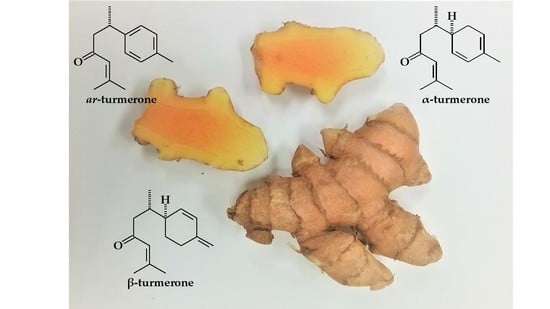
Variation in the Chemical Composition of Five Varieties of Curcuma longa Rhizome Essential Oils Cultivated in North Alabama
Abstract
1. Introduction
2. Materials and Methods
2.1. Curcuma longa Varieties
2.2. Cultivation of Curcuma longa
2.3. Essential Oils
2.4. Gas Chromatographic–Mass Spectral (GC–MS) Analysis
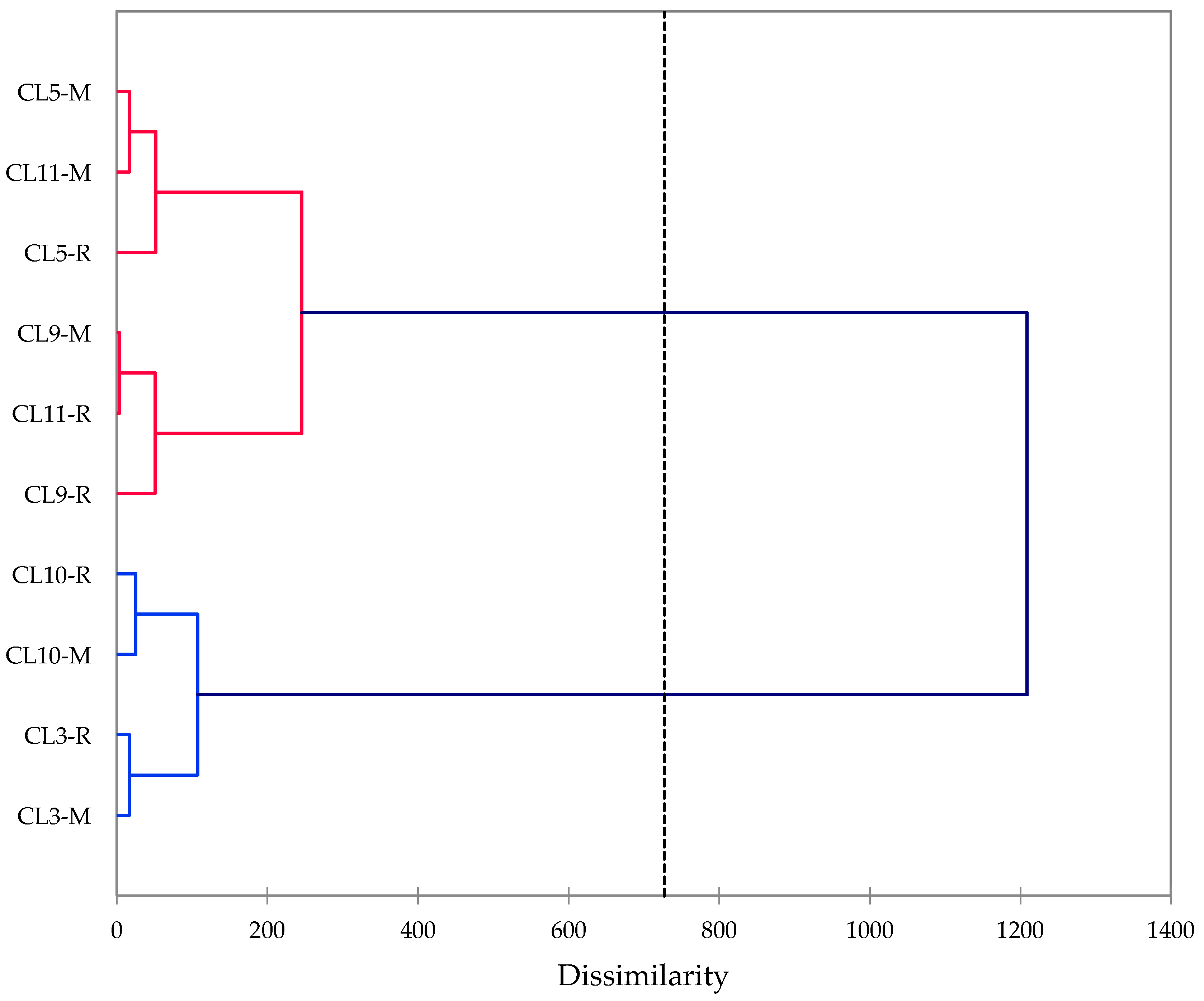
2.5. Hierarchical Cluster Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, F.D.; Kemmelmeier, C.; Arrotéia, C.C.; Da Costa, C.L.; Mallmann, C.A.; Janeiro, V.; Ferreira, F.M.D.; Mossini, S.A.G.; Silva, E.L.; Machinski, M. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013, 136, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.K.; Dixit, S.C. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of northern India. J. Young Pharm. 2009, 1, 312–316. [Google Scholar]
- Dahal, K.R.; Idris, S. Curcuma longa L. In Plant Resources of South-East Asia No 13: Spices; de Guzman, C.C., Siemonsma, J.S., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 111–116. [Google Scholar]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Helen, C.F.; Su, R.H.; Ghulam, J. Isolation, purification and characterization of insect repellents from Curcuma longa L. J. Agric. Food Chem. 1982, 30, 290–292. [Google Scholar]
- Govindarajan, V.S. Turmeric- chemistry, technology and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine to modern medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 263–288. ISBN 978-1439807132. [Google Scholar]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Yamada, M. Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010, 16, 285–297. [Google Scholar] [CrossRef]
- Shahbandeh, M. Trade Value of Turmeric Imported to the United States in 2019, by Country of Origin. Available online: https://www.statista.com/statistics/798301/us-turmeric-imports-by-country/ (accessed on 5 October 2020).
- Hossain, M.A. Effects of harvest time on shoot biomass and yield of turmeric (Curcuma longa L.) in Okinawa, Japan. Plant Prod. Sci. 2015, 13, 97–103. [Google Scholar] [CrossRef]
- Shannon, D.A.; van Santen, E.; Salmasi, S.Z.; Murray, T.J.; Duong, L.T.; Greenfield, J.T.; Gonzales, T.; Foshee, W. Shade, establishment method, and varietal effects on rhizome yield and curcumin content in turmeric in Alabama. Crop Sci. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Rathore, P.; Dohare, P.; Varma, S.; Ray, A.; Sharma, U.; Jaganathanan, N.R.; Ray, M. Curcuma oil: Reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008, 33, 1672–1682. