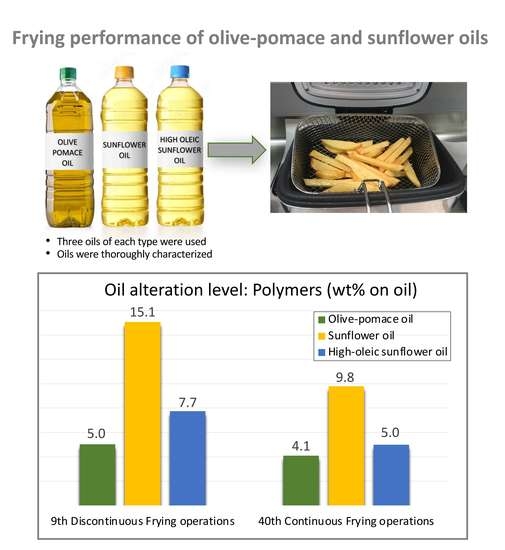
Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comparative Behavior with Sunflower Oils and High-Oleic Sunflower Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples
2.2. Frying Experiments
2.3. Quality and Characterization Parameters of Fresh Oils
2.4. Total Content and Distribution of Polar Compounds
2.5. Color of Oils and Fried Potatoes
2.6. Acceptability Test of Fried Potatoes
2.7. Lipid Extraction of Fried Potatoes
2.8. Analysis of Oils and Oils Extracted from Fried Potatoes during Frying
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization and Quality Parameters of Fresh Oils
3.2. Total Content and Distribution of Polar Compounds in the Fresh Oils
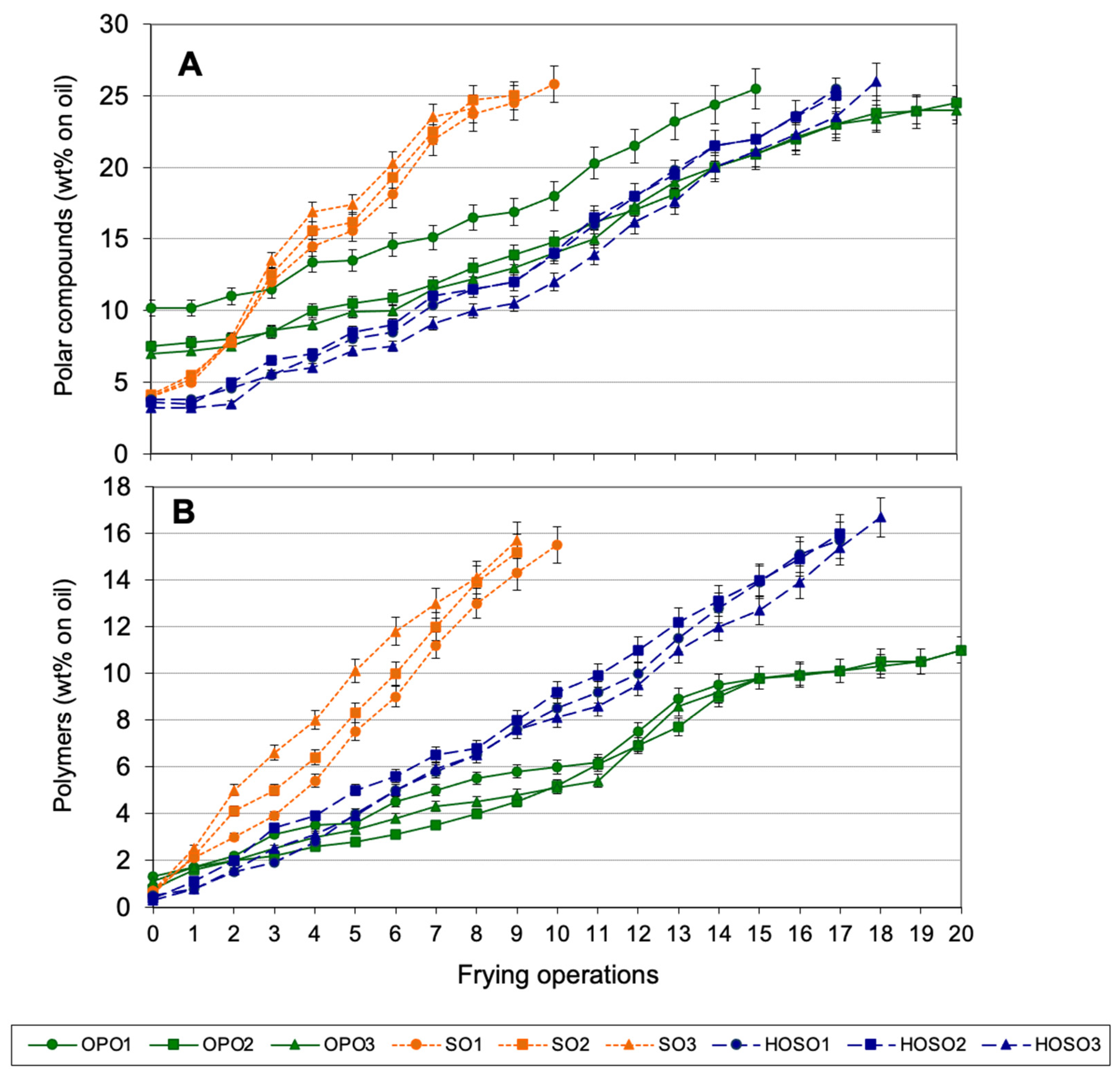
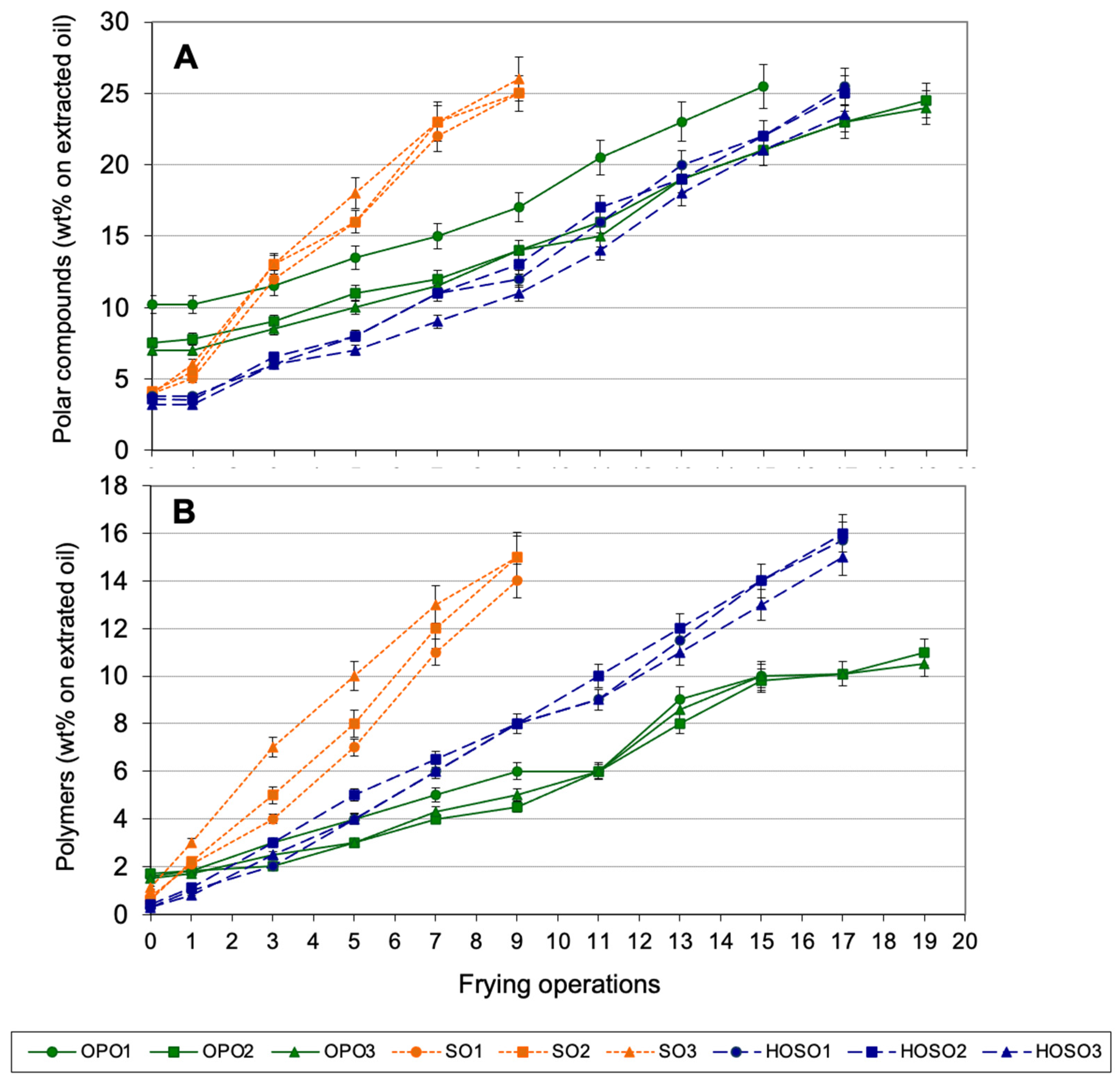
3.3. Discontinuous Frying Experiments
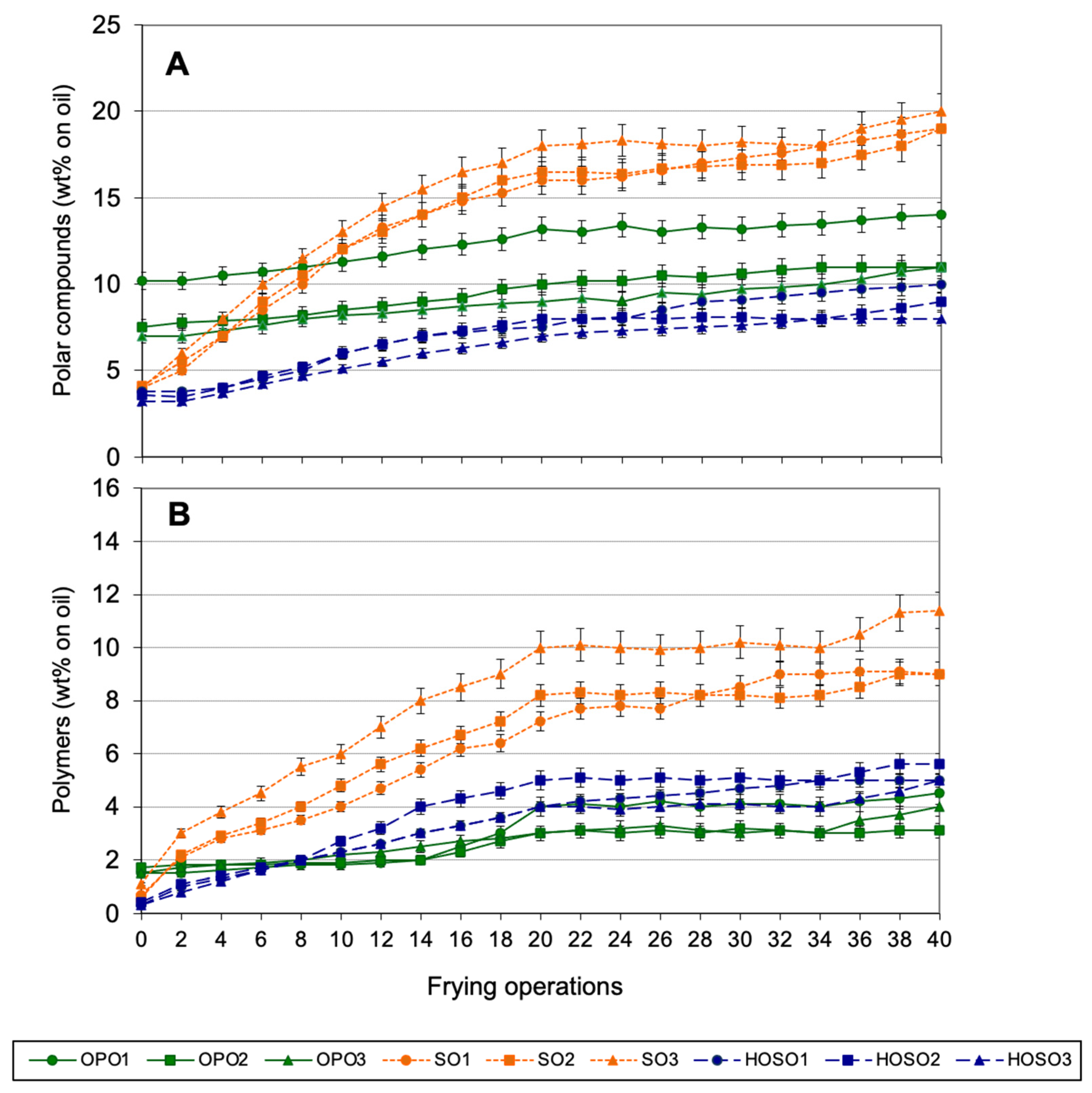
3.4. Continuous Frying Experiments
3.5. Evaluation of Fried Potatoes from Discontinuous Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Márquez-Ruiz, G.; Holgado, F. Frying performance of olive extracted oils. Grasas Aceites 2018, 69, e264. [Google Scholar] [CrossRef]
- Cecchi, L.; Innocenti, M.; Melani, F.; Migliorini, M.; Conte, L.; Mulinacci, N. New isobaric lignans from refined olive oils as quality markers for virgin olive oils. Food Chem. 2017, 219, 148–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruiz-Méndez, M.V.; Aguirre, M.R.; Marmesat, S. Olive Oil Refining Process. In Handbook of Olive Oil: Analysis and Properties; Aparicio, R., Harwood, J., Eds.; Springer: Boston, MA, USA, 2013; pp. 715–738. ISBN 978-1-4899-7724-3. [Google Scholar] [CrossRef]
- Mateos, R.; Sarriá, B.; Bravo, L. Nutritional and other Health Properties of Olive Pomace Oil. Crit. Rev. Food Sci. 2020, 60, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miana, M.; Jurado-Lopez, R.; Martínez-Martínez, E.; Gómez-Hurtado, N.; Delgado, C.; Bartolomé, M.V.; San Román, J.A.; Cordova, C.; Lahera, V.; et al. DIOL triterpenes block profibrotic effects of angiotensin II and protect from cardiac hypertrophy. PLoS ONE 2012, 7, e41545. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef]
