Polyphenol Loaded W1/O/W2 Emulsions Stabilized with Lesser Mealworm (Alphitobius diaperinus) Protein Concentrate Produced by Membrane Emulsification: Stability under Simulated Storage, Process, and Digestion Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lesser Mealworm Protein Concentrate (LMPC)
2.3. Osmolality of W1 and W2
2.4. W1/O/W2 Emulsions Production
2.4.1. Coarse W1/O/W2 Emulsion
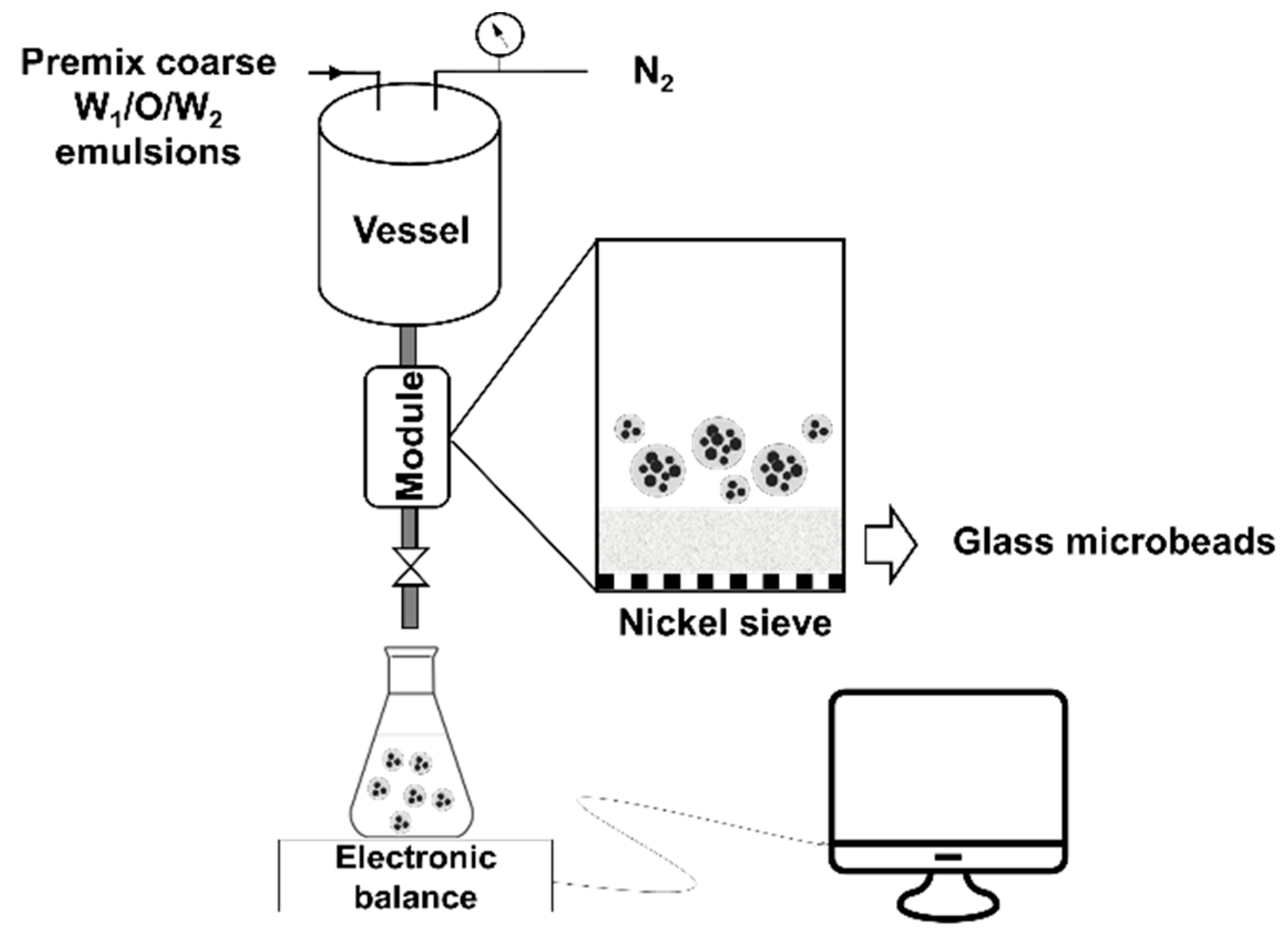
2.4.2. Refinement of W1/O/W2 emulsions by Dynamic Membranes of Tunable Pore Size (DMTS)
