1. Introduction
Pasta dishes have been known since the beginning of the first century BC [
1]. Traditionally, pasta is made from durum wheat (
Triticum durum). Although durum wheat contains B vitamins, macro- and microelements, some essential acids (phenylalanine, tryptophan, and isoleucine), the main component is starch, providing a low glycemic index [
2,
3]. Durum wheat pasta, due to its nutritional properties, low cost, and long shelf life, remains a traditional food product in the diet of many countries. The popularity of pasta is promoted by the variety of dishes that can be prepared from them—as an independent dish [
4] or (carbonara pasta, pasta with bolognese sauce, trenette with pesto sauce) or be an ingredient in various dishes, such as lasagna, pizzoccheri ravioli, tortellini, soups.
The nutritional value of pasta can be improved by adding unconventional raw materials rich in dietary fiber [
5,
6], vitamins, and polyunsaturated fatty acids [
7,
8,
9]. Plant pigments of unconventional raw materials change the sensory properties of pasta (tomato, carrot, spinach), with their simultaneous enrichment, but they cannot enrich PUFA [
10,
11]. Omega-3 fatty acids, including eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, are essential since they cannot be synthesized by the human body. Fatty fish such as salmon, mackerel and sardines, fish oil, some nuts, and vegetable oils are the main sources of EPA and DHA in diets, while rapeseed, walnut, soy, and flaxseed oils contain α-linolenic acid (ALA) [
12].
A natural source of EPA and DHA is also macroalgae of the
Isochrysidaceae families (genus
Isochrysis galbana (Ig) and
Pavlovaceae (genus
Diacronema vlkianum (Dv)), which accumulate omega-3 fatty acids, making them an important food raw material obtained in aquaculture [
13,
14].
Microalgae of the genus
Chlorella are a promising source of biologically active substances.
Chlorella biomass is rich in polyunsaturated fatty acids [
15], contains protein up to 50%, folic acid, niacin, choline, pantothenic acid, as well as more than 10 types of vitamins, micro- and macroelements, including Ca, K, Fe, Na, Mg, Zn, Cu, P, and Se [
16,
17,
18,
19,
20]. The chlorophyll content in
Chlorella reaches 4%, which is 5–10 times more than in the algae
Spirulina and
Alfalfa (
Medicago) [
21,
22]. Currently
Chlorella is classified as a food supplement [
23,
24]. It is widely used in food products for the prevention of iron deficiency, anemia, lowering blood cholesterol levels, etc.
There are some results of clinical trials of
Chlorella biomass [
25] and analysis of its biotechnological potential. The author Muller-Feuga [
25] proposed
Chlorella biomass as an additive for yogurt in order to increase the viability of bacterial probiotics. Emulsions enriched with carotenoids and polyunsaturated fatty acids obtained from the biomass of
Chlorella microalgae [
26,
27,
28,
29], thickened desserts [
30], biscuits [
31], and pasta [
32] have been developed. There is an experience in using
Chlorella protein hydrolyzate as a food additive [
33]. Most often,
Chlorella biomass is used in the form of a dry powder since in this form it is most bioavailable for human digestive enzymes [
34,
35].
The aim of this study is to develop pasta recipe with additives of microalgae biomass C. sorokiniana and study of their quality indicators.
2. Materials and Methods
To obtain the biomass of the
Chlorella microalgae, a pre-culture of
C. sorokiniana (
strain 211-8k) from the collection of algae of the University of Göttingen (Culture Collection of Algae at Göttingen University, international acronym SAG) was used. Biomass cultivation was carried out in a laboratory photobioreactor in the mode of illumination with fluorescent lamps (luminous flux 2500 ± 300 Lx, T (K) 400, daylight, photoperiod—12 h) [
34,
36]. The cultivation temperature was (23 ± 1) °C; the intensity of aeration of the mixture—1.2–1.8 L/min; mixing mode–periodic (15 min once a day); stirring speed—500 rpm. For the cultivation of microalgae, a culture medium, well-balanced in the content of macro- and microelements, for example MgSO
4·7H
2O, KH
2PO
4, MnCl
2·4H
2O, ZnSO
4·7H
2O and other. [
37]. The concentration of the cell suspension was carried out by centrifugation at 6000 rpm for 5 min, after which the moist biomass was dehydrated by lyophilization with air cooling Alpha 2–4 Ldplus (pressure 1 MBar, freezing temperature −55 °C). The residual moisture content in dry biomass of microalgae ranged from 3.5 to 4.0%.
