Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis in the Human Hepatoma Cell Line HepaRG
Abstract
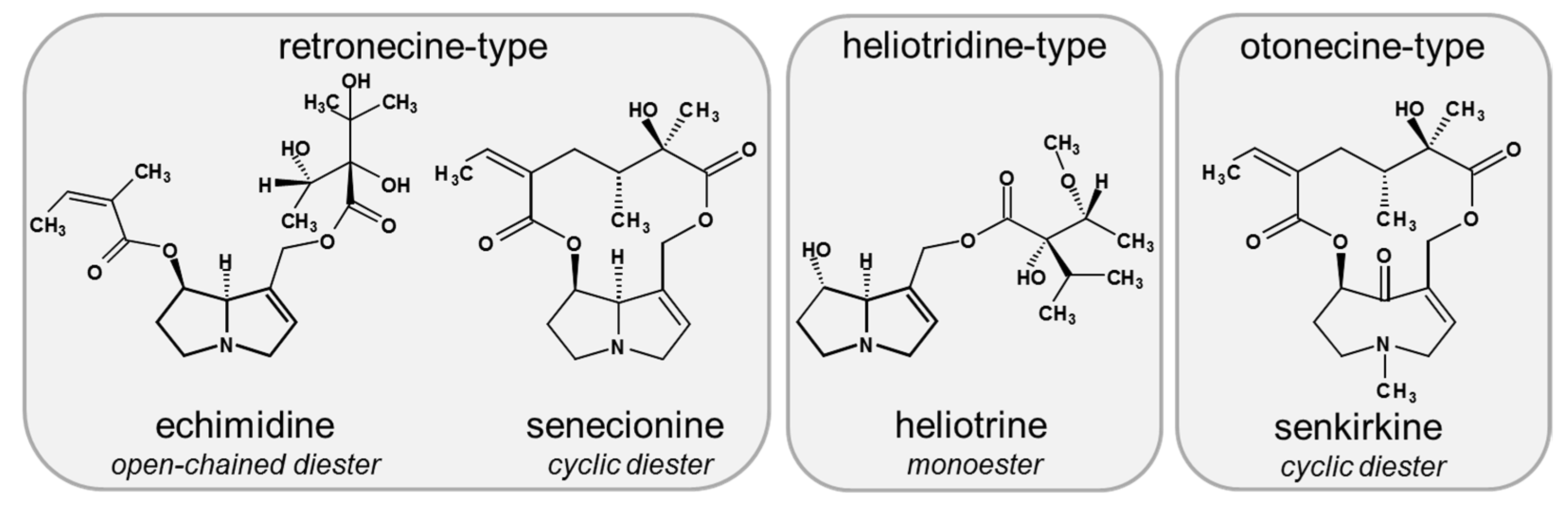
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plasmids
2.3. Cell Culture
2.4. Preparation of RNA and Quantitative Real-Time PCR Analysis (qRT-PCR)
2.5. Transcriptional Activation of CYP7A1 by Dual Luciferase Reporter Gene Assay
2.6. Staining of Bile Canaliculi to Assay Canalicular Efflux
2.7. Staining of the Tight Junction Protein Zonula Occludens-1 (ZO-1) Combined with Nuclei Staining
2.8. Analysis of Bile Acid Content
2.9. Statistical Analysis
3. Results
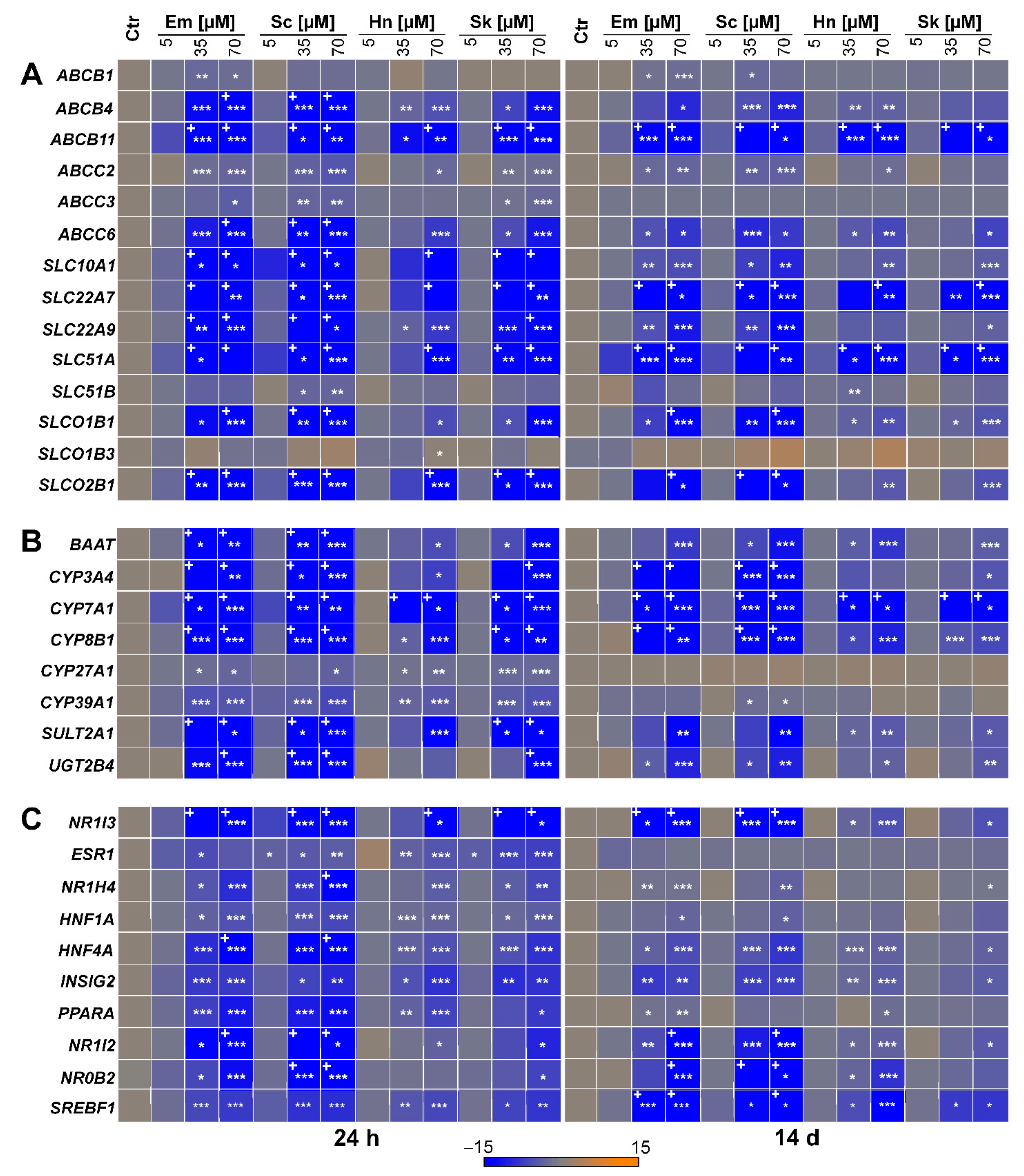
3.1. PA-Dependent Alterations of Gene Expression of Transporters, Enzymes, and Transcription Regulators Involved in Bile Acid Homeostasis
3.2. PA-Dependent Inhibition of CYP7A1 Promoter Activity and Gene Expression
3.3. Effect of PAs on ABCC2-Driven Canalicular Efflux
3.4. Influence of PAs on the Tight Junction Protein ZO-1
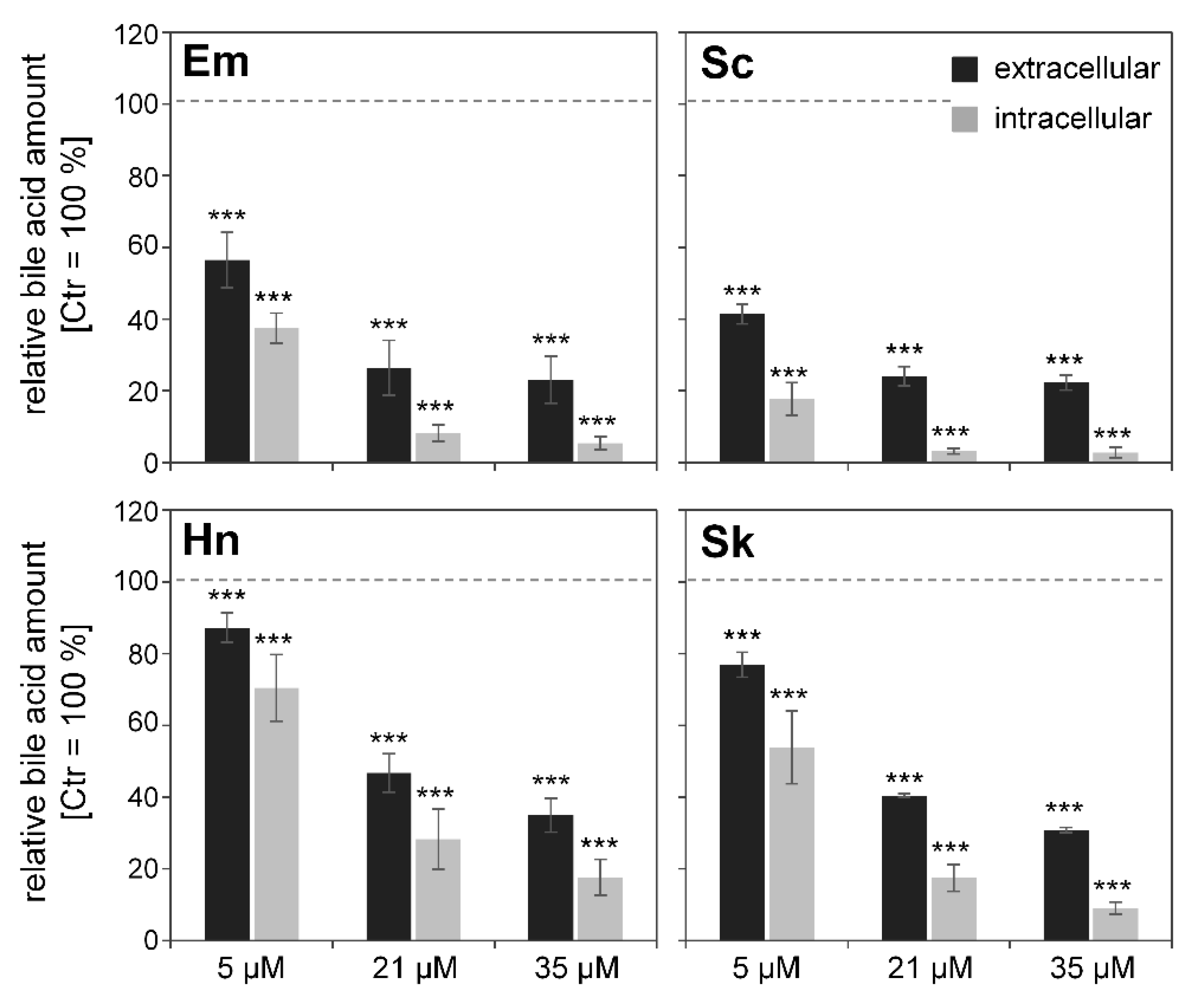
3.5. Effects of PAs on Bile Acid Content
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hartmann, T.; Witte, L. Chapter Four—Chemistry, Biology and Chemoecology of the Pyrrolizidine Alkaloids. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 155–233. [Google Scholar]
- Smith, L.W.; Culvenor, C.C.J. Plant Sources of Hepatotoxic Pyrrolizidine Alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef]
- Roeder, E. Medicinal plants in China containing pyrrolizidine alkaloids. Die Pharm. 2000, 55, 711–726. [Google Scholar]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar] [PubMed]
- Cheeke, P.R. Toxicity and Metabolism of Pyrrolizidine Alkaloids. J. Anim. Sci. 1988, 66, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huo, J.R. Hepatic veno-occlusive disease associated with toxicity of pyrrolizidine alkaloids in herbal prepara-tions. Neth. J. Med. 2010, 68, 252–260. [Google Scholar] [PubMed]
