Their Products Effect on Neurodegeneration and Alzheimer’s Disease. Coll. Antropol. 2017, 41, 181–187. [Google Scholar]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, e188. [Google Scholar] [CrossRef]
- Gajęcka, M.; Przybylska-Gornowicz, B.; Zakłos-Szyda, M.; Dąbrowski, M.; Michalczuk, L.; Koziołkiewicz, M.; Babuchowski, A.; Zielonka, Ł.; Lewczuk, B.; Gajęcki, M.T. The influence of a natural triterpene preparation on the gastrointestinal tract of gilts with streptozocin-induced diabetes and on cell metabolic activity. J. Funct. Foods 2017, 33, 11–20. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic compounds in apple (Malus x domestica borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef]
- Jakobek, L.; Boc, M.; Barron, A.R. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds from Apples. Food Anal. Methods 2015, 8, 2612–2625. [Google Scholar] [CrossRef]
- Carbone, K.; Giannini, B.; Picchi, V.; Lo Scalzo, R.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 2011, 127, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene Acid and Phenolics from Ancient Apples of Friuli Venezia Giulia as Nutraceutical Ingredients: LC-MS Study and In Vitro Activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Tedesco, I.; Nappo, A.; Russo, G.L.; Malorni, A.; Carbone, V. Phenolic compound characterisation and antiproliferative activity of ‘Annurca’ apple, a southern Italian cultivar. Food Chem. 2010, 123, 157–164. [Google Scholar] [CrossRef]
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Schneider, P.; Hosseiny, S.S.; Szczotka, M.; Jordan, V.; Schlitter, K. Rapid solubility determination of the triterpenes oleanolic acid and ursolic acid by UV-spectroscopy in different solvents. Phytochem. Lett. 2009, 2, 85–87. [Google Scholar] [CrossRef]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Geană, E.I.; Ionete, R.E.; Ciocarlan, A.; Aricu, A.; Fulga, A.; Ungur, N.; Podogova, M.; Nikolaeva, D. HPLC determination of oleanolic and ursolic acids in apples and by-products. Prog. Cryog. Isot. Sep. 2014, 17, 53–62. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Hosu, A.; Floare-Avram, V.; Magdas, D.A.; Feher, I.; Inceu, M.; Cimpoiu, C. The Influence of the Variety, Vineyard, and Vintage on the Romanian White Wines Quality. J. Anal. Methods Chem. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Morresi, C.; Cianfruglia, L.; Armeni, T.; Mancini, F.; Tenore, G.C.; D’Urso, E.; Micheletti, A.; Ferretti, G.; Bacchetti, T. Polyphenolic compounds and nutraceutical properties of old and new apple cultivars. J. Food Biochem. 2018, 42, e12641. [Google Scholar] [CrossRef]
- Panzella, L.; Petriccione, M.; Rega, P.; Scortichini, M.; Napolitano, A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2020, 1, 3. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Bondonno, N.P.; Shinde, S.; Shafaei, A.; Boyce, M.C.; Swinny, E.; Jacob, S.R.; Lacey, K.; Woodman, R.J.; Croft, K.D.; et al. Phenolic composition of 91 Australian apple varieties: Towards understanding their health attributes. Food Funct. 2020, 11, 7115–7125. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an Optimized HPLC Method for the Detection of Various Phenolic Compounds in Apples from Lithuanian Cultivars. J. Chem. 2014, 542121, 10. [Google Scholar] [CrossRef]
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Bozzi Nising, A.; Hurrell, R.F. Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. J. Food Compos. Anal. 2012, 26, 128–135. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.; Jeong, M.; Kim, S.S.; Mitchell, A.E.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early- to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Ku, K.H.; Jeong, M.C.; Kwon, S.I.; Lee, J. Metabolite profiling and antioxidant activity of 10 new early- to mid-season apple cultivars and 14 traditional cultivars. Antioxidants 2020, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, K.; Raudonis, R.; Viškelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.; Matumba, M. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Gutierrez-Salmean, G.; Ciaraldi, T.P.; Nogueira, L.; Barboza, J.; Taub, P.R.; Hogan, M.C.; Henry, R.R.; Meaney, E.; Villarreal, F.; Ceballos, G.; et al. Effects of (-)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. J. Nutr. Biochem. 2014, 25, 91–94. [Google Scholar] [CrossRef]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Caffeic Acid: Benefits, Sources, and Foods. Available online: https://www.healthline.com/health/caffeic-acid (accessed on 6 January 2021).