While analyzing the chemical composition and biologically active substances in the obtained samples of air-dry biomass of microalgae
C. sorokiniana, the following characteristics were determined: moisture content by drying [
38]; the content of protein, fat and carbohydrates [
39]; the fatty acid composition of the allocated lipids [
40]; the content of chlorophylls and carotenoids according to the method of the authors Nayek et al. [
41]; the content of phenolic compounds by the Folin–Ciocalteu method [
42]; microbiological indicators such as
Escherichia coli, Staphylococcus aureus, and
Salmonella [
43]. When developing pasta recipes with partial substitution of the flour with biomass of microalgae, organoleptic characteristics of pasta after culinary processing was determined [
44].
The lipid fraction extraction from C. sorokiniana microalgae biomass was carried out on the Büchi E-812 SOX Soxhlet apparatus. For this, 3 g of dry biomass was placed in a cellulose glass (33 mm × 94 mm). A mixture of a solution of ethanol: n-hexane (1:9) was used as an extractant. For 3 g of dry biomass, 100 mL of extractant was used
The composition of higher fatty acids in the sum of lipids obtained from the Chlorella sorokiniana microalgae biomass was determined using the gas chromatograph with a flame ionization detector Agilent Technologies Sales & Services GmbH & Co.KG (Waldbronn, Germany), on a BPX70 column (60 m × 0.25 mm × 0.25 μm), SGE Analytical Science, VWR International GmbH; carrier gas was nitrogen.
To prepare the pasta dough, we used commercial durum wheat flour Molino Grassi (manufactured by Molino Grassi S.p.A., Parma, Italy), drinking water, and egg melange. During the development of pasta recipes, we used the replacement of flour with dry biomass of microalgae in an amount of 2.5 to 7.5% by weight of the flour mixture. The preparation of pasta control samples was carried out without the use of microalgae biomass additives. The technology for preparing pasta dough includes the processes of mixing dry components with water, extrusion (extrusion into the required shape), and dehydration (drying under controlled conditions). The dough pieces were molded in the form of a Tagliatelle 7 mm wide and 500 mm long, then dried in an Electrolux oven at 60 °C for 5 h. In dry pasta, the titratable acidity index was determined. The culinary processing of experimental and control samples of pasta was carried out by boiling in water in a ratio of 1:10 (by weight) at a temperature of ~90 °C for 5–7 min until cooked.
The quality of finished pasta was assessed by the increase in the mass and volume of the products during cooking. The preservation of the shape of the products, and the loss of protein substances [
45] and chlorophyll [
41] was determined.
The determination of the concentration of metal ions in the samples was carried out by anodic stripping voltammetry on the TA-lab analyzer (NPO Tomyanalit LLC, Khimreaktiv, Russia) after mineralization. The concentration of nitrates was determined by photometric method according to the state standard. Statistical processing of the results was carried out. Reagents are manufactured by JSC “Khimreaktiv” Russia.
Samples of pasta were prepared in distilled water until the optimum cooking time, and after drying for 2 min, they were served to experts. Experts rated the products for flavor, appearance, texture, and overall acceptability using a 7-point hedonic scale ranging from 7 (like) to 1 (strongly dislike) for each sensory characteristic [
46,
47].
3. Results
Table 1 shows the general chemical composition of dry biomass of microalgae
C. sorokiniana obtained by cultivation in the photobioreactor [
48,
49].
It was determined that dry biomass of microalgae C. sorokiniana contains about 48% protein, which is significantly higher than in soy, an alternative source of vegetable protein. Lipid content reached 13% of the biomass sample, which is comparable to or slightly higher than in other aquacultures such as spirulina.