- Fu, P.P.; Xia, Q.; Lin, G.; Chou, M.W. Pyrrolizidine Alkaloids—Genotoxicity, Metabolism Enzymes, Metabolic Activation, and Mechanisms. Drug Metab. Rev. 2004, 36, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Herbal Teas and Toxins: Novel Aspects of Pyrrolizidine Poisoning in the United States. Perspect. Biol. Med. 1980, 24, 1–14. [Google Scholar] [CrossRef]
- Chauvin, P.; Dillon, J.C.; Moren, A. An outbreak of Heliotrope food poisoning, Tadjikistan, November 1992–March 1993. Sante 1994, 4, 263–268. [Google Scholar]
- Kakar, F.; Akbarian, Z.; Leslie, T.; Mustafa, M.L.; Watson, J.; Van Egmond, H.P.; Omar, M.F.; Mofleh, J. An Outbreak of Hepatic Veno-Occlusive Disease in Western Afghanistan Associated with Exposure to Wheat Flour Contaminated with Pyrrolizidine Alkaloids. J. Toxicol. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Krishnamachari, K.A.; Bhat, R.V.; Krishnamurthi, D.; Krishnaswamy, K.; Nagarajan, V. Aetiopathogenesis of endemic ascites in Surguja district of Madhya Pradesh. Indian J. Med Res. 1977, 65, 672–678. [Google Scholar]
- Steenkamp, V.; Stewart, M.J.; Van der Merwe, S.; Zuckerman, M.; Crowther, N.J. The effect of Senecio latifolius a plant used as a South African traditional medicine, on a human hepatoma cell line. J. Ethnopharmacol. 2001, 78, 51–58. [Google Scholar] [CrossRef]
- Tandon, H.D.; Tandon, B.N.; Mattocks, A.R. An epidemic of veno-occlusive disease of the liver in Afghanistan. Pathologic features. Am. J. Gastroenterol. 1978, 70, 607–613. [Google Scholar] [PubMed]
- Wiedenfeld, H.; Edgar, J. Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochem. Rev. 2011, 10, 137–151. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Pyrrolizidine alkaloids in food and feed—EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2011, 9, 2406–2540. [Google Scholar] [CrossRef]
- Luckert, C.; Hessel-Pras, S.; Lenze, D.; Lampen, A. Disturbance of gene expression in primary human hepatocytes by hepatotoxic pyrrolizidine alkaloids: A whole genome transcriptome analysis. Toxicol. In Vitro 2015, 29, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Hessel-Pras, S.; Braeuning, A.; Guenther, G.; Adawy, A.; Enge, A.