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds—Biological Activity; Intechopen: London, UK, 2017; pp. 1–21. [Google Scholar]
- Epicatechin Supplements for Bodybuilding Shop—Myostatin Inhibitor Benefits & Reviews. Available online: https://www.mz-store.com/epicatechin (accessed on 6 January 2021).
- Schwarz, N.A.; Theodore, A.P.; Funderburg, B.R.; Waldhelm, A.; McKinley-Barnard, S.K.; Hudson, G.M. Acute (-)-Epicatechin Consumption: Effects on Local Vasodilation Following Resistance Exercise and High-Intensity Exercise Performance. Sports 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Sibel Kılıc, C.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, P.; Hariri, M.; Ghiasvand, R.; Askari, G.; Darvishi, L.; Mashhadi, N.S.; Khosravi-Boroujeni, H. Effect of eight weeks of quercetin supplementation on exercise performance, muscle damage and body muscle in male badminton players. Int. J. Prev. Med. 2013, 4, S53–S57. [Google Scholar] [PubMed]
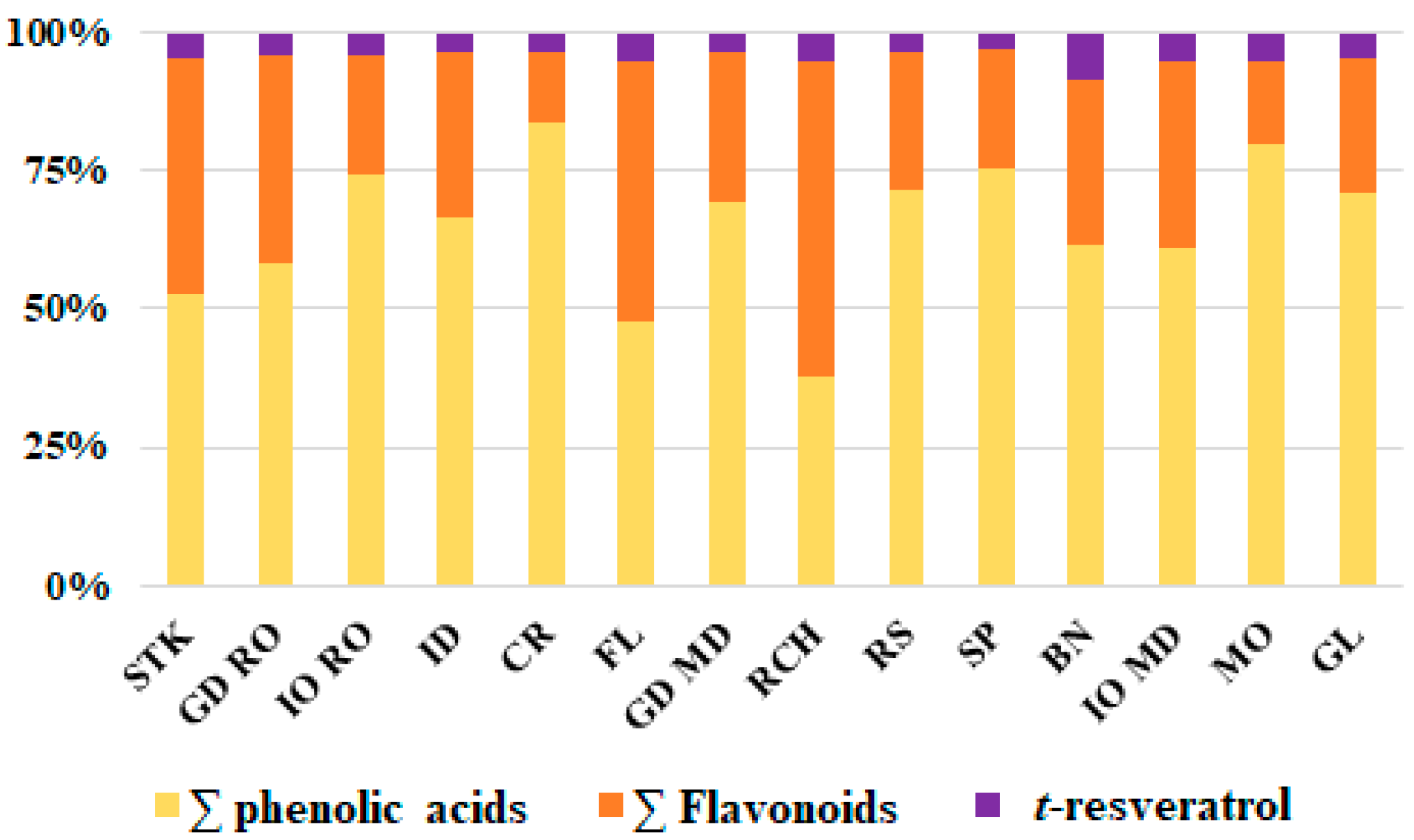
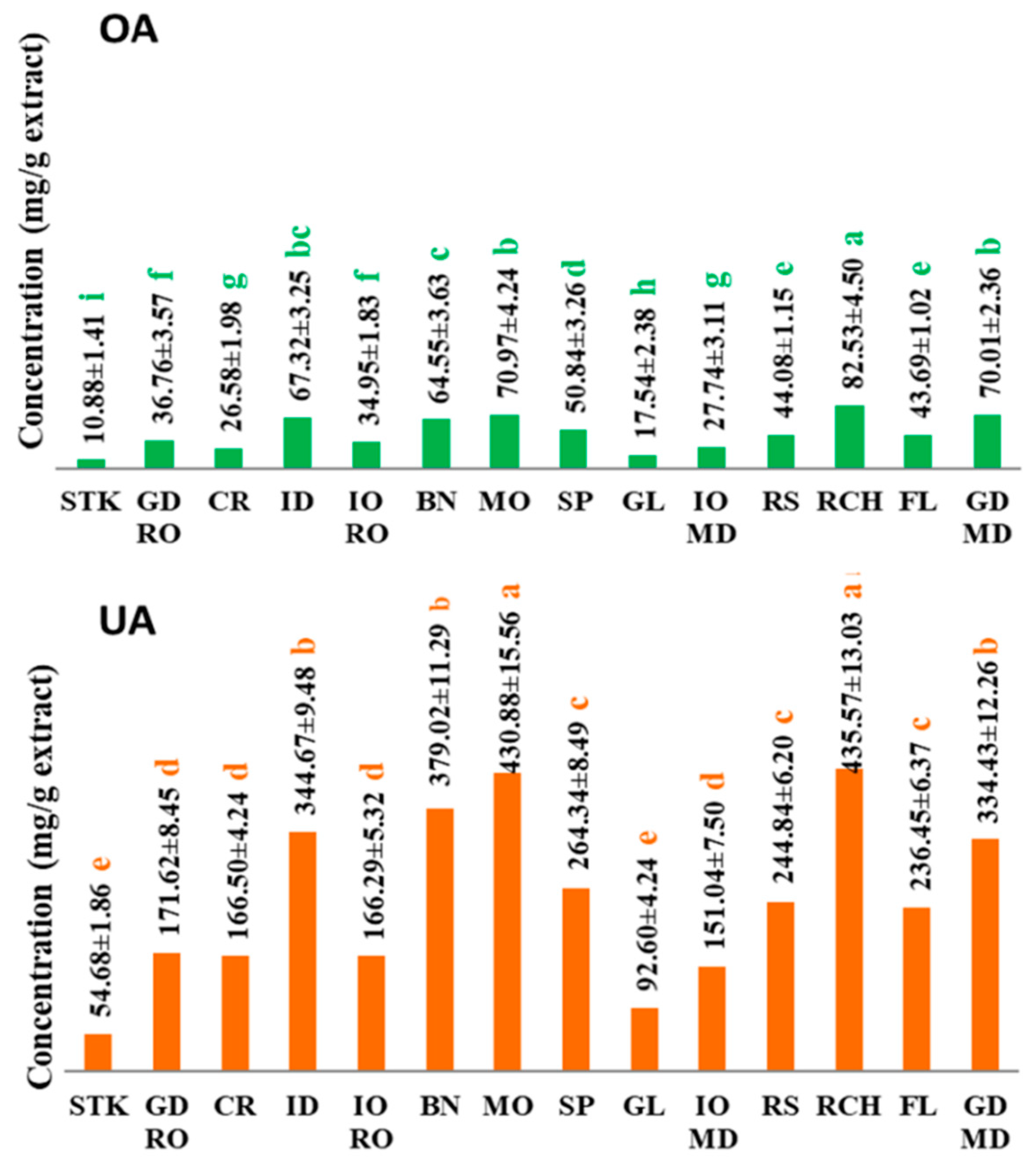
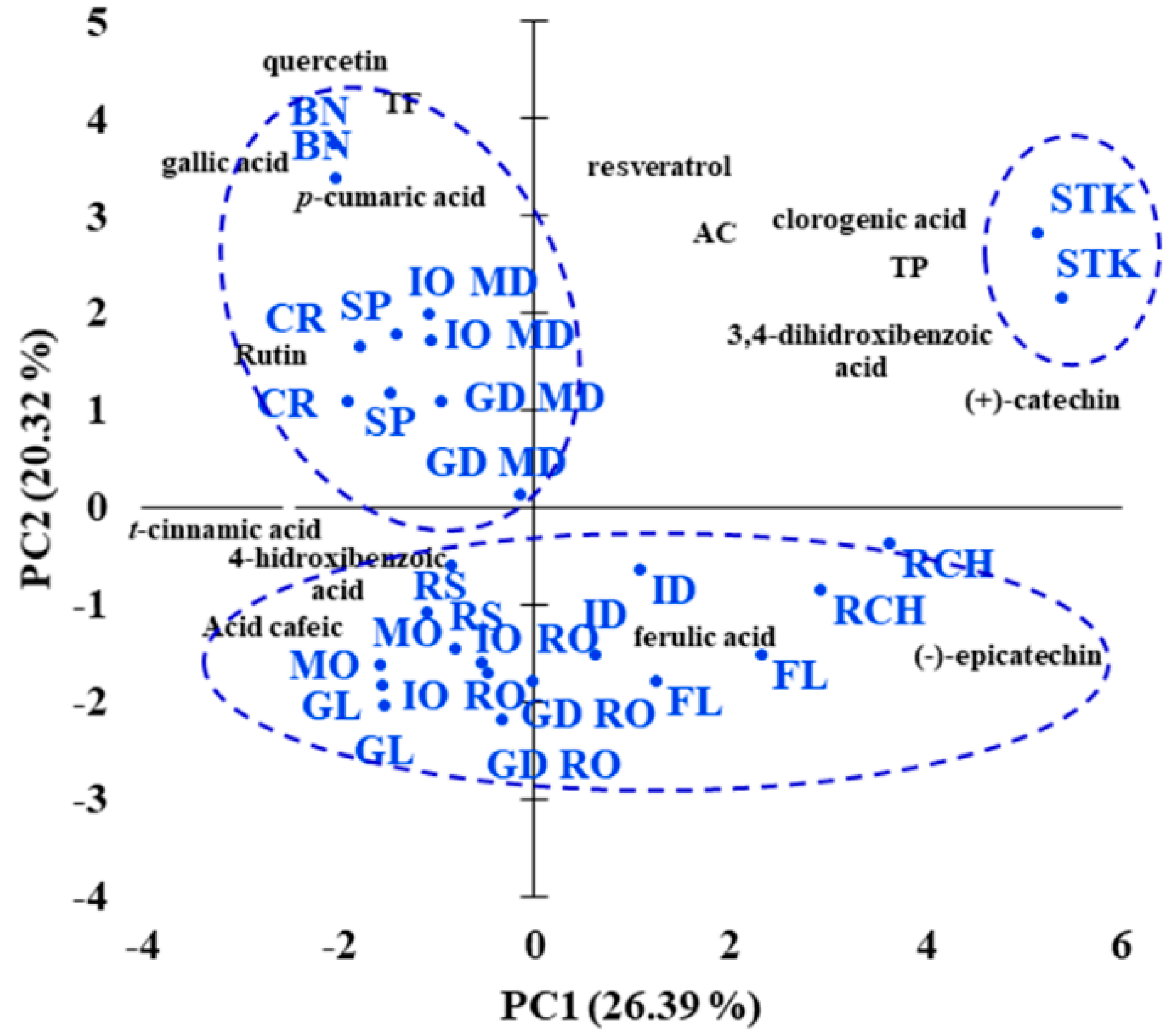
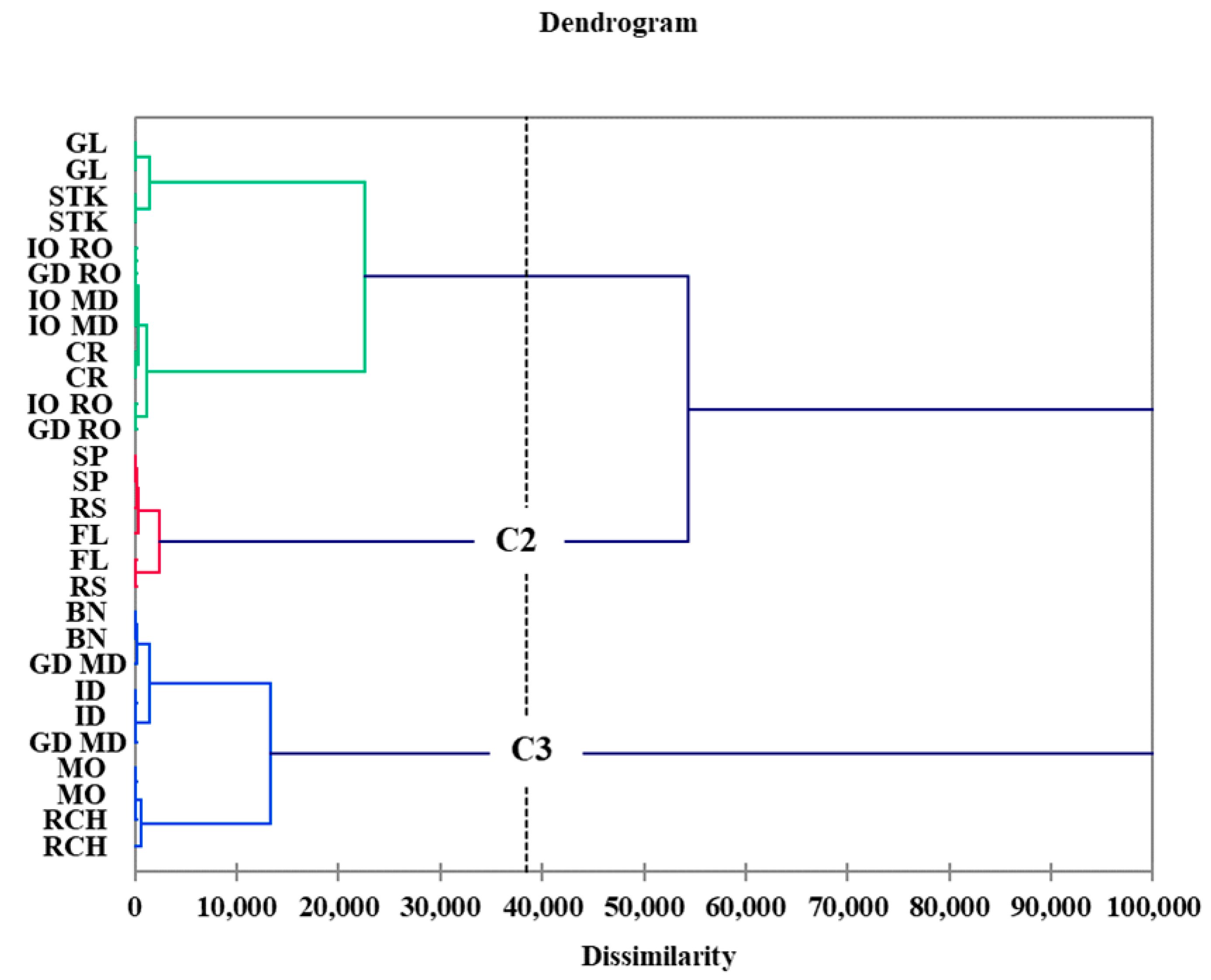