During cultivation, microalgae are able to accumulate metals from the nutrient medium and introduced additives. For further use of biomass in food recipes, it is necessary to establish compliance with the safety indicators of the obtained samples in accordance with the Customs Union Technical Regulations on the safety of food products CU TR 021/2011, according to which the content of toxic elements, nitrates, and microbiological indicators are normalized.
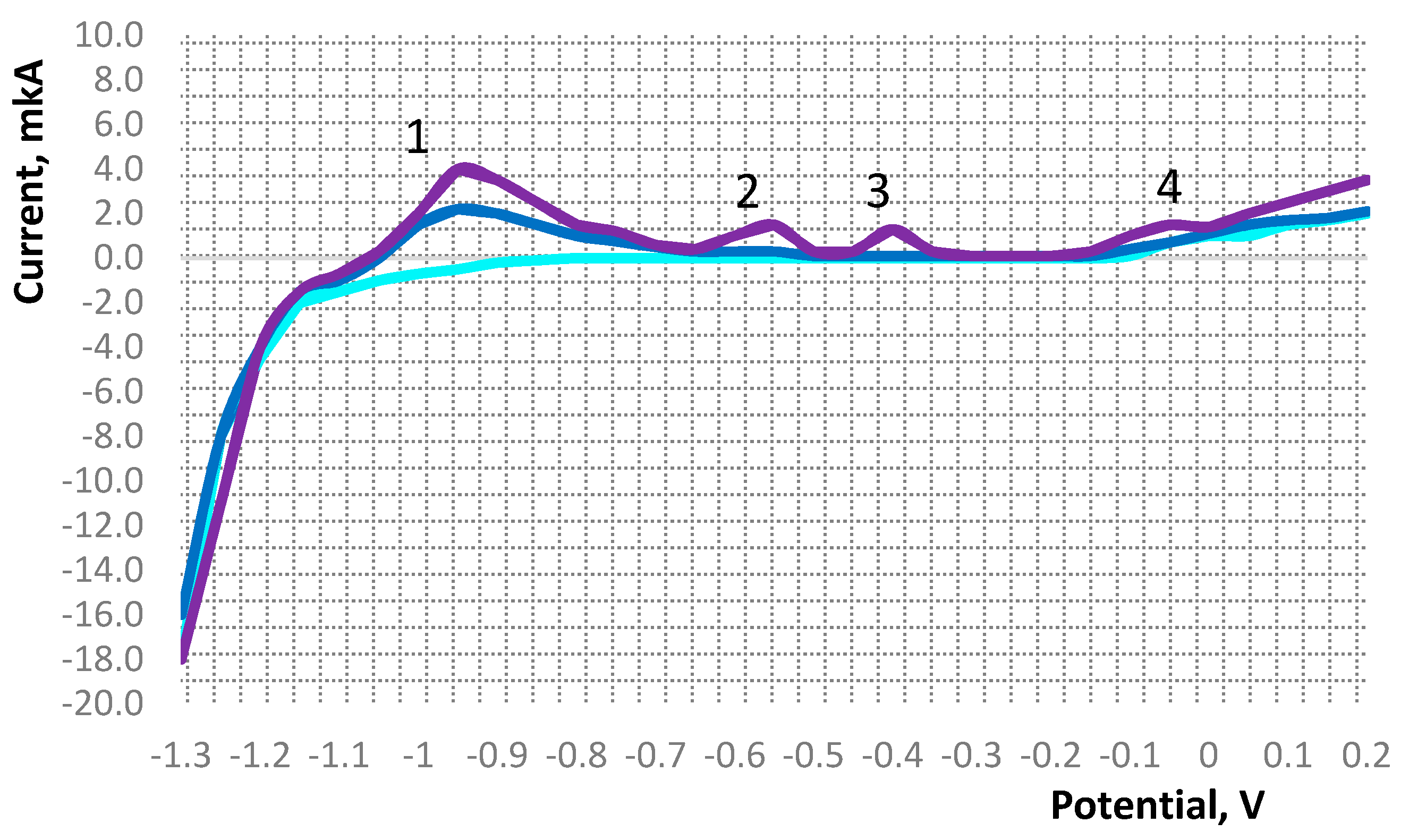
Figure 1 shows the results of studying the content of metals in biomass samples by the voltametric method.