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Variations in the volatile compositions of Curcuma species. Foods 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, T.J.; Sasikumar, B.; Nirmal Babu, K. Variation for quality components in ginger and turmeric and their interaction with environments. Biotechnol. Plant. Crop. 1999, 3, 230–251. [Google Scholar]
- Sandeep, I.S.; Sanghamitra, N.; Sujata, M. Differential effect of soil and environment on metabolic expression of turmeric (Curcuma longa cv. Roma). Indian J. Exp. Biol. 2015, 53, 406–411. [Google Scholar] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- National Institute of Standards and Technology. NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Garg, S.N.; Bansal, R.P.; Gupta, M.M.; Kumar, S. Variation in the rhizome essential oil and curcumin contents and oil quality in the land races of turmeric Curcuma longa of North Indian plains. Flavour Fragr. J. 1999, 14, 315–318. [Google Scholar] [CrossRef]
- Raina, V.K.; Srivastava, S.K.; Syamasundar, K.V. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan region of northern India. J. Essent. Oil Res. 2005, 17, 556–559. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Leela, N.K.; Tava, A.; Shafi, P.M.; John, S.P.; Chempakam, B. Chemical composition of essential oils of turmeric (Curcuma longa L.). Acta Pharm. 2002, 52, 137–141. [Google Scholar]
- Hwang, K.-W.; Son, D.; Jo, H.-W.; Kim, C.H.; Seong, K.C.; Moon, J.-K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 2016, 59, 209–215. [Google Scholar] [CrossRef]
- Xu, L.; Shang, Z.; Lu, Y.; Li, P.; Sun, L.; Guo, Q.; Bo, T.; Le, Z.; Bai, Z.; Zhang, X.; et al. Analysis of curcuminoids and volatile components in 160 batches of turmeric samples in China by high-performance liquid chromatography and gas chromatography mass spectrometry. J. Pharm. Biomed. Anal. 2020, 188, 113465. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakatani, K.; Fujihara, T.; Yamada, H. Aroma of turmeric: Dependence on the combination of groups of several odor constituents. Nat. Prod. Commun. 2015, 10, 1047–1050. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Choi, J.; Lee, J.; Lee, Y. Induction of apoptosis by ar-turmerone on various cell lines. Int. J. Mol. Med. 2004, 14, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-B.; Wu, L.-C.; Hsieh, Y.-C.; Wu, C.-H.; Chan, Y.-J.; Chang, L.-H.; Chang, C.-M.J.; Hsu, S.