-M.; Ebmeyer, J.; Henderson, C.J.; Hengstler, J.G.; Lampen, A.; Reif, R. The pyrrolizidine alkaloid senecionine induces CYP-dependent destruction of sinusoidal endothelial cells and cholestasis in mice. Arch. Toxicol. 2019, 94, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Arrese, M.; Ananthananarayanan, M.; Suchy, F.J. Hepatobiliary Transport: Molecular Mechanisms of Development and Cholestasis. Pediatr. Res. 1998, 44, 141–147. [Google Scholar] [CrossRef]
- Trauner, M.; Boyer, J.L. Bile Salt Transporters: Molecular Characterization, Function, and Regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef]
- Cañaveras, J.C.G.; Donato, M.T.; Castell, J.V.; Lahoz, A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J. Lipid Res. 2012, 53, 2231–2241. [Google Scholar] [CrossRef]
- Sharanek, A.; Burban, A.; Humbert, L.; Azzi, P.B.-E.; Felix-Gomes, N.; Rainteau, M.; Guillouzo, A. Cellular Accumulation and Toxic Effects of Bile Acids in Cyclosporine A-Treated HepaRG Hepatocytes. Toxicol. Sci. 2015, 147, 573–587. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Beuers, U.; Paumgartner, G. Hepatobiliary transport. J. Hepatol. 2000, 32, 3–18. [Google Scholar] [CrossRef]
- Trauner, M.; Meier, P.J.; Boyer, J.L. Molecular Pathogenesis of Cholestasis. N. Engl. J. Med. 1998, 339, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Antherieu, S.; Azzi, P.B.-E.; Dumont, J.; Fromenty, B.; Robin, M.-A.; Guillouzo, A.; Abdel-Razzak, Z.; Guguen-Guillouzo, C. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 2013, 57, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M.; Landesmann, B.; Goumenou, M.; Vinken, S.; Shah, I.; Jaeschke, H.; Willett, C.; Whelan, M.; Rogiers, V. Development of an Adverse Outcome Pathway From Drug-Mediated Bile Salt Export Pump Inhibition to Cholestatic Liver Injury. Toxicol. Sci. 2013, 136, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; McGill, M.R.; Yan, H.; Jaeschke, H. Bile Acid-Induced Toxicity in HepaRG Cells Recapitulates the Response in Primary Human Hepatocytes. Basic Clin. Pharmacol. Toxicol. 