- Agra, L.C.; Lins, M.P.; da Silva Marques, P.; Smaniotto, S.; Bandeira de Melo, C.; Lagente, V.; Barreto, E. Uvaol attenuates pleuritis and eosinophilic inflammation in ovalbumin-induced allergy in mice. Eur. J. Pharmacol. 2016, 780, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.H.; Solís-Gutiérrez, M.; Rojas-Molina, J.I.; Rivero-Cruz, F. Role of nitric oxide and hydrogen sulfide in the vasodilator effect of ursolic acid and uvaol from black cherry Prunus serotina fruits. Molecules 2016, 21, 78. [Google Scholar] [CrossRef]
- Suárez Montenegro, Z.J.; Álvarez-Rivera, G.; Sánchez-Martínez, J.D.; Gallego, R.; Valdés, A.; Bueno, M.; Cifuentes, A.; Ibáñez, E. Neuroprotective Effect of Terpenoids Recovered from Olive Oil By-Products. Foods 2021, 10, 1507. [Google Scholar] [CrossRef]
- Fernández-Arche, A.; Marquez-Martín, A.; de la Puerta Vazquez, R.; Perona, J.S.; Terencio, C.; Pérez-Camino, C.; Ruiz-Gutierrez, V. Long-chain fatty alcohols from pomace olive oil modulate the release of proinflammatory mediators. J. Nutr. Biochem. 2009, 20, 155–162. [Google Scholar] [CrossRef]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med. 2004, 229, 215–226. [Google Scholar] [CrossRef]
- Tekin, L.; Aday, M.S.; Yilmaz, E. Physicochemical changes in hazelnut, olive pomace, grapeseed and sunflower oils heated at frying temperatures. Food Sci. Technol. Res. 2009, 15, 519–524. [Google Scholar] [CrossRef]
- Bulut, E.; Yilmaz, E. Comparison of the Frying Stability of Sunflower and Refined Olive Pomace Oils with/without Adsorbent Treatment. J. Am. Oil Chem. Soc. 2010, 87, 1145–1153. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Ruiz-Méndez, M.V.; Velasco, J.; Dobarganes, M.C. Preventing oxidation during frying of foods. In Oxidation in Foods and Beverages and Antioxidant Applications; Decker, E., Elias, R., McClements, D.J., Eds.; Woodhead Publishing: Lancaster, PA, USA, 2010; Volume 2, pp. 239–273. ISBN 978-1-84569-983-3. [Google Scholar]
- Grompone, M.A. Sunflower and high-oleic sunflower oils. In Bailey´s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2020; Volume 2, pp. 685–738. ISBN 9781-119-25788-2. [Google Scholar]