-L.; Teng, C.-L.; Wu, C.-C. Supercritical carbon dioxide extraction of aromatic turmerone from Curcuma longa Linn. induces apoptosis through reactive oxygen species-triggered intrinsic and extrinsic pathways in human hepatocellular carcinoma HepG2 cells. J. Agric. Food Chem. 2012, 60, 9620–9630. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.-J. Aromatic-turmerone attenuates invasion and expression of MMP-9 and COX-2 through inhibition of NF-κB in TPA-induced breast cancer cells. J. Cell. Biochem. 2012, 113, 3653–3662. [Google Scholar] [CrossRef]
- Nair, A.; Amalraj, A.; Jacob, J.; Kunnumakkara, A.B.; Gopi, S. Non-curcuminoids from turmeric and their potential in cancer therapy and anticancer drug delivery formulations. Biomolecules 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int. Immunopharmacol. 2012, 14, 13–20. [Google Scholar] [CrossRef]
- Li, Y.-L.; Du, Z.-Y.; Li, P.-H.; Yan, L.; Zhou, W.; Tang, Y.-D.; Liu, G.-R.; Fang, Y.-X.; Zhang, K.; Dong, C.-Z.; et al. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Negi, P.S.; Anandharamakrishnan, C.; Sakariah, K.K. Chemical composition of turmeric oil—A byproduct from turmeric oleoresin industry and its inhibitory activity against different fungi. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2001, 56, 40–44. [Google Scholar] [CrossRef]
- Jankasem, M.; Wuthi-udomlert, M.; Gritsanapan, W. Antidermatophytic properties of ar-turmerone, turmeric oil, and Curcuma longa preparations. ISRN Dermatol. 2013, 2013, 250597. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.O.; Sama, W.; Essien, E.E.; Olayemi, J.O.; Ekundayo, O.; Walker, T.M.; Setzer, W.N. Larvicidal activity of turmerone-rich essential oils of Curcuma longa leaf and rhizome from Nigeria on Anopheles gambiae. Pharm. Biol. 2008, 46, 279–282. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Zoghroban, A.A.M.; Kassem, S.M.I. Insecticidal and antifungal activities of crude extracts and pure compounds from rhizomes of Curcuma longa L. (Zingiberaceae). J. Agric. Sci. Technol. 2019, 21, 1049–1061. [Google Scholar]
- de Tavares, W.S.; de Freitas, S.S.; Grazziotti, G.H.; Parente, L.M.L.; Lião, L.M.; Zanuncio, J.C. Ar-turmerone from Curcuma longa (Zingiberaceae) rhizomes and effects on Sitophilus zeamais (Coleoptera: Curculionidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae). Ind. Crops Prod. 2013, 46, 158–164. [Google Scholar] [CrossRef]

| C. longa Cultivar (Rhizome) | Source (Origin) of Rhizome | Mass of Rhizome (g) | Mass of Essential Oil (g) | % Yield | Color |