The voltammogram shows peaks of current, which serves as an analytical signal, characterizes the nature of the determined element and its concentration in the solution of the electrochemical cell. This figure shows the current peaks for zinc (1), cadmium (2), lead (3), and copper (4). The intensity of the peak signals is the sum of the value of the background current and the electric dissolution current of the determined element concentrate from the surface of the electrode [
50].
Table 2 shows the results of studies of the residual content of zinc, copper, and iron in samples of the biomass of microalgae
C. sorokiniana.
In acceptable amounts, Zn, Cu, and Fe are trace elements, necessary for normal functioning of the human body [
51], while lead, cadmium, arsenic, and mercury are toxic elements. Determination of the concentration of toxic elements is necessary to determine the safety of biomass along with the determination of the concentration of nitrates and microbiological indicators.
Table 3 and
Table 4 show the results of determining the content of toxic elements and microbiological parameters in the obtained samples of the
C. sorokiniana biomass.
The content of toxic elements does not exceed the permissible values according to the Customs Union Technical Regulations on safety of food products CU TR 021/2011.
The content of nitrates in biomass is 768 ± 80 mg/kg, which is an acceptable value for biologically active substances.
The content of microbiological indicators complies with the safety standards CU TR 021/2011 and confirms the safety of microalgae biomass.
It is equally important to determine the amino acid and fatty acid composition for understanding the value of food raw materials.
Table 5 shows the composition of a sample of microalgae
C. sorokiniana dry biomass, obtained by cultivation in a pilot bioreactor [
47].
Dry biomass of microalgae C. sorokiniana contains all the amino acids necessary for the development of living organisms.
About 80% of the total fatty acid content of C. sorokiniana lipids are unsaturated fatty acids. In addition, the biomass is rich in biologically active phytochemicals, along which chlorophylls (22.13 mg/g dry biomass) and carotenoids (6.04 mg/g dry biomass) prevail.
Thus, the inclusion of
C. sorokiniana biomass additives in pasta recipes will allow enriching products with essential lipids and minor nutritional components, as well as achieving the required pasta color range without the use of synthetic dyes. It was found that the content of phenolic compounds in the of dry biomass samples is about 0.05 mg/g. In previous studies we examined the antioxidant properties of
C. sorokiniana microalgae and analyzed the total content of phenolic antioxidants in microalgae samples obtained under various light conditions [
52]. The content of organic acids in the resulting biomass was about 2.70 mg/g.
Figure 2 shows the results of a sensory evaluation of finished pasta with the replacement of a part of the flour in the formulations with dry biomass of
C. sorokiniana microalgae and pasta samples.
Pasta with microalgae biomass additives had a color from light green to intense green, depending on the amount of additive. It was noted that the samples of pasta with the addition of more than 5% of biomass had a significant fishy flavor. Thus, the addition of microalgae in an amount of more than 5% is impractical.
The results of studies of physicochemical quality indicators of pasta with additives of microalgae
C. sorokiniana dry biomass are presented in
Table 6.
It was determined that replacing flour with dry biomass of microalgae in the range from 2.5 to 7.5% practically does not affect the moisture loss during drying of pasta, and the loss of dry matter during pasta cooking does not exceed the established values [
44].
Protein losses during pasta cooking increase in proportion to the amount of added microalgae additives and vary from 2.6 to 3.4 g per 100 g of finished products.
The introduction of
C. sorokiniana biomass additives into pasta makes it possible to enrich them with chlorophyll, and its content increases in proportion to the amount of the additive and reaches from 25% to 77% [
53]. Valuable composition is possessed not only by
Chlorella, but also by other types of microalgae [
54].