| Apple Variety | Phenolic Acids (mg/100 g DW) | |||||||
|---|---|---|---|---|---|---|---|---|
| Caffeic | Gallic | Ferulic | p-Coumaric | p-Hydroxybenzoic | 3,4-Dihydroxybenzoic | t-Cinnamic | Chlorogenic | |
| Beliy Naliv | 9.90 ± 0.71 e | 13.44 ± 0.11 b | 2.28 ± 0.10 cd | 5.32 ± 0.11 bcde | 0.59 ± 0.06 a | 0.94 ± 0.08 a | 0.35 ± 0.07 a | 0.84 ± 0.11 c |
| Cretesc | 9.94 ± 0.23 e | 14.13 ± 0.27 b | 2.67 ± 0.16 cd | 16.31 ± 0.07 a | 0.43 ± 0.11 a | 0.84 ± 0.11 a | 0.35 ± 0.04 a | 1.02 ± 0.08 c |
| Florina | 13.57 ± 1.25 cde | 0.62 ± 0.18 h | 7.27 ± 0.94 a | 1.33 ± 0.94 e | 0.73 ± 0.13 a | 0.78 ± 0.13 a | <LOQ b | 0.82 ± 0.21 c |
| Gloster | 9.43 ± 0.13 e | 7.73 ± 0.06 cd | 1.69 ± 0.06 de | 3.53 ± 0.04 de | 0.78 ± 0.07 a | 1.04 ± 0.06 a | 0.36 ± 0.06 a | 0.86 ± 0.06 c |
| Golden Delicious MD | 18.79 ± 0.73 bcd | 14.94 ± 0.09 b | 3.27 ± 0.29 bcd | 8.14 ± 1.14 bcd | 0.46 ± 0.01 a | 0.82 ± 0.10 a | 0.34 ± 0.01 a | 0.78 ± 0.18 c |
| Golden Delicious RO | 13.99 ± 3.3 cde | 2.84 ± 0.33 fg | 2.51 ± 0.61 cd | 4.59 ± 0.26 cde | 0.48 ± 0.07 a | 0.73 ± 0.11 a | 0.35 ± 0.01 a | <LOQ d |