2.5. Environmental Stress Test
2.5.1. pH
2.5.2. Temperature
2.5.3. Osmotic Stress
2.6. Characterization of Emulsions
2.6.1. Droplet Size Distribution
2.6.2. Zeta Potential
2.6.3. Encapsulation Efficiency of Polyphenols
2.6.4. Microstructure Analysis
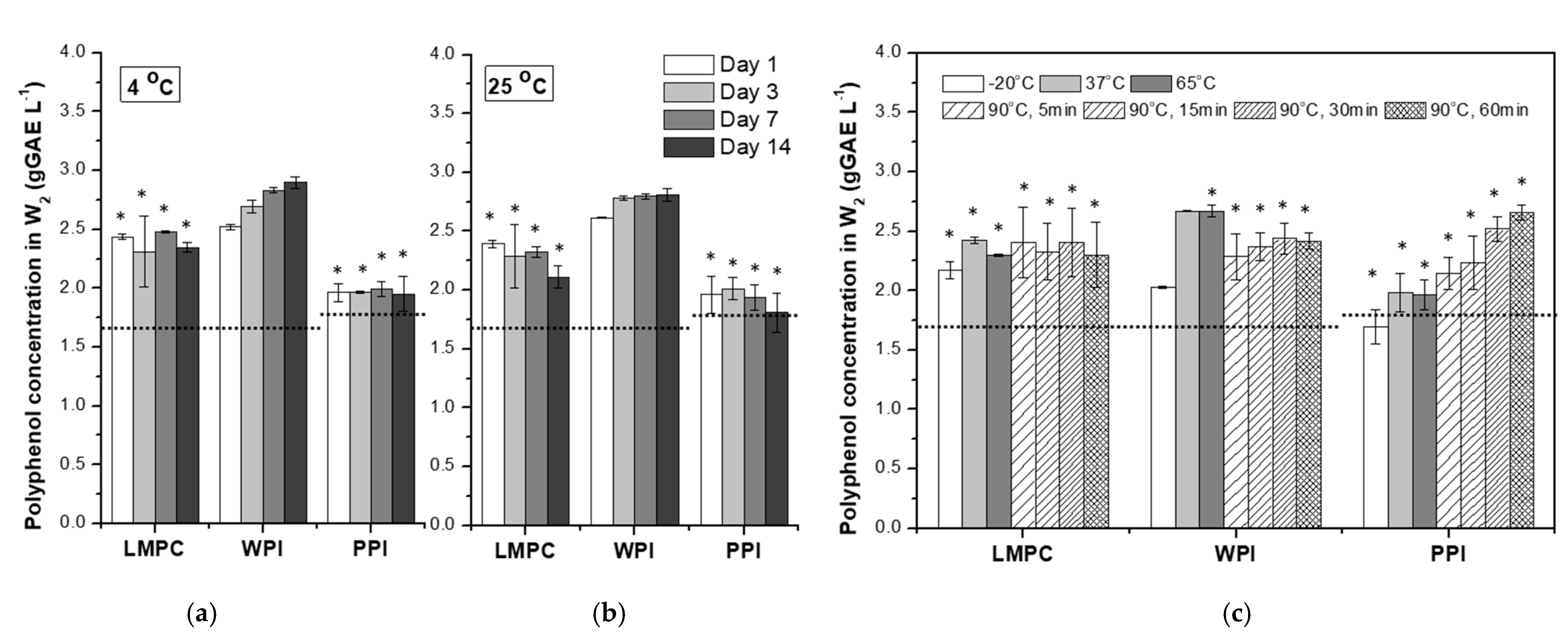
2.7. Interaction of Protein and Polyphenols
2.8. Statistical Analysis
3. Results