2016, 118, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jossé, R.; Aninat, C.; Glaise, D.; Dumont, J.; Fessard, V.; Morel, F.; Poul, J.-M.; Guguen-Guillouzo, C.; Guillouzo, A. Long-Term Functional Stability of Human HepaRG Hepatocytes and Use for Chronic Toxicity and Genotoxicity Studies. Drug Metab. Dispos. 2008, 36, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Waizenegger, J.; Braeuning, A.; Templin, M.; Lampen, A.; Hessel-Pras, S. Structure-dependent induction of apoptosis by hepatotoxic pyrrolizidine alkaloids in the human hepatoma cell line HepaRG: Single versus repeated exposure. Food Chem. Toxicol. 2018, 114, 215–226. [Google Scholar] [CrossRef]
- Behr, A.-C.; Kwiatkowski, A.; Ståhlman, M.; Schmidt, F.F.; Luckert, C.; Braeuning, A.; Buhrke, T. Impairment of bile acid metabolism by perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in human HepaRG hepatoma cells. Arch. Toxicol. 2020, 94, 1673–1686. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Yim, H.-S.; Ryu, J.-Y.; Lee, H.S.; Lee, J.-H.; Seen, D.-S.; Kang, S.G. One-Step Sequence- and Ligation-Independent Cloning as a Rapid and Versatile Cloning Method for Functional Genomics Studies. Appl. Environ. Microbiol. 2012, 78, 5440–5443. [Google Scholar] [CrossRef]
- Li, M.Z.; Elledge, S.J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 2007, 4, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Luckert, C.; Ehlers, A.; Buhrke, T.; Seidel, A.; Lampen, A.; Hessel, S. Polycyclic aromatic hydrocarbons stimulate human CYP3A4 promoter activity via PXR. Toxicol. Lett. 2013, 222, 180–188. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Crestani, M.; Stroup, D.; Chiang, J.Y. Hormonal regulation of the cholesterol 7 alpha-hydroxylase gene (CYP7). J. Lipid Res. 1995, 36, 2419–2432. [Google Scholar] [CrossRef]