| Idared | 9.80 ± 0.85 e | 6.53 ± 1.06 de | 4.07 ± 0.30 bc | 4.21 ± 0.10 de | 0.60 ± 0.11 a | 1.22 ± 0.30 a | 0.35 ± 0.08 a | 2.30 ± 0.14 b |
| Jonathan MD | 10.41 ± 0.10 de | 13.14 ± 0.10 b | 0.39 ± 0.10 e | 5.42 ± 0.14 bcde | 0.84 ± 0.06 a | 0.86 ± 0.04 a | 0.33 ± 0.04 a | 1.06 ± 0.06 c |
| Jonathan RO | 24.19 ± 6.82 b | 2.24 ± 0.76 gh | 1.49 ± 0.50 de | 1.41 ± 0.06 e | 0.61 ± 0.15 a | 0.72 ± 0.03 a | 0.29 ± 0.07 a | <LOQ d |
| Montuan | 47.23 ± 0.14 a | 3.04 ± 0.06 fg | 2.23 ± 0.04 cde | 1.09 ± 0.06 e | 0.60 ± 0.06 a | 0.84 ± 0.03 a | <LOQ b | <LOQ d |
| Renet Simirenco | 12.29 ± 0.46 cde | 9.80 ± 1.43 c | 4.87 ± 0.88 b | 5.43 ± 1.96 bcde | 0.48 ± 0.15 a | 0.62 ± 0.29 a | 0.33 ± 0.01 a | 0.48 ± 0.15 cd |
| Richard | 10.92 ± 1.38 cde | 0.49 ± 0.01 h | 2.02 ± 0.53 de | 2.22 ± 0.42 e | 0.38 ± 0.01 a | 1.09 ± 0.15 a | <LOQ b | 0.44 ± 0.21 cd |
| Spartan | 9.83 ± 0.07 e | 21.74 ± 0.48 a | 1.82 ± 0.08 de | 9.45 ± 0.10 b | 0.46 ± 0.11 a | 0.72 ± 0.06 a | <LOQ b | 0.96 ± 0.08 c |
| Starkrimson | 19.40 ± 1.18 bc | 4.50 ± 0.03 ef | 1.93 ± 0.69 de | 9.18 ± 3.58 bc | 0.53 ± 0.26 a | 1.38 ± 0.57 a | <LOQb | 3.77 ± 0.53 a |