- Giuffrè, A.M.; Caracciolo, M.; Zappia, C.; Capocasale, M.; Poiana, M. Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil. Ital. J. Food Sci. 2018, 30, 715–739. [Google Scholar] [CrossRef]
- Firestone, D. Regulation of Frying fats and oils. In Deep Frying: Chemistry, Nutrition and Practical Applications; Erickson, M.D., Ed.; AOCS Press: Champaign, IL, USA, 2007; pp. 373–385. ISBN 978-1-893997-92-9. [Google Scholar]
- Giuffrè, A.M.; Capocasale, M.; Macrì, R.; Caracciolo, M.; Zappia, C.; Poiana, M. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. Lebensm-Wiss Technol. 2020, 117, 108631. [Google Scholar] [CrossRef]
- Hammouda, I.B.; Triki, M.; Matthäus, B.; Bouaziz, M. A comparative study on formation of polar components, fatty acids and sterols during frying of refined olive pomace oil pure and its blend coconut oil. J. Agric. Food Chem. 2018, 66, 3514–3523. [Google Scholar] [CrossRef]
- Hammouda, I.B.; Márquez-Ruiz, G.; Holgado, F.; Freitas, F.; Gomes Da Silva, M.D.R.; Bouaziz, M. Comparative study of polymers and total polar compounds as indicators of refined oil degradation during frying. Eur. Food Res. Technol. 2019, 245, 967–976. [Google Scholar] [CrossRef]
- Ozkan, K.S.; Ketenoglu, O.; Yorulmaz, A.; Tekin, A. Utilization of molecular distillation for determining the effects of some minor compounds on the quality and frying stability of olive pomace oil. J. Food Sci. Technol. 2019, 56, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, I.B.; Márquez-Ruiz, G.; Holgado, F.; Sonda, A.; Skalicka-Wozniak, K.; Bouaziz, M. RP-UHPLC-DAD-QTOF-MS as a Powerful Tool of Oleuropein and Ligstroside Characterization in Olive-leaf Extract and their Contribution to the Improved Performance of Refined Olive-Pomace Oil during Heating. J. Agric. Food Chem. 2020, 68, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; Márquez-Ruiz, G.; Martín-Polvillo, M.; Ruiz-Méndez, M.V.; Dobarganes, M.C. Influence dimethylpolyxilosane addition to frying oils: Performance of Sunflower Oils in Discontinuous and Continuous Laboratory Frying. Grasas Aceites 1996, 47, 20–25. [Google Scholar] [CrossRef]
- COI Consejo Oleícola Internacional (International Olive Council); Madrid, Spain. Available online: https://www.internationaloliveoil.org/ (accessed on 10 December 2021).