3.1. Production of W1/O/W2 Emulsions Stabilized with LMPC, WPI, and PPI
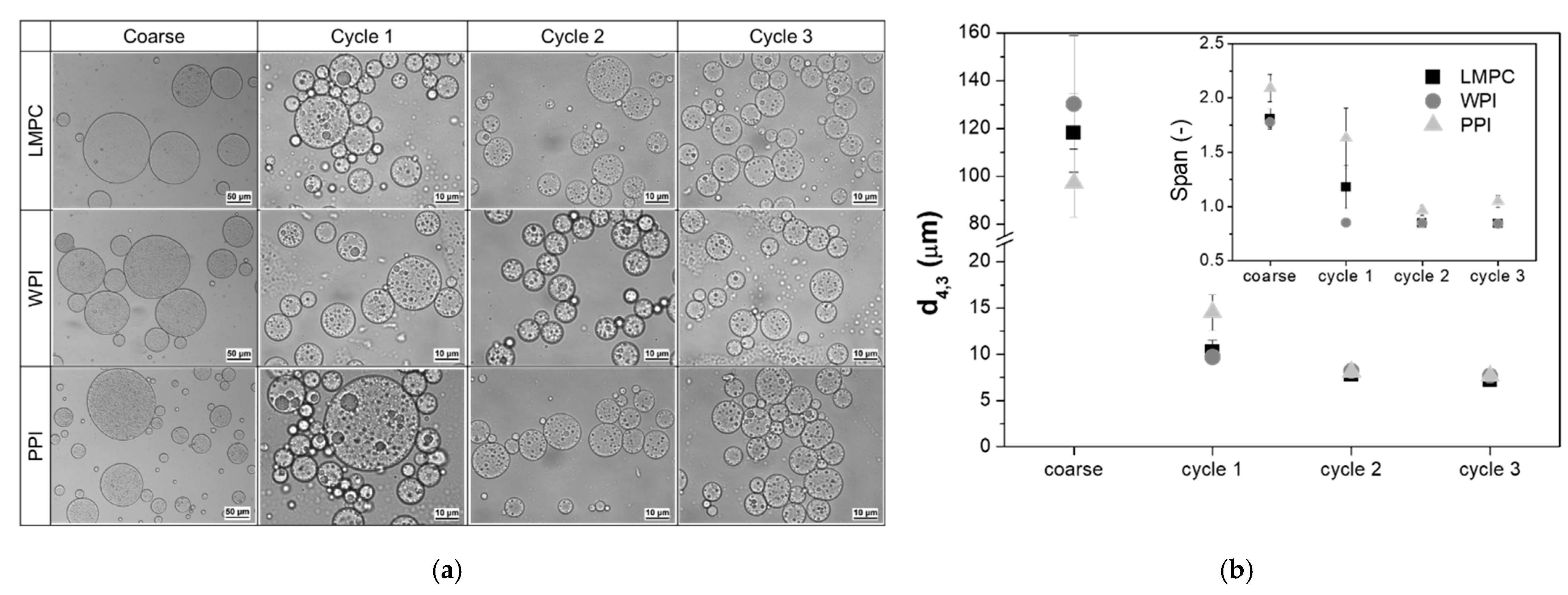
3.1.1. Particle Size Distribution and Transmembrane Flux