|---|---|---|---|---|---|
| CL3 (mother) | Horizon Herbs, 3350 Cedar Flat Road, Williams, Oregon (Hawaii) | 236.2 | 0.5935 | 0.251 | pale yellow |
| CL3 (lateral) | 268.7 | 0.6797 | 0.253 | pale yellow | |
| CL5 (mother) | James Simon, Rutgers University, New Jersey (India) | 214.0 | 1.3120 | 0.613 | yellow |
| CL5 (lateral) | 281.0 | 1.5540 | 0.553 | pale yellow | |
| CL9 (mother) | Lam T. Duong (Dak Lak Province, Vietnam) | 207.1 | 1.3645 | 0.659 | yellow |
| CL9 (lateral) | 188.3 | 0.8050 | 0.428 | yellow | |
| CL10 (mother) | International farmers Market, Chamblee, Georgia (unknown) | 232.0 | 0.6715 | 0.289 | pale yellow |
| CL10 (lateral) | 282.7 | 0.5763 | 0.204 | pale yellow | |
| CL11 (mother) | Dr. Anand Yadav, Fort Valley State University, Georgia (unknown) | 215.0 | 1.0760 | 0.500 | pale yellow |
| CL11 (lateral) | 264.6 | 1.4126 | 0.534 | pale yellow |
| RIcalc | RIdb | Compound | CL3-M | CL3-L | CL5-M | CL5-L | CL9-M | CL9-L | CL10-M | CL10-L | CL11-M | CL11-L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 924 | 925 | α-Thujene | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 931 | 933 | α-Pinene | 0.1 | 0.2 | 0.2 | 0.3 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 | 0.1 |
| 971 | 972 | Sabinene | 0.1 | 0.1 | 0.1 | tr | tr | tr | 0.1 | 0.1 | tr | tr |
| 976 | 978 | β-Pinene | tr | tr | tr | tr | tr | tr | 0.1 | 0.1 | tr | tr |
| 988 | 991 | Myrcene | 0.4 | 0.6 | 0.3 | 0.4 | 0.2 | 0.3 | 0.6 | 0.7 | 0.2 | 0.2 |
| 999 | 1000 | δ-2-Carene | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1007 | 1007 | α-Phellandrene | 5.9 | 9.1 | 7.3 | 11.8 | 3.7 | 6.7 | 8.7 | 7.8 | 4.4 | 5.8 |
| 1009 | 1009 | δ-3-Carene | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| 1016 | 1017 | α-Terpinene | 0.2 | 0.3 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.2 | 0.2 | 0.2 |
| 1019 | 1022 | m-Cymene | --- | --- | --- | --- | --- | --- | --- | tr | --- | --- |
| 1024 | 1025 | p-Cymene | 0.9 | 2.2 | 1.0 | 1.2 | 0.5 | 1.2 | 1.7 | 3.0 | 0.6 | 0.7 |
| 1029 | 1030 | Limonene | 0.5 | 0.7 | 0.5 | 0.6 | 0.3 | 0.4 | 0.9 | 0.8 | 0.4 | 0.4 |
| 1033 | 1032 | 1,8-Cineole | 9.2 | 5.1 | 6.1 | 3.5 | 3.4 | 2.6 | 11.7 | 6.2 | 8.5 | 3.6 |
| 1034 | 1034 | (Z)-β-Ocimene | --- | tr | tr | tr | tr | tr | tr | tr | --- | --- |
| 1045 | 1045 | (E)-β-Ocimene | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1057 | 1058 | γ-Terpinene | 0.3 | 0.4 | 0.3 | 0.5 | 0.2 | 0.3 | 0.4 | 0.4 | 0.3 | 0.3 |