- AOCS American Oil Chemists´ Society. Official Methods and Recommended Practices of the American Oil Chemists´ Society, 7th ed.; AOCS Press: Urbana, IL, USA, 2003; ISBN 13 978-0935584899. [Google Scholar]
- IUPAC International Union of Pure and Applied Chemistry. Standard Methods for the Analysis of Oil and Derivatives, 1st supplement to 7th ed.; Dieffenbacher, A., Pocklington, W.D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1992; ISBN 0-632-03337-I. [Google Scholar]
- ISO International Organization for Standardization. Geneva, Switzerland. Available online: https://www.iso.org/standards.html (accessed on 10 December 2021).
- Commission Regulation (EEC) N° 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis—Annex XIX Determination of Aliphatic Alcohols Content by Capillary Gas Chromatography. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991R2568-20191020 (accessed on 10 December 2021).
- Dobarganes, M.C.; Pérez-Camino, M.C.; Márquez-Ruiz, G. High performance size exclusion chromatography of polar compounds in heated and non-heated fats. Fat Sci. Technol. 1998, 90, 308–311. [Google Scholar] [CrossRef]
- Santos, C.S.P.; Molina-Garcia, L.; Cunha, S.C.; Casal, S. Fried potatoes: Impact of prolonged frying in monounsaturated oils. Food Chem. 2018, 243, 192–201. [Google Scholar] [CrossRef]
- Lim, J. Hedonic Scaling: A Review of Methods and Theory. Food Qual. Prefer. 2011, 22, 733–747. [Google Scholar] [CrossRef]
- AENOR Asociación Española de Normalización (Spanish Association for Standardization). Catálogo de Normas; UNE Asociación Española de Normalización Press: Madrid, Spain, 1991; ISBN 8486688469. [Google Scholar]
- Joint FAO/WHO Codex Alimentarius Commission. Codex standards for fats and oils from vegetable sources. In Codex Alimentarius, Fats and Oils and Related Products, 2nd ed.; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001; Volume 8, ISBN 92-5-104682-4. [Google Scholar]
- Erickson, D.R. Production and composition of frying fats. In Deep Frying: Chemistry, Nutrition and Practical Applications, 2nd ed.; Erickson, M.D., Ed.; AOCS Press: Champaign, IL, USA, 2007; pp. 3–24. ISBN 9781893997929. [Google Scholar]
- White, P. Fatty acids in oilseeds (vegetable oils). In Fatty Acids in Foods and Their Health Implications, 3rd ed.; Chow, K., Ed.; CRC Press Taylor and Francis: Boca Raton, FL, USA, 2008; pp. 227–262. ISBN 9780849372612. [Google Scholar]
- Pérez-Camino, M.C.; Cert, A. quantitative determination of hydroxyl pentacyclic triterpene acids in vegetable oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef]
- Brenes, M.; Romero, C.; García, A.; Hidalgo, F.J.; Ruiz Méndez, M.V. Phenolic compounds in olive oils intended for refining: Formation of 4-ethylphenol during olive paste storage. J. Agric. Food Chem. 2004, 52, 8177–8181. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Brenes, M.; Dobarganes, M.C.; Romero, C.; Ruiz-Méndez, M.V. Enrichment of pomace oil in triterpenic acids during storage of “Alperujo” olive paste. Eur. J. Lipid Sci. Technol. 2008, 110, 1136–1141. [Google Scholar] [CrossRef]
- Ruiz-Méndez, M.V.; Márquez-Ruiz, G.; Dobarganes, M.C. Relationships between quality of crude and refined edible oils based on quantitation of minor glyceridic compounds. Food Chem. 1997, 60, 549–554. [Google Scholar] [CrossRef]
- Giannoutso, E.P.; Meintanis, C.; Karagouni, A.D. Identification of yeast strains isolated from a two-phase decanter system olive oil waste and investigation on their ability for its fermentation. Bioresour. Technol. 2004, 93, 301–306. [Google Scholar] [CrossRef]
- Marmesat, S.; Morales, A.; Velasco, J.; Dobarganes, M.C. Influence of fatty acid composition on chemical changes in blends of sunflower oils during thermoxidation and frying. Food Chem. 2012, 135, 2333–2339. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Camino, M.C.; Márquez-Ruiz, G.; Ruiz-Méndez, M.V.; Dobarganes, M.C. Lipid changes during frying of frozen prefried foods. J. Food Sci. 1991, 56, 1644–1650. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N. Virgin olive oil as frying oil. Comp. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef]