Figure 3 shows the chlorophyll content depending on the amount of microalgae biomass additive and chlorophyll loss during pasta cooking.
It was found that the total loss of chlorophyll during pasta cooking is about 10%.
As a result of studies of the shape retention of pasta during cooking, it turned out that the addition of microalgae biomass more than 5% leads to an increase in the digestibility of products.
The presence of gluten in wheat flour promotes the process of swelling and water retention, which contributes to an increase in the volume and weight of products during cooking. The absence of gluten in the composition of microalgae leads to a decrease in water-holding capacity, which entails a slight decrease in the coefficient of increase in the mass of pasta.
Thus, it can be concluded that replacing more than 5% of flour with dry biomass of C. sorokiniana microalgae is not advisable.
The nutritional value of pasta is presented in
Table 7 [
55].
4. Discussion
The issue of using microalgae biomass for increasing the nutritional value of pasta has been covered in the literature. There is an experience of using food additives from broccoli [
56], amaranth [
57], cumini pulp [
58], and gac fruit [
59] in pasta. The unique composition of
Chlorella biomass makes it possible to obtain products enriched with the various essential macro- and micronutrients. The addition of 5% dry biomass of
C. sorokiniana microalgae to flour allowed to increase the protein content by 18% and lipids by 10% in comparison with the control sample; chlorophyll by 34.3%, and carotenoids by 23.6% of the recommended daily intake of these micronutrients according to MR 2.3.1.0253-21.
The authors Lemes et al. [
60] note an increase in the content of proteins in pasta up to 14.5% in the case of the addition of
Spirulina platensis (Cyanobacteria) biomass in the amount of 10%. In the work of the authors Farouk et al. [
61], proposed adding the algae
Dunaliella salina (Chlorophyta) biomass to pasta. The results of studying the composition of pasta showed that the maximum introduction of biomass additive (3% per 100 g of products) allows reaching a protein content of 13.6 ± 0.46%, fats—1.18 ± 0.05%, carbohydrates—83.12 ± 0.75%.
Authors Ozyurt et al. [
47], who enriched pasta with the addition of
Arthrospira (for-merly Spirulina) microalgae biomass in an amount of 10% and obtained a sample with optimal organoleptic properties. The authors Fradique et al. [
32] pointed to an improvement in the appearance of pasta with the addition of the biomass of microalgae
Chlorella vulgaris (Chlorophyta) and
Limnospira maxima (formerly Spirulina maxima) (Cyano-bacteria), without negative changes in the culinary and textural properties of the finished product. The results obtained on the content of proteins and lipids in the samples of macaroni products with the supplement of 5% microalgae biomass corresponds the literature review.
The carbohydrate content in pasta decreases in proportion to the amount of flour with microalgae biomass substitution. The proposed amount of carbohydrates in flour is about 68%, and in the biomass of C. sorokiniana microalgae is about 7% and the calorie content of pasta is 332.8 kcal. Thus, the addition of dry biomass of C. sorokiniana microalgae to pasta in the amount of 5% flour mixture does not affect the increase in the calorie content of pasta.
According to research conducted by the authors Klejdus et al. [
62], microalgae synthesize isoflavones, flavanones, flavonols, and dihydrohalcones. As a result of the analysis of the total content of phenols and flavonoids in green unicellular algae
Chlorella vulgaris it has been shown that methanol extracts of
C. vulgaris contain 220 mg-eq. gallic acid and 131.15 mg-eq. quercitin [
63,
64]. It is known that the content of phenols in microalgae depends on the composition of the medium and growing conditions. We have previously obtained results indicating that the amount of antioxidant activity of
C. sorokiniana microalgae biomass correlates with the total content of phenolic compounds and depends on the illumination mode [
52].
The disadvantage of using microalgae biomass in the pasta production is the in-stability of unsaturated fatty acids which are subjected to oxidation during the storage. To increase the storage duration, it is proposed to pack products in vacuum bags made of light-proof matte polymer material.