| Apple Variety | Flavonoids | t-Resveratrol | |||
|---|---|---|---|---|---|
| (+)-Catechin | (-)-Epicatechin | Quercetin | Rutin | ||
| Beliy Naliv | 0.96 ± 0.07 cd | <LOQ f | 11.61 ± 0.10 a | 3.81 ± 0.07 a | 4.79 ± 0.06 a |
| Cretesc | 1.01 ± 0.04 cd | <LOQ f | 3.67 ± 0.16 bcd | 2.15 ± 0.18 bc | 2.05 ± 0.10 c |
| Florina | 1.89 ± 0.32 bc | 19.65 ± 2.40 ab | 1.63 ± 0.92 de | 1.48 ± 0.18 cd | 2.69 ± 0.38 bc |
| Gloster | 0.64 ± 0.06 d | 4.69 ± 0.13 ef | 2.19 ± 0.10 cde | 1.04 ± 0.06 de | 1.70 ± 0.06 c |
| Golden Delicious MD | 0.90 ± 0.31 cd | 12.12 ± 3.63 cd | 5.45 ± 1.90 b | 0.38 ± 0.14 ef | 2.40 ± 0.12 bc |
| Golden Delicious RO | 1.24 ± 0.15 cd | 13.46 ± 1.25 bc | 1.28 ± 0.57 de | 0.48 ± 0.07 ef | 1.90 ± 0.13 c |
| Idared | 1.24 ± 0.21 cd | 6.90 ± 0.62 cdef | 5.03 ± 0.35 b | <LOQ f | 1.25 ± 0.42 c |
| Jonathan MD | 1.00 ± 0.03 cd | 6.75 ± 0.07 cdef | 9.67 ± 0.04 a | 0.49 ± 0.08 ef | 2.76 ± 0.07 bc |
| Jonathan RO | 0.87 ± 0.18 cd | 6.75 ± 0.36 cdef | 1.10 ± 0.11 e | 0.27 ± 0.38 ef | 1.90 ± 0.13 c |
| Montuan | 0.83 ± 0.01 cd | 5.03 ± 0.07 def | 2.06 ± 0.06 cde | 2.50 ± 0.06 b | 3.42 ± 0.11 b |
| Renet Simirenco | 0.69 ± 0.17 d | 8.22 ± 0.83 cde | 1.28 ± 0.36 de | 1.87 ± 0.50 bcd | 1.60 ± 0.85 c |
| Richard | 2.88 ± 0.59 b | 22.36 ± 1.99 a | 1.54 ± 0.05 de | <LOQ f | 2.38 ± 0.09 bc |
| Spartan | 1.04 ± 0.08 cd | 1.81 ± 0.06 ef | 9.91 ± 0.16 a | <LOQ f | 1.72 ±0.07 c |
| Starkrimson | 4.08 ± 0.55 a | 25.03 ± 4.67 a | 4.17 ± 0.35 bc | <LOQ f | 3.43 ± 0.60 b |
| ∑ Phenolic Acids | ∑ Flavonoids | TP | TF | AC | |
|---|---|---|---|---|---|
| ∑ phenolic acids | 1 | ||||
| ∑ phenolic acids | −0.261 | 1 | |||
| TP | 0.547 | −0.028 | 1 | ||
| TF | −0.126 | 0.776 | 0.009 | 1 | |
| AC | −0.137 | 0.524 | 0.457 | 0.519 | 1 |
| Main Bioactive Compounds | Main Associated Biological and Pharmacological Activities That Contribute to Human Health | References |
|---|---|---|
| caffeic acid | biological and pharmacological activities: antioxidant, anticancer, as part of a cancer treatment regime, treat certain viruses, including herpes and HIV | [38] |
| marketed as a supplement to boost athletic performance and to aid in weight loss or as skin care serums | [41] | |
| gallic acid | biological and pharmacological activities: antioxidant, antimicrobial, anti-inflammatory, anticancer, cardio-protective, gastro-protective, and neuroprotective effects, metabolic diseases | [35,42] |
| marketed as flavouring agents and preservatives in the food industry | [43] | |
| epicatechin | biological activities: myostatin inhibition, increase muscle growth and strength, improve blood pressure and reduce insulin resistance | [39,40] |
| marketed as a supplement to enhance athletic performance | [44,45] | |
| quercetin | effects on diabetes complications, Alzheimer’s, cardiovascular and liver diseases, Arthritis, Microbial Infections | [46] |
| mediate anti-oxidative and anti-inflammatory activity and shows potential to decrease body fat percent and may improve exercise performance | [47] | |
| ursolic acid | potential biological activities: reduce fat accumulation and increase muscle mass—obesity control, inhibits the proliferation of various cancer cell types, cardioprotective, anti-inflammatory, antibacterial, antidiabetic and neuroprotective activities. NOTE: there are not enough clinical studies to demonstrate the benefits to human health. | [36] |
| oleanolic acid | biological activities: hepato-protective, antitumor, antidiabetic, antimicrobial, antihypertensive and anti-inflammatory properties | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geană, E.-I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars. Foods 2021, 10, 267. https://doi.org/10.3390/foods10020267
Geană E-I, Ciucure CT, Ionete RE, Ciocârlan A, Aricu A, Ficai A, Andronescu E. Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars. Foods. 2021; 10(2):267. https://doi.org/10.3390/foods10020267
Chicago/Turabian StyleGeană, Elisabeta-Irina, Corina Teodora Ciucure, Roxana Elena Ionete, Alexandru Ciocârlan, Aculina Aricu, Anton Ficai, and Ecaterina Andronescu. 2021. "Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars" Foods 10, no. 2: 267. https://doi.org/10.3390/foods10020267
APA StyleGeană, E.-I., Ciucure, C. T., Ionete, R. E., Ciocârlan, A., Aricu, A., Ficai, A., & Andronescu, E. (2021). Profiling of Phenolic Compounds and Triterpene Acids of Twelve Apple (Malus domestica Borkh.) Cultivars. Foods, 10(2), 267. https://doi.org/10.3390/foods10020267