3.1.2. Encapsulation Efficiency (EE)
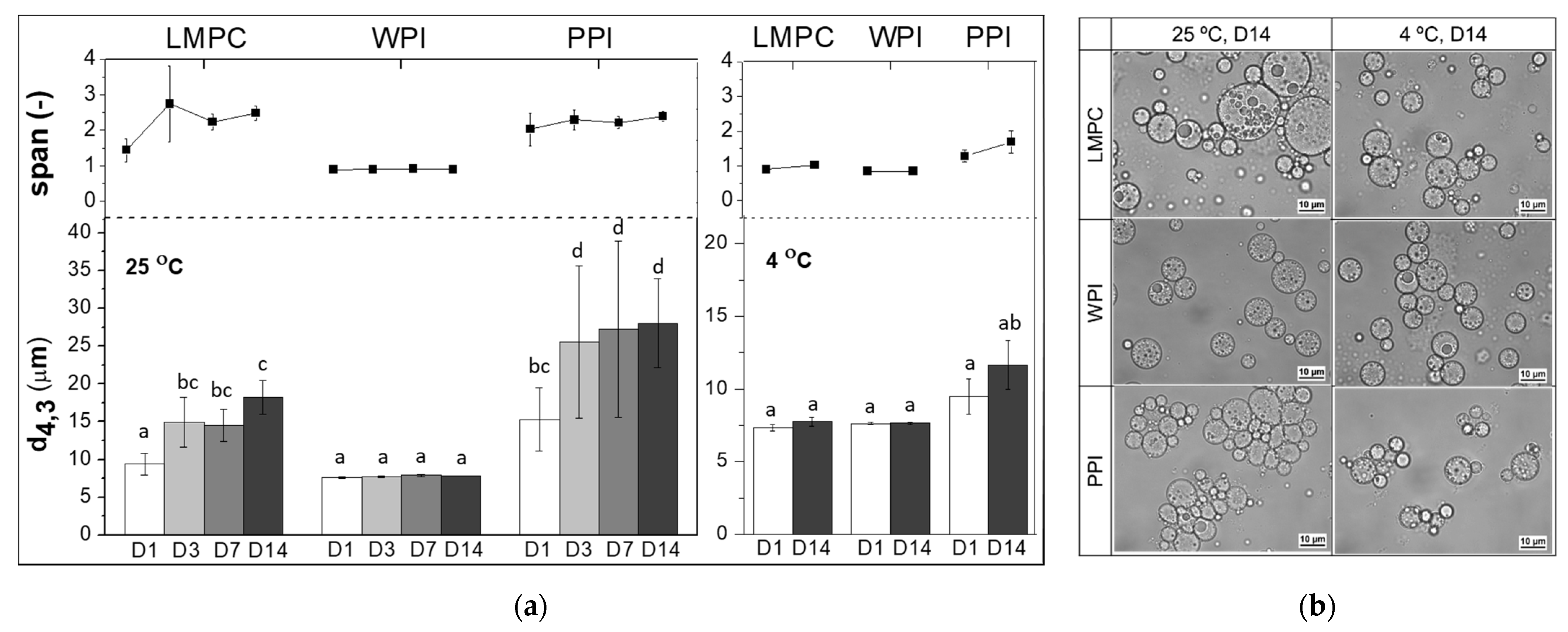
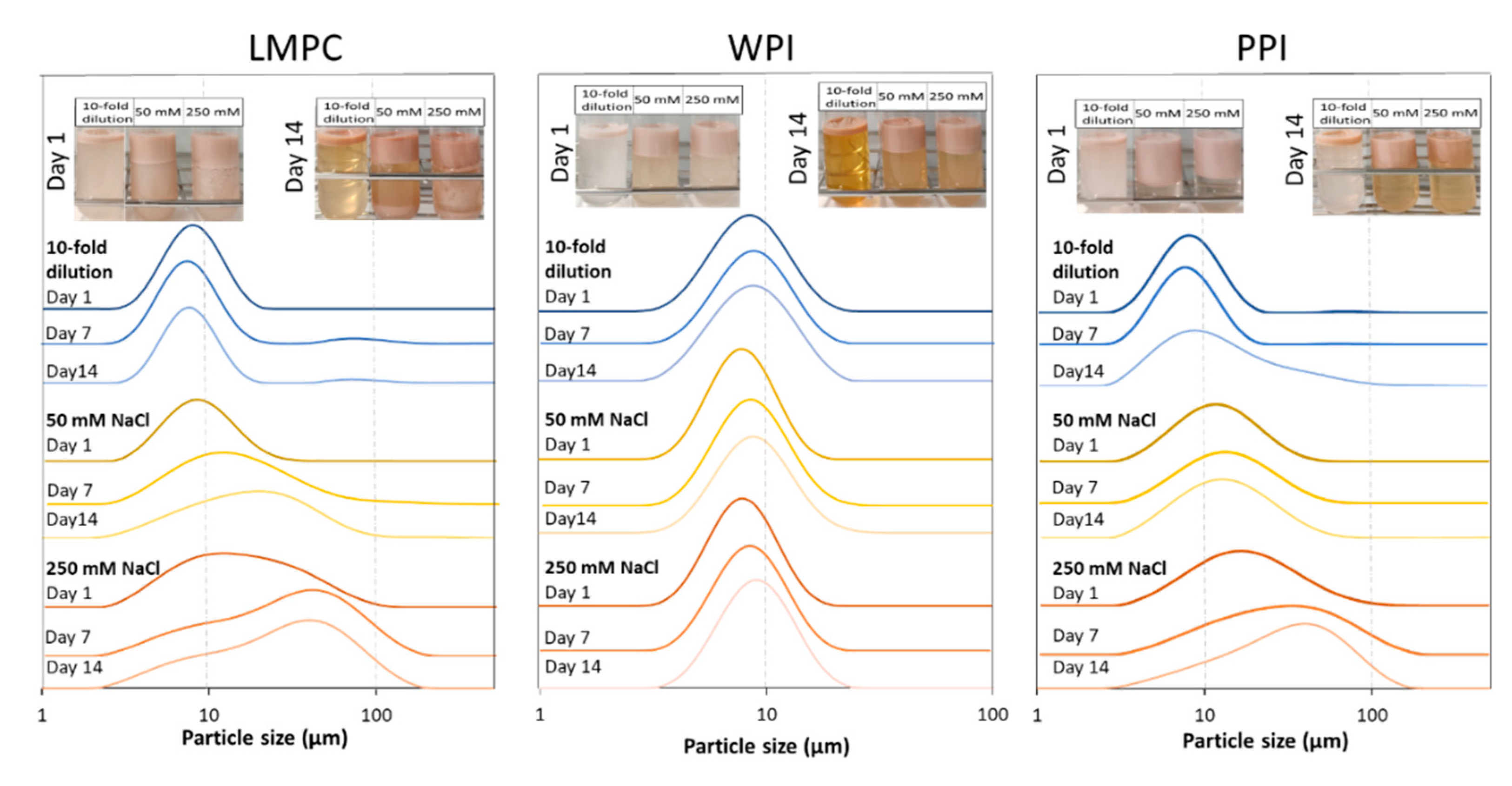
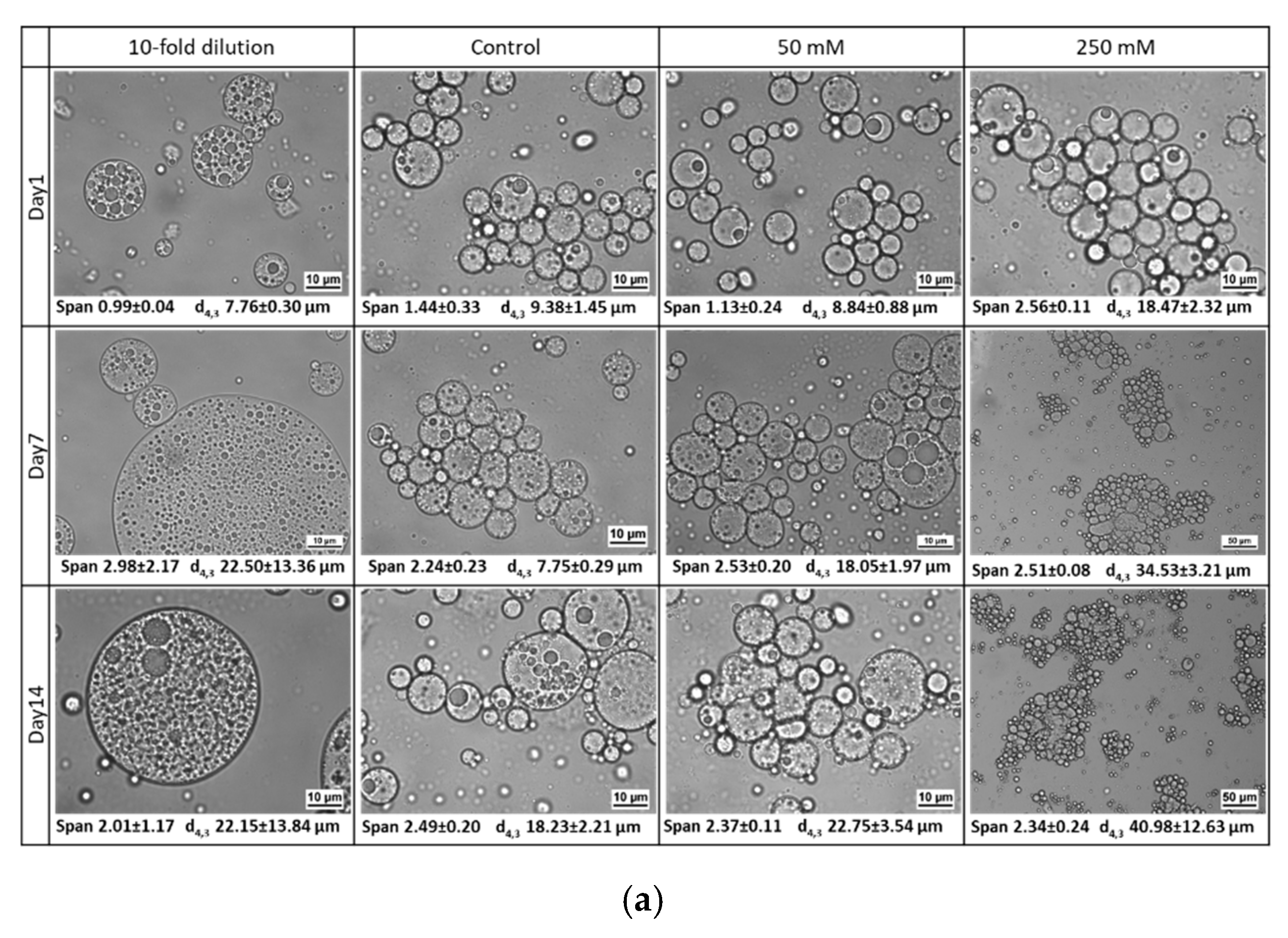
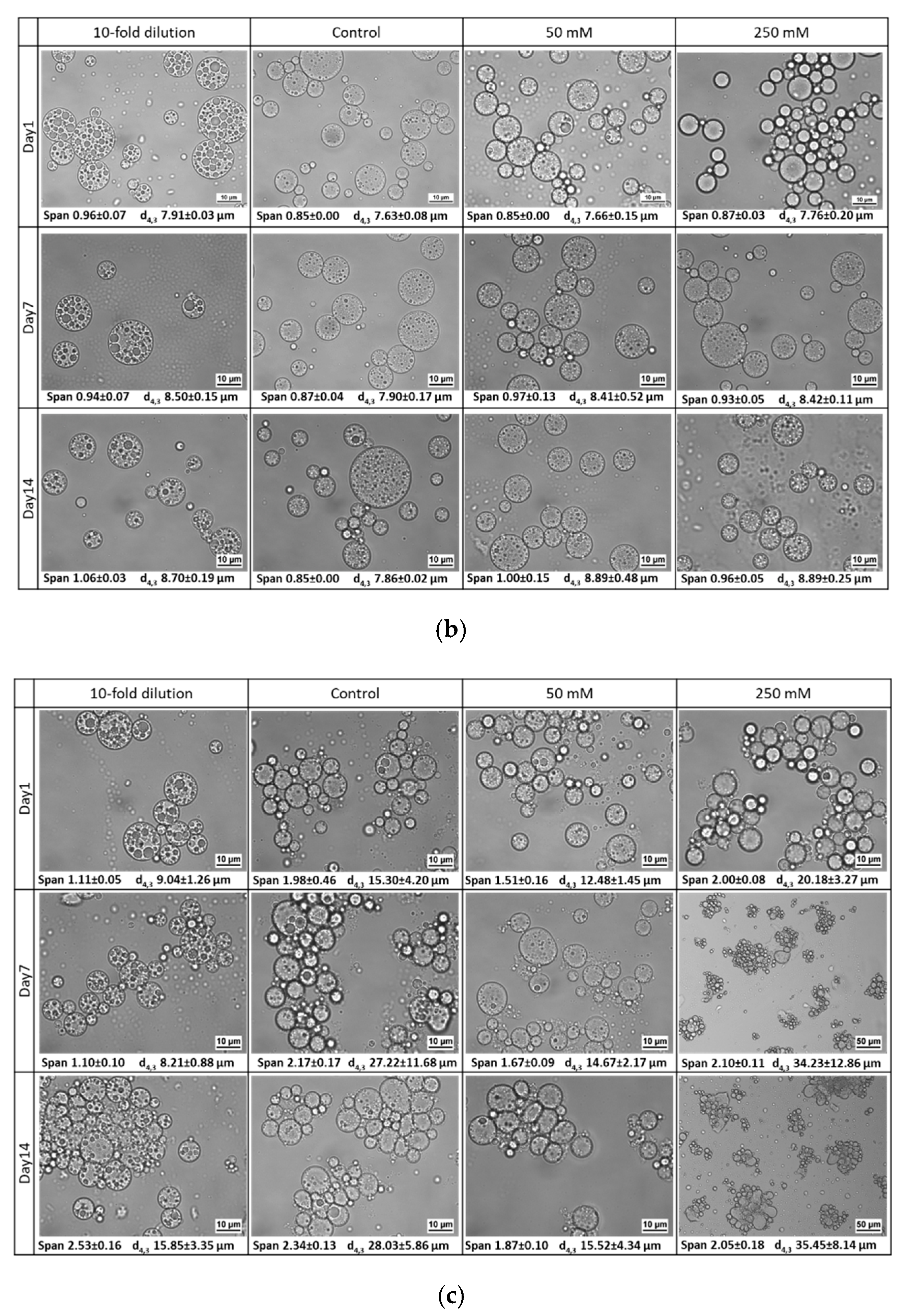