- Hampf, M.; Gossen, M. A protocol for combined Photinus and Renilla luciferase quantification compatible with protein assays. Anal. Biochem. 2006, 356, 94–99. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; Le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Zamek-Gliszczynski, M.J.; Xiong, H.; Patel, N.J.; Turncliff, R.Z.; Pollack, G.M.; Brouwer, K.L.; Leslie, E.M.; Bowers, R.J.; Deeley, R.G.; Cole, S.P.C. Pharmacokinetics of 5 (and 6)-Carboxy-2′,7′-Dichlorofluorescein and Its Diacetate Promoiety in the Liver. J. Pharmacol. Exp. Ther. 2003, 304, 801–809. [Google Scholar] [CrossRef]
- Xiong, A.; Yang, F.; Fang, L.; Yang, L.; He, Y.; Wan, Y.Y.J.; Xu, Y.; Qi, M.; Wang, X.; Yu, K.; et al. Metabolomic and genomic evidence for compromised bile acid homeostasis by senecionine, a hepatotoxic pyrrolizidine alkaloid. Chem. Res. Toxicol. 2014, 27, 775–786. [Google Scholar] [CrossRef]
- Xiong, A.; Fang, L.; Yang, X.; Yang, F.; Qi, M.; Kang, H.; Yang, L.; Tsim, K.W.K.; Wang, Z. An application of target profiling analyses in the hepatotoxicity assessment of herbal medicines: Comparative characteristic fingerprint and bile acid profiling of Senecio vulgaris L. and Senecio scandens Buch.-Ham. Anal. Bioanal. Chem. 2014, 406, 7715–7727. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Meier, P.J. Hepatobiliary transporters and drug-induced cholestasis. Hepatology 2006, 44, 778–787. [Google Scholar] [CrossRef]
- Paumgartner, G. Medical treatment of cholestatic liver diseases: From pathobiology to pharmacological targets. World J. Gastroenterol. 2006, 12, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Ebmeyer, J.; Franz, L.; Lim, R.; Niemann, B.; Glatt, H.; Braeuning, A.; Lampen, A.; Hessel-Pras, S. Sensitization of Human Liver Cells Toward Fas-Mediated Apoptosis by the Metabolically Activated Pyrrolizidine Alkaloid Lasiocarpine. Mol. Nutr. Food Res. 2019, 63, e1801206. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Glade, J.L.; Stevenson, B.R.; Boyer, J.L.; Mooseker, M.S. Hepatic immunohistochemical localization of the tight junction protein ZO-1 in rat models of cholestasis. Am. J. Pathol. 1989, 134, 1055–1062. [Google Scholar] [PubMed]
- Fallon, M.B.; Mennone, A.; Anderson, J.M. Altered expression and localization of the tight junction protein ZO-1 after common bile duct ligation. Am. J. Physiol. Physiol. 1993, 264 Pt 1, C1439–C1447. [Google Scholar] [CrossRef]