| 1069 | 1069 | cis-Sabinene hydrate | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1085 | 1086 | Terpinolene | 4.0 | 4.4 | 0.7 | 0.7 | 0.5 | 0.7 | 3.6 | 3.0 | 2.0 | 1.8 |
| 1090 | 1093 | 2-Nonanone | 0.1 | --- | --- | --- | --- | --- | 0.1 | --- | --- | --- |
| 1090 | 1093 | p-Cymenene | --- | tr | tr | tr | tr | tr | --- | tr | tr | tr |
| 1099 | 1099 | Linalool | 0.1 | 0.1 | tr | tr | tr | tr | 0.1 | tr | tr | tr |
| 1100 | 1101 | trans-Sabinene hydrate | --- | --- | tr | tr | tr | tr | --- | --- | tr | tr |
| 1101 | 1101 | 2-Nonanol | 0.2 | 0.1 | --- | --- | --- | --- | 0.2 | 0.2 | --- | --- |
| 1124 | 1124 | cis-p-Menth-2-en-1-ol | 0.1 | 0.1 | tr | 0.1 | tr | tr | 0.1 | 0.1 | tr | tr |
| 1141 | 1146 | Ipsdienol | --- | --- | --- | --- | --- | --- | --- | --- | tr | tr |
| 1142 | 1142 | trans-p-Menth-2-en-1-ol | 0.1 | 0.1 | 0.1 | 0.1 | tr | 0.1 | 0.1 | 0.1 | tr | tr |
| 1145 | 1146 | Myrcenone | tr | 0.1 | 0.1 | 0.1 | tr | 0.1 | --- | --- | 0.1 | 0.1 |
| 1149 | 1146 | trans-Limonene oxide | tr | 0.3 | tr | tr | tr | tr | 0.2 | 0.3 | tr | tr |
| 1167 | 1169 | trans-β-Terpineol | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1170 | 1170 | δ-Terpineol | 0.1 | 0.1 | 0.1 | tr | tr | tr | 0.1 | 0.1 | 0.1 | tr |
| 1171 | 1165 | iso-Borneol | tr | tr | --- | --- | --- | --- | tr | tr | --- | --- |
| 1171 | 1171 | p-Mentha-1,5-dien-8-ol | --- | --- | tr | tr | tr | tr | --- | --- | tr | tr |
| 1173 | 1173 | Borneol | tr | tr | tr | tr | tr | tr | tr | 0.1 | --- | --- |
| 1180 | 1180 | Terpinen-4-ol | 0.4 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.4 | 0.2 | 0.3 | 0.1 |
| 1187 | 1188 | p-Cymen-8-ol | 0.1 | 0.2 | tr | tr | tr | tr | 0.1 | 0.2 | 0.1 | tr |
| 1195 | 1195 | α-Terpineol | 0.7 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.7 | 0.4 | 0.6 | 0.2 |
| 1196 | 1196 | cis-Piperitol | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1203 | 1202 | cis-Sabinol | 0.1 | 0.2 | 0.1 | 0.1 | tr | 0.1 | 0.2 | 0.3 | 0.1 | tr |
| 1203 | 1203 | p-Cumenol | 0.1 | 0.1 | --- | --- | --- | --- | --- | --- | tr | tr |
| 1208 | 1209 | trans-Piperitol | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1289 | 1289 | Thymol | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | --- | --- | 0.1 | 0.1 |
| 1292 | 1293 | 2-Undecanone | tr | tr | --- | --- | --- | --- | tr | tr | --- | --- |
| 1297 | 1300 | Carvacrol | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1309 | 1312 | Livescone | --- | --- | --- | --- | --- | --- | --- | --- | tr | tr |