3.2. Influence of Environmental Factors
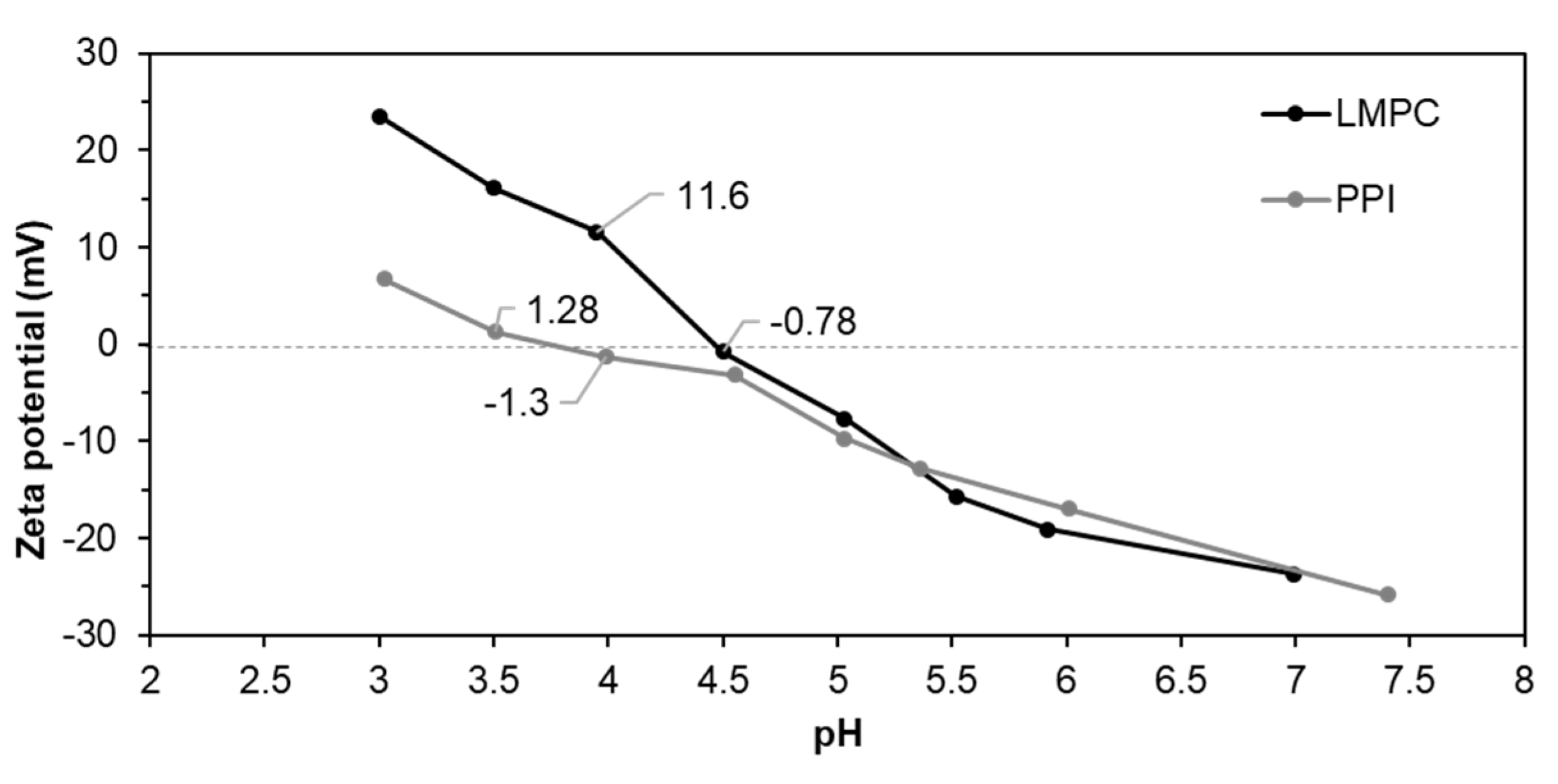
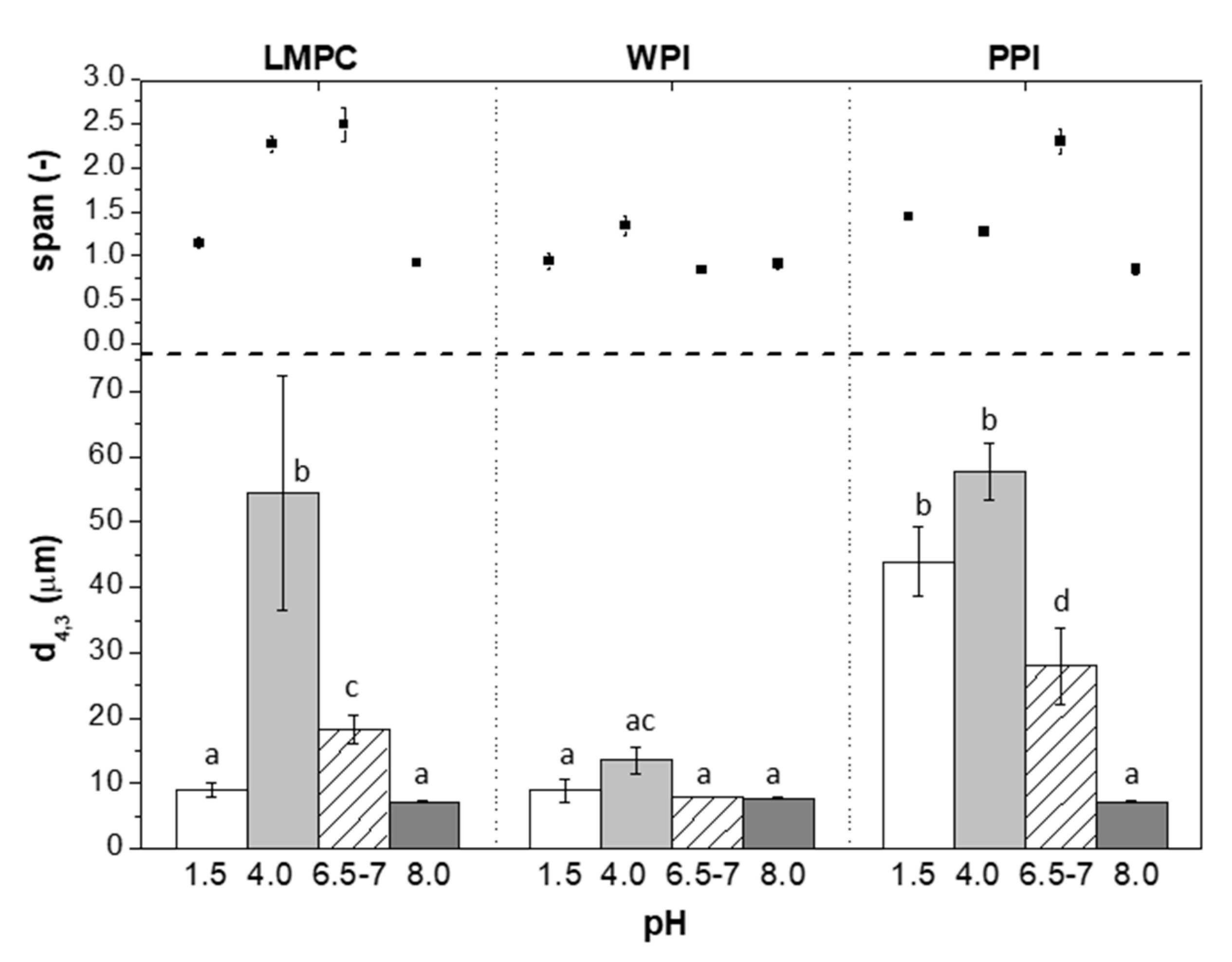
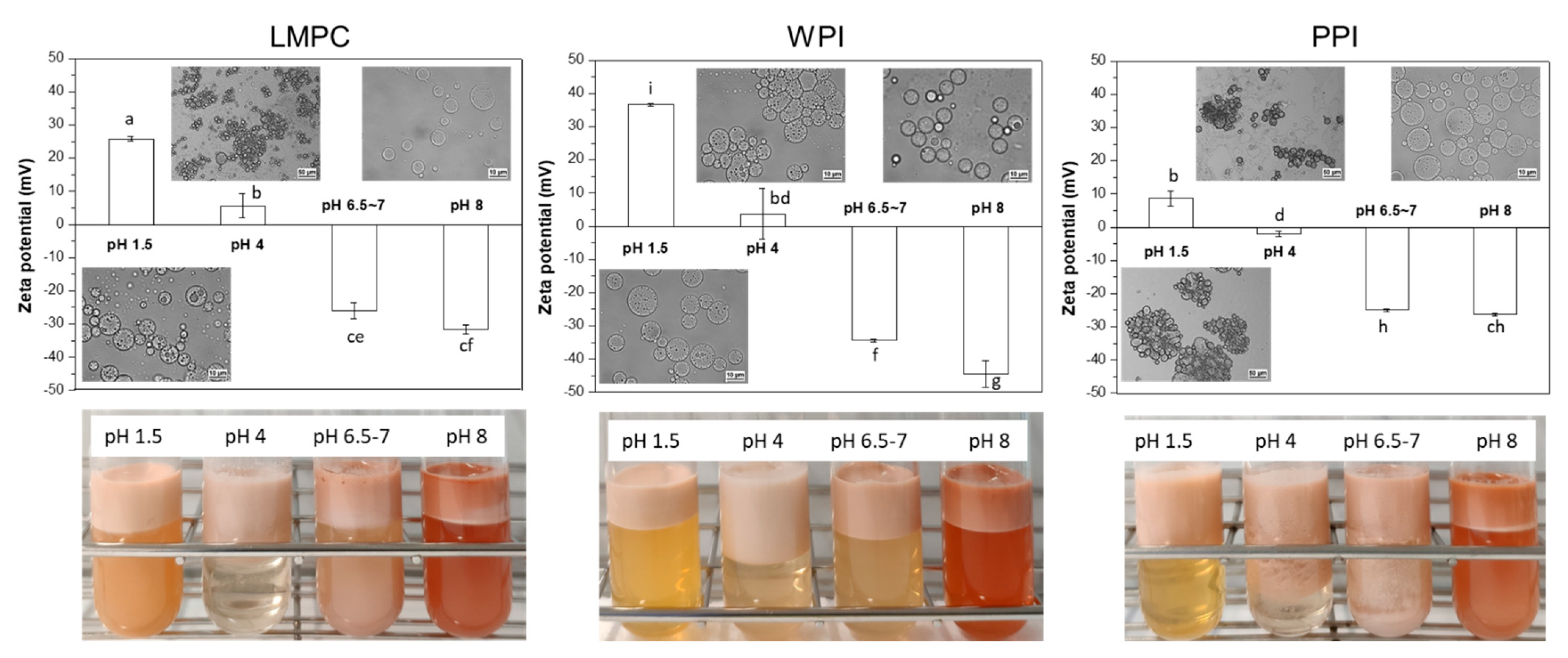