- Abenoza, M.; de las Heras, P.; Benito, M.; Oria, R.; Sánchez-Gimeno, A.C. Changes in the physicochemical and nutritional parameters of Piqual and Arbequina olive oils during frying. J. Food Process Preserv. 2016, 40, 353–361. [Google Scholar] [CrossRef]
- Olivero-David, R.; Mena, C.; Pérez-Jimenez, M.A.; Sastre, B.; Bastida, S.; Márquez-Ruiz, G.; Sánchez-Muniz, F.J. Influence of Picual olive ripening on virgin olive oil alteration and stability during potato frying. J. Agric. Food Chem. 2014, 62, 11637–11646. [Google Scholar] [CrossRef] [PubMed]
- Olivero-David, R.; Mena, C.; Sánchez-Muniz, F.J.; Pérez-Jimenez, M.A.; Holgado, F.; Bastida, S.; Velasco, J. Frying performance of two virgin oils from Cornicabra olives with different ripeness indices. Grasas Aceites 2017, 68, e223. [Google Scholar] [CrossRef]
- Zribi, A.; Jabeur, H.; Aladedunye, F.; Rebai, A.; Matthäus, B.; Bouaziz, M. Monitoring of quality and stability characteristics and fatty acid compositions of refined olive and seed oils during repeated pan- and deep-frying using GC, FT-NIRS, and chemometrics. J. Agric. Food Chem. 2014, 62, 10357–10367. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.S.P.; Cunha, S.C.; Casal, S. Deep or air frying? A comparative study with different vegetable oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1600375. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Márquez Ruiz, G.; Pérez Camino, M.C. Thermal stability and frying performance of genetically modified sunflower seed (Helianthus annuus L.) oils. J. Agric. Food Chem. 1993, 41, 678–681. [Google Scholar] [CrossRef]
- Barrera-Arellano, D.; Ruiz-Méndez, M.V.; Velasco, J.; Márquez-Ruiz, G.; Dobarganes, M.C. Loss of tocopherols and formation of degradation compounds at frying temperatures in oils differing in unsaturation degree and natural antioxidant content. J. Sci. Food Agric. 2002, 82, 1696–1702. [Google Scholar] [CrossRef]
- Winkler, J.K.; Warner, K. The Effect of Phytosterol Structure on Thermal Polymerization of Heated Soybean Oil. Eur. J. Lipid Sci. Technol. 2008, 110, 1068–1077. [Google Scholar] [CrossRef]
- Singh, A. Sitosterol as an antioxidant in frying oils. Food Chem. 2013, 137, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Malecka, M. The effect of squalene on the thermostability of rapeseed oil. Nahrung 1994, 38, 135–140. [Google Scholar] [CrossRef]
- Manzi, P.; Panfili, G.; Esti, M.; Pizzoferrato, L. Natural antioxidants in the unsaponifiable fraction of virgin olive oils from different cultivars. J. Sci. Food Agric. 1998, 77, 115–120. [Google Scholar] [CrossRef]
- Totani, N.; Yawata, M.; Yasaki, N. Polydimethylsiloxane shows strong protective effects in continuous deep-frying operations. J. Oleo Sci. 2018, 67, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Banks, D. Industrial frying. In Deep Frying: Chemistry, Nutrition and Practical Applications, 2nd ed.; Erickson, M.D., Ed.; AOCS Press: Champaign, IL, USA, 2007; pp. 291–304. ISBN 9781893997929. [Google Scholar]
- Mesías, M.; Holgado, F.; Marquez-Ruiz, G.; Morales, F.J. Impact of the characteristics of fresh potatoes available in-retail on exposure to acrylamide: Case study for French fries. Food Control 2017, 73, 1407–1414. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Márquez-Ruiz, G.; Velasco., J. Interactions between fat and food during deep-frying. Eur. J. Lipid Sci. Technol. 2000, 102, 521–528. [Google Scholar] [CrossRef]
- Arslan, M.; Xiaobo, Z.; Shi, J.; Rakha, A.; Hu, X.; Zareef, M.; Zhai, X.; Basheer, S. Oil uptake by potato chips or french fries: A review. Eur. J. Lipid Sci. Technol. 2018, 120, 1800058. [Google Scholar] [CrossRef]