- Baker, D.C.; Pfister, J.A.; Molyneux, R.J.; Kechele, P. Cynoglossum officinale toxicity in calves. J. Comp. Pathol. 1991, 104, 403–410. [Google Scholar] [CrossRef]
- Craig, A.; Pearson, E.; Meyer, C.; Schmitz, J. Clinicopathologic studies of tansy ragwort toxicosis in ponies: Sequential serum and histopathological changes. J. Equine Veter. Sci. 1991, 11, 261–271. [Google Scholar] [CrossRef]
- Mendel, V.E.; Witt, M.R.; Gitchell, B.S.; Gribble, D.N.; Rogers, Q.R.; Segall, H.J.; Knight, H.D. Pyrrolizidine alkaloid-induced liver disease in horses: An early diagnosis. Am. J. Veter. Res. 1988, 49, 572–578. [Google Scholar]
- Stegelmeier, B.L.; Gardner, D.R.; James, L.F.; Molyneux, R.J. Pyrrole detection and the pathologic progression of Cynoglossum officinale (houndstongue) poisoning in horses. J. Vet. Diagn. Investig. 1996, 8, 81–90. [Google Scholar] [CrossRef]
- Merz, K.-H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waizenegger, J.; Glück, J.; Henricsson, M.; Luckert, C.; Braeuning, A.; Hessel-Pras, S. Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis in the Human Hepatoma Cell Line HepaRG. Foods 2021, 10, 161. https://doi.org/10.3390/foods10010161
Waizenegger J, Glück J, Henricsson M, Luckert C, Braeuning A, Hessel-Pras S. Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis in the Human Hepatoma Cell Line HepaRG. Foods. 2021; 10(1):161. https://doi.org/10.3390/foods10010161
Chicago/Turabian StyleWaizenegger, Julia, Josephin Glück, Marcus Henricsson, Claudia Luckert, Albert Braeuning, and Stefanie Hessel-Pras. 2021. "Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis in the Human Hepatoma Cell Line HepaRG" Foods 10, no. 1: 161. https://doi.org/10.3390/foods10010161
APA StyleWaizenegger, J., Glück, J., Henricsson, M., Luckert, C., Braeuning, A., & Hessel-Pras, S. (2021). Pyrrolizidine Alkaloids Disturb Bile Acid Homeostasis in the Human Hepatoma Cell Line HepaRG. Foods, 10(1), 161. https://doi.org/10.3390/foods10010161