| 1319 | 1318 | 3-Hydroxycineole | 0.1 | 0.1 | tr | tr | tr | tr | 0.1 | 0.3 | tr | tr |
| 1400 | 1405 | Sesquithujene | 0.1 | 0.1 | --- | --- | --- | --- | 0.2 | 0.2 | --- | --- |
| 1417 | 1417 | (E)-Caryophyllene | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 |
| 1431 | 1432 | trans-α-Bergamotene | 0.1 | tr | --- | --- | --- | --- | 0.1 | 0.1 | --- | --- |
| 1442 | 1439 | (Z)-β-Farnesene | tr | tr | tr | tr | tr | 0.1 | --- | --- | 0.1 | 0.1 |
| 1450 | 1452 | (E)-β-Farnesene | 0.3 | 0.2 | tr | tr | tr | tr | 0.3 | 0.3 | tr | 0.1 |
| 1453 | 1454 | α-Humulene | tr | tr | tr | tr | tr | tr | --- | --- | tr | tr |
| 1476 | 1482 | γ-Curcumene | 0.1 | tr | tr | tr | tr | tr | 0.1 | 0.1 | tr | tr |
| 1480 | 1482 | ar-Curcumene | 1.1 | 1.5 | 0.3 | 0.3 | 0.4 | 0.5 | 1.9 | 3.2 | 0.3 | 0.5 |
| 1482 | 1483 | trans-β-Bergamotene | 0.1 | tr | tr | tr | tr | tr | 0.1 | 0.1 | tr | tr |
| 1495 | 1494 | α-Zingiberene | 9.2 | 7.8 | 0.9 | 0.9 | 1.2 | 0.9 | 12.5 | 10.4 | 0.9 | 1.0 |
| 1507 | 1508 | β-Bisabolene | 1.3 | 1.2 | 0.2 | 0.2 | 0.2 | 0.2 | 2.0 | 2.2 | 0.2 | 0.2 |
| 1508 | 1511 | β-Curcumene | tr | tr | tr | tr | tr | tr | --- | --- | tr | tr |
| 1524 | 1523 | β-Sesquiphellandrene | 5.5 | 4.9 | 0.7 | 0.8 | 0.9 | 0.9 | 7.7 | 8.0 | 0.8 | 1.0 |
| 1526 | 1528 | (E)-γ-Bisabolene | 0.1 | 0.2 | tr | 0.1 | tr | tr | 0.1 | 0.1 | tr | tr |
| 1553 | 1555 | 7-epi-cis-Sesquisabinene hydrate | 0.4 | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.5 | 0.5 | 0.2 | 0.2 |
| 1559 | 1561 | (E)-Nerolidol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1577 | 1578 | ar-Tumerol | 0.4 | 0.5 | 0.5 | 0.5 | 0.6 | 0.8 | 0.3 | 0.3 | 0.5 | 0.9 |
| 1589 | 1590 | 7-epi-trans-Sesquisabinene hydrate | 0.9 | 0.8 | 0.5 | 0.4 | 0.5 | 0.4 | 1.1 | 1.2 | 0.4 | 0.4 |
| 1600 | 1594 | anti-anti-anti-Helifolen-12-al B | 0.2 | 0.2 | 0.3 | 0.4 | 0.4 | 0.4 | 0.2 | 0.2 | 0.2 | 0.4 |
| 1615 | 1615 | Zingiberenol | 2.0 | 1.7 | 0.3 | 0.5 | 0.6 | 0.5 | 2.8 | 3.0 | 0.5 | 0.6 |
| 1620 | 1620 | anti-syn-syn-Helifolen-12-al C | 0.2 | 0.3 | 0.3 | 0.3 | 0.5 | 0.3 | --- | --- | --- | 0.6 |
| 1623 | 1624 | 10-epi-γ-Eudesmol | 0.2 | 0.2 | --- | 0.2 | --- | 0.4 | 0.2 | 0.4 | --- | 0.1 |
| 1643 | 1647 | Camphenone | --- | --- | --- | --- | --- | --- | --- | 0.3 | --- | --- |
| 1670 | 1668 | ar-Turmerone | 15.4 | 15.3 | 18.3 | 15.5 | 26.3 | 32.5 | 6.8 | 9.5 | 21.8 | 27.0 |
| 1675 | 1668 | α-Turmerone | 17.6 | 18.7 | 30.1 | 31.5 | 24.9 | 18.9 | 15.4 | 13.6 | 27.8 | 24.8 |