- Dana, D.; Saguy, I.S. Review: Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. Adv. Colloid Interface Sci. 2006, 128–130, 267–272. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of intermittent frying on chemical properties, fatty acid composition, and oxidative stability of 10 different vegetable oil blends. J. Food Process. Preserv. 2021, 44, 16015. [Google Scholar] [CrossRef]
| OPO1 | OPO2 | OPO3 | SO1 | SO2 | SO3 | HOSO1 | HOSO2 | HOSO3 | |
|---|---|---|---|---|---|---|---|---|---|
| Acidity (% oleic acid) | 0.21 ± 0.03 d | 0.12 ± 0.02 c | 0.12 ± 0.01 c | 0.06 ± 0.01 b | 0.05 ± 0.01 ab | 0.06 ± 0.01 b | 0.05 ± 0.01 ab | 0.05 ± 0.01 ab | <0.05 a |
| Peroxide value (meq O2/kg oil) | 3.0 ± 0.6 a | 2.8 ± 0.5 a | 2.4 ± 0.5 a | 5.7 ± 1.1 bc | 6.6 ± 1.3 c | 7.6 ± 1.2 c | 3.8 ± 0.8 ab | 3.6 ± 0.6 ab | 3.2 ± 0.6 a |
| Oxidative Stability Index (h) | 40.7 ± 1.5 cd | 44.0 ± 1.9 d | 42.8 ± 2.1 d | 11.6 ± 1.1 a | 10.9 ± 1.0 a | 10.9 ± 0.9 a | 36.7 ± 1.2 bc | 32.9 ± 1.3 b | 41.0 ± 1.5 d |
| Smoke point (°C) | 190 ± 2 a | 194 ± 2 a | 192 ± 3 b | 230 ± 1 c | 234 ± 2 c | 233 ± 2 c | 233 ± 3 c | 233 ± 2 c | 233 ± 2 c |
| Fatty acid composition (%) | |||||||||
| C16:0 | 11.03 ± 0.44 c | 11.44 ± 0.31 c | 11.45 ± 0.22 c | 6.45 ± 0.15 b | 6.40 ± 0.11 b | 6.59 ± 0.23 b | 4.51 ± 0.20 a | 4.75 ± 0.18 a | 4.22 ± 0.21 a |
| C16:1 | 0.85 ± 0.04 b | 0.91 ± 0.05 b | 0.83 ± 0.06 b | 0.13 ± 0.04 a | 0.10 ± 0.03 a | 0.10 ± 0.04 a | 0.18 ± 0.03 a | 0.14 ± 0.01 a | 0.14 ± 0.02 a |
| C18:0 | 3.16 ± 0.14 abc | 2.92 ± 0.10 ab | 2.86 ± 0.12 a | 3.40 ± 0.09 cd | 3.53 ± 0.15 cd | 3.71 ± 0.20 d | 3.23 ± 0.14 abc | 3.28 ± 0.12 bc | 3.27 ± 0.13 bc |
| C18:1 | 73.80 ± 0.89 e | 72.02 ± 0.65 d | 72.06 ± 0.60 d | 32.16 ± 0.38 c | 30.04 ± 0.29 b | 28.38 ± 0.20 a | 80.35 ± 0.70 g | 78.04 ± 0.67 f | 81.21 ± 0.44 g |
| C18:2 | 9.54 ± 0.26 a | 11.00 ± 0.16 b cd | 11.12 ± 0.10 cd | 56.56 ± 0.77 e | 58.70 ± 0.80 f | 60.00 ± 0.98 f | 10.15 ± 0.10 abc | 12.31 ± 0.12 d | 9.56 ± 0.10 ab |
| C18:3 | 0.62 ± 0.04 c | 0.40 ± 0.03 b | 0.45 ± 0.02 b | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.09 ± 0.01 a | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.09 ± 0.00 a |
| C20:0 | 0.46 ± 0.04 d | 0.19 ± 0.02 a | 0.23 ± 0.02 a | 0.31 ± 0.02 b | 0.29 ± 0.03 b | 0.29 ± 0.04 b | 0.35 ± 0.03 c | 0.33 ± 0.04 c | 0.36 ± 0.02 c |
| C20:1 | 0.37 ± 0.04 d | 0.16 ± 0.01 a | 0.18 ± 0.01 a | 0.26 ± 0.02 b | 0.24 ± 0.02 b | 0.23 ± 0.01 b | 0.31 ± 0.02 c | 0.30 ± 0.0.03 c | 0.31 ± 0.02 c |
| C22:0 | 0.17 ± 0.02 b | 0.05 ± 0.01 a | 0.05 ± 0.00 a | 0.65 ± 0.03 c | 0.61 ± 0.02 c | 0.62 ± 0.04 c | 0.84 ± 0.05 d | 0.78 ± 0.03 d | 0.83 ± 0.05 d |
| Total trans fatty acids | 0.21 ± 0.01 b | 0.32 ± 0.01 c | 0.29 ± 0.03 bc | 0.10 ± 0.01 a | 0.12 ± 0.01 a | 0.08 ± 0.01 a | 0.20 ± 0.01 b | 0.19 ± 0.02 b | 0.16 ± 0.02 b |
| OPO1 | OPO2 | OPO3 | SO1 | SO2 | SO3 | HOSO1 | HOSO2 | HOSO3 | |
|---|---|---|---|---|---|---|---|---|---|
| Unsaponifiable matter (wt.% on oil) | 1.28 ± 0.15 cd | 1.32 ± 0.10 cd | 1.49 ± 0.08 d | 1.08 ± 0.13 bc | 0.85 ± 0.08 ab | 0.83 ± 0.07 ab | 0.77 ± 0.06 a | 0.78 ± 0.05 a | 0.95 ± 0.10 ab |
| Sterols (wt.% on total) | |||||||||
| Cholesterol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Brassicasterol | <0.1 | <0.1 | <0.1 | 0.0 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Campesterol | 2.9 | 3.0 | 3.1 | 9.5 | 8.9 | 8.9 | 9.2 | 9.2 | 9.9 |
| Stigmasterol | 0.8 | 1.0 | 1.0 | 7.5 | 7.7 | 7.7 | 7.8 | 7.7 | 8.6 |
| β-Sitosterol | 88.6 | 87.5 | 85.8 | 54.6 | 55.8 | 55.5 | 53.3 | 53.0 | 55.2 |
| ∆7-Stigmastenol | 0.4 | 0.5 | 0.4 | 14.7 | 14.3 | 14.5 | 15.6 | 15.3 | 13.6 |
| 24-Methylen cholesterol | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 |
| Campestanol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 |
| ∆7-Campesterol | 0.0 | 0.0 | 0.0 | 2.7 | 2.5 | 2.5 | 3.2 | 3.2 | 2.8 |
| ∆5,23-Stigmastadienol | 0.2 | 0.2 | 0.1 | 0.0 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Clerosterol | 1.2 | 1.5 | 2.1 | 0.9 | 0.7 | 0.7 | 1.2 | 1.1 | 1.0 |
| Sitostanol | 1.4 | 1.7 | 1.9 | 0.5 | 0.5 | 0.4 | 0.4 | 0.5 | 0.4 |
| ∆5-Avenasterol | 1.2 | 2.0 | 2.0 | 2.8 | 2.9 | 2.9 | 2.4 | 2.6 | 2.5 |
| ∆5,24-Stigmastadienol | 1.6 | 1.7 | 2.4 | 1.4 | 1.1 | 1.3 | 1.5 | 1.6 | 1.1 |
| ∆7-Avenasterol | 1.4 | 0.6 | 0.9 | 5.1 | 5.3 | 5.4 | 5.1 | 5.4 | 4.5 |
| Total (mg/kg oil) | 3348 ± 35 f | 2756 ± 24 b | 2373 ± 15 a | 3328 ± 30 f | 3152 ± 26 e | 3136 ± 36 de | 2982 ± 18 c | 3066 ± 32 d | 3162 ± 35 e |
| Triterpenic alcohols (Erythrodiol + Uvaol) (mg/kg oil) | 579 ± 18 a | 647 ± 30 b | 648 ± 27 b | ||||||
| Aliphatic alcohols (C22 + C24 + C26 + C28) (mg/kg oil) | 2269 ± 71 b | 1677 ± 58 a | 1749 ± 39 a | ||||||
| Squalene (mg/kg oil) | 742 ± 26 a | 1538 ± 38 b | 816 ± 30 a | ||||||
| Triterpenic acids (Oleanoic acid + Maslinic acid) (mg/kg) | 102 ± 12 a | 126 ± 18 a | 123 ± 15 a | ||||||
| Tocopherols (mg/kg oil) | |||||||||
| α-Tocopherol | 415 | 350 | 272 | 473 | 493 | 482 | 424 | 413 | 431 |
| β-Tocopherol | 8 | 11 | 13 | 28 | 28 | 26 | 26 | 25 | 27 |
| γ -Tocopherol | 23 | 17 | 16 | 20 | 15 | 11 | 15 | 15 | 16 |
| δ -Tocopherol | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Total | 446 ± 24 c | 378 ± 18 b | 301 ± 10 a | 521 ± 28 de | 536 ± 32 e | 519 ± 13 de | 465 ± 21 cd | 453 ± 19 c | 474 ± 18 cd |
| Phenols (mg/kg oil) | |||||||||
| Hydroxytyrosol | 1 | <1 | <1 | ||||||
| Tyrosol | 1 | 1 | <1 | ||||||
| Vanillic acid | <1 | <1 | <1 | ||||||
| Vanillin | <1 | <1 | <1 | ||||||
| p-coumaric acid | <1 | <1 | <1 | ||||||
| Hydroxytyrosol acetate | 1 | <1 | <1 | ||||||
| Dialdehydic form of decarboxymethyl oleuropein aglycone | 1 | 2 | 3 | ||||||
| Tyrosol acetate | <1 | <1 | <1 | ||||||
| Dialdehydic form of decarboxymethyl ligstroside aglycone | 2 | 1 | 1 | ||||||
| Pinoresinol | 2 | 2 | 2 | ||||||
| Cinnamic acid | <1 | <1 | <1 | ||||||
| 1-Acetoxypinoresinol | 1 | 1 | 1 | ||||||
| Oleuropein aglycone | 6 | 1 | 1 | ||||||
| Ligstroside aglycone | 1 | 1 | 2 | ||||||
| Ferulic acid | <1 | <1 | <1 | ||||||
| Luteolin | 1 | <1 | <1 | ||||||
| Apigenin | <1 | <1 | <1 | ||||||
| Total polyphenols | 15 ± 1 b | 8 ± 1 a | 10 ± 1 a | ||||||
| Total orthodiphenols | 9 ± 1 b | 3 ± 0 a | 4 ± 1 a | ||||||
| Total secoiridoids | 9 ± 1 b | 5 ± 1 a | 7 ± 1 ab |
| OPO1 | OPO2 | OPO3 | SO1 | SO2 | SO3 | HOSO1 | HOSO2 | HOSO3 | |
|---|---|---|---|---|---|---|---|---|---|
| Total polar compounds (% on oil) | 10.3 ± 0.1 a | 7.5 ± 0.1 b | 7.0 ± 0.1 b | 4.0 ± 0.1 c | 4.1 ± 0.1 c | 4.0 ± 0.1 c | 3.7 ± 0.1 c | 3.6 ± 0.1 c | 3.2 ± 0.1 d |
| Oxidized triacylglycerol monomers | 1.1 ± 0.1 a | 1.2 ± 0.1 a | 1.2 ± 0.1a | 1.9 ± 0.2 b | 2.2 ± 0.1 b | 2.1 ± 0.1 b | 1.6 ± 0.1 a | 1.3 ± 0.1 a | 1.4 ± 0.1 a |
| Triacylglycerol dimers | 1.3 ± 0.1 a | 0.8 ± 0.1 a | 1.0 ± 0.1 a | 0.6 ± 0.1 b | 0.6 ± 0.1 b | 0.6 ± 0.1 b | 0.7 ± 0.1 b | 0.3 ± 0.1 b | 0.3 ± 0.0 b |
| Diacylglycerols | 7.2 ± 0.2 a | 5.0 ± 0.1 b | 4.4 ± 0.1 b | 1.1 ± 0.1 c | 1.0 ± 0.1 c | 0.9 ± 0.1 c | 1.3 ± 0.1 c | 1.2 ± 0.1 c | 1.1 ± 0.1 c |
| Monoacylglycerols | 0.3 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | nd | nd | nd | nd | nd | nd |
| Free fatty acids * | 0.2 ± 0.1 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | <0.1 |
| Oil | k PC (%/h) | r | Relative Rate | k Pol (%/h) | r | Relative Rate |
|---|---|---|---|---|---|---|
| OPO1 | 2.110 ± 0.10 | 0.986 | 1.11 | 1.089 ± 0.04 | 0.985 | 1.04 |
| OPO2 | 1.903 ± 0.08 | 0.994 | 1.00 | 1.074 ± 0.04 | 0.983 | 1.03 |
| OPO3 | 1.961 ± 0.07 | 0.990 | 1.03 | 1.043 ± 0.04 | 0.983 | 1.00 |
| SO1 | 4.687 ± 0.07 | 0.991 | 2.46 | 3.109 ± 0.05 | 0.995 | 2.98 |
| SO2 | 5.055 ± 0.05 | 0.991 | 2.66 | 3.269 ± 0.06 | 0.998 | 3.13 |
| SO3 | 5.158 ± 0.09 | 0.984 | 2.71 | 3.264 ± 0.07 | 0.997 | 3.13 |
| HOSO1 | 2.691 ± 0.05 | 0.991 | 1.41 | 1.874 ± 0.03 | 0.996 | 1.80 |
| HOSO2 | 2.633 ± 0.04 | 0.993 | 1.38 | 1.821 ± 0.02 | 0.998 | 1.75 |
| HOSO3 | 2.602 ± 0.04 | 0.987 | 1.37 | 1.772 ± 0.03 | 0.995 | 1.70 |
| OPO1 | OPO2 | OPO3 | SO1 | SO2 | SO3 | HOSO1 | HOSO2 | HOSO3 | |
|---|---|---|---|---|---|---|---|---|---|