| 1687 | 1687 | Himachal-4-en-1β-ol | 0.7 | 0.7 | --- | --- | --- | --- | 0.9 | 1.0 | --- | --- |
| 1688 | 1687 | α-Bisabolol | 0.5 | 0.3 | --- | --- | --- | --- | 0.5 | 0.8 | --- | --- |
| 1695 | 1695 | (2Z,6Z)-Farnesol | 0.2 | 0.3 | --- | --- | --- | --- | 0.4 | 0.6 | --- | --- |
| 1702 | 1699 | β-Turmerone (= Curlone) | 8.9 | 8.8 | 17.1 | 16.8 | 18.4 | 17.0 | 5.0 | 4.8 | 17.9 | 17.5 |
| 1712 | 1712 | Curcuphenol | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 |
| 1743 | 1742 | (6S,7R)-Bisabolone | 2.0 | 1.7 | 0.6 | 0.7 | 0.8 | 0.7 | 3.1 | 3.4 | 0.7 | 0.7 |
| 1773 | 1775 | trans-α-Atlantone | 0.3 | 0.2 | 0.2 | 0.4 | 0.4 | 0.4 | 0.2 | 0.2 | 0.3 | 0.4 |
| 1807 | 1807 | Eudesm-11-en-4α,6α-diol | 0.4 | 0.4 | --- | --- | --- | --- | --- | --- | --- | --- |
| 1985 | 1983 | Methyl-β-(E)-ionyl tiglate | 0.5 | 0.4 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.5 | 0.1 | 0.1 |
| Monoterpene hydrocarbons | 12.6 | 18.3 | 10.6 | 15.9 | 5.6 | 9.9 | 17.0 | 16.6 | 8.3 | 9.5 | ||
| Oxygenated monoterpenoids | 2.0 | 2.0 | 0.9 | 0.8 | 0.5 | 0.7 | 2.1 | 1.9 | 1.4 | 0.6 | ||
| Sesquiterpene hydrocarbons | 17.9 | 16.1 | 2.2 | 2.5 | 2.8 | 2.7 | 25.0 | 24.8 | 2.3 | 2.9 | ||
| Oxygenated sesquiterpenoids | 51.1 | 51.1 | 68.9 | 67.8 | 74.0 | 73.0 | 37.9 | 40.5 | 70.5 | 73.8 | ||
| Others | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.2 | 0.0 | 0.0 | ||
| Total Identified | 83.8 | 87.6 | 82.7 | 86.9 | 82.9 | 86.2 | 82.2 | 84.0 | 82.4 | 86.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setzer, W.N.; Duong, L.; Poudel, A.; Mentreddy, S.R. Variation in the Chemical Composition of Five Varieties of Curcuma longa Rhizome Essential Oils Cultivated in North Alabama. Foods 2021, 10, 212. https://doi.org/10.3390/foods10020212
Setzer WN, Duong L, Poudel A, Mentreddy SR. Variation in the Chemical Composition of Five Varieties of Curcuma longa Rhizome Essential Oils Cultivated in North Alabama. Foods. 2021; 10(2):212. https://doi.org/10.3390/foods10020212
Chicago/Turabian StyleSetzer, William N., Lam Duong, Ambika Poudel, and Srinivasa Rao Mentreddy. 2021. "Variation in the Chemical Composition of Five Varieties of Curcuma longa Rhizome Essential Oils Cultivated in North Alabama" Foods 10, no. 2: 212. https://doi.org/10.3390/foods10020212
APA StyleSetzer, W. N., Duong, L., Poudel, A., & Mentreddy, S. R. (2021). Variation in the Chemical Composition of Five Varieties of Curcuma longa Rhizome Essential Oils Cultivated in North Alabama. Foods, 10(2), 212. https://doi.org/10.3390/foods10020212