| Total polar compounds (wt.% on oil) | 25.5 ± 0.1 b | 26.0 ± 0.2 b | 25.7 ± 0.1 b | 25.8 ± 0.2 b | 25.0 ± 0.1 a | 25.7 ± 0.1 b | 25.5 ± 0.2 ab | 25.0 ± 0.2 a | 26.0 ± 0.2 b |
| Oxidized triacylglycerol monomers | 8.1 ± 0.1 a | 9.2 ± 0.2 b | 8.9 ± 0.2 b | 9.0 ± 0.1 b | 8.8 ± 0.2 b | 9.0 ± 0.1 b | 8.6 ± 0.1 b | 7.7 ± 0.2 a | 8.2 ± 0.1 a |
| Triacylglycerol polymers * | 9.8 ± 0.2 a | 11.3 ± 0.1 b | 11.2 ± 0.1 b | 15.5 ± 0.1 c | 15.2 ± 0.1 c | 15.7 ± 0.2 d | 15.7 ± 0.3 d | 16.0 ± 0.2 d | 16.7 ± 0.1 d |
| Diacylglycerols | 7.1 ± 0.2 a | 5.3 ± 0.3 b | 5.2 ± 0.3 b | 1.1 ± 0.2 c | 0.9 ± 0.1 c | 0.9 ± 0.3 c | 1.2 ± 0.1 c | 1.2 ± 0.3 c | 1.1 ± 0.1 c |
| Monoacylglycerols | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | nd | nd | nd | nd | nd | nd |
| Free fatty acids § | 0.3 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.0 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a |
| Oils | Fried Potatoes | ||||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | Oil Content (%) | |
| OPO1 | 92.50 ± 1.90 a | −7.90 ± 0.50 a | 31.93 ± 3.10 a | 67.63 ± 1.49 a | 3.52 ± 0.88 a | 31.56 ± 1.95 a | 12.0 ± 0.3 a |
| OPO2 | 94.38 ± 1.70 b | −6.96 ± 0.51 b | 37.50 ± 1.21 b | 64.09 ± 2.37 a | 4.32 ± 1.31 a | 32.18 ± 1.55 a | 11.9 ± 0.2 a |
| OPO3 | 95.32 ± 1.55 b | −5.37 ± 0.70 b | 36.97 ± 1.18 b | 65.82 ± 2.38 a | 3.61 ± 1.02 a | 32.26 ± 1.70 a | 12.1 ± 0.6 a |
| SO1 | 98.93 ± 3.10 c | −2.11 ± 0.30 c | 7.71 ± 1.00 c | 66.72 ± 3.01 a | 3.64 ± 1.00 a | 32.44 ± 1.92 a | 12.3 ± 0.5 a |
| SO2 | 99.08 ± 2.94 c | −1.72 ± 0.42 c | 6.70 ± 1.00 c | 65.23 ± 0.28 a | 3.18 ± 0.67 a | 32.67 ± 1.97 a | 12.1 ± 0.3 a |
| SO3 | 99.11 ± 2.78 c | −1.74 ± 0.31 c | 6.39 ± 1.00 c | 65.08 ± 2.09 a | 4.33 ± 0.52 a | 31.81 ± 2.08 a | 11.9 ± 0.7 a |
| HOSO1 | 98.91 ± 3.10 c | −1.95 ± 0.50 c | 7.31 ± 1.30 c | 66.30 ± 1.52 a | 2.98 ± 0.47 a | 33.84 ± 1.59 a | 12.3 ± 0.7 a |
| HOSO2 | 98.64 ± 3.40 c | −2.19 ± 0.41 c | 8.20 ± 1.10 c | 66.50 ± 0.80 a | 2.99 ± 0.90 a | 34.86 ± 1.85 a | 12.1 ± 0.8 a |
| HOSO3 | 98.32 ± 2.90 c | −2.30 ± 0.34 c | 8.21 ± 0.90 c | 66.56 ± 3.75 a | 4.44 ± 0.92 a | 33.67 ± 2.14 a | 11.9 ± 0.3 a |
| Sensory Attribute | |||||
|---|---|---|---|---|---|
| Texture | Oiliness | Taste | Color | Global Appreciation | |
| OPO1 | 4.0 ± 1.3 a | 4.7 ± 2.3 a | 4.7 ± 1.2 a | 4.3 ± 0.6 a | 5.2 ± 1.2 a |
| OPO2 | 4.0 ± 1.9 a | 3.9 ± 1.3 a | 5.6 ± 1.5 a | 4.5 ± 0.9 a | 5.4 ± 1.3 a |
| OPO3 | 4.4 ± 1.8 a | 4.3 ± 1.8 a | 5.4 ± 1.5 a | 4.5 ± 0.5 a | 5.3 ± 1.5 a |
| SO1 | 4.3 ± 1.9 a | 4.3 ± 1.9 a | 5.1 ± 1.5 a | 4.6 ± 1.0 a | 5.0 ± 1.2 a |
| SO2 | 3.8 ± 1.7 a | 4.5 ± 1.6 a | 4.7 ± 1.4 a | 4.3 ± 1.0 a | 4.8 ± 1.2 a |
| SO3 | 5.1 ± 2.3 a | 5.0 ± 1.3 a | 5.8 ± 1.1 a | 4.4 ± 2.0 a | 5.3 ± 1.1 a |
| HOSO1 | 3.5 ± 2.1 a | 3.8 ± 1.8 a | 5.2 ± 1.0 a | 3.7 ± 1.2 a | 4.9 ± 0.9 a |
| HOSO2 | 4.9 ± 2.0 a | 4.8 ± 1.7 a | 5.4 ± 1.2 a | 4.7 ± 1.3 a | 5.7 ± 1.3 a |
| HOSO3 | 4.7 ± 1.1 a | 5.2 ± 1.6 a | 5.2 ± 1.8 a | 4.5 ± 2.1 a | 5.3 ± 1.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holgado, F.; Ruiz-Méndez, M.V.; Velasco, J.; Márquez-Ruiz, G. Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comparative Behavior with Sunflower Oils and High-Oleic Sunflower Oils. Foods 2021, 10, 3081. https://doi.org/10.3390/foods10123081
Holgado F, Ruiz-Méndez MV, Velasco J, Márquez-Ruiz G. Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comparative Behavior with Sunflower Oils and High-Oleic Sunflower Oils. Foods. 2021; 10(12):3081. https://doi.org/10.3390/foods10123081
Chicago/Turabian StyleHolgado, Francisca, María Victoria Ruiz-Méndez, Joaquín Velasco, and Gloria Márquez-Ruiz. 2021. "Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comparative Behavior with Sunflower Oils and High-Oleic Sunflower Oils" Foods 10, no. 12: 3081. https://doi.org/10.3390/foods10123081
APA StyleHolgado, F., Ruiz-Méndez, M. V., Velasco, J., & Márquez-Ruiz, G. (2021). Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comparative Behavior with Sunflower Oils and High-Oleic Sunflower Oils. Foods, 10(12), 3081. https://doi.org/10.3390/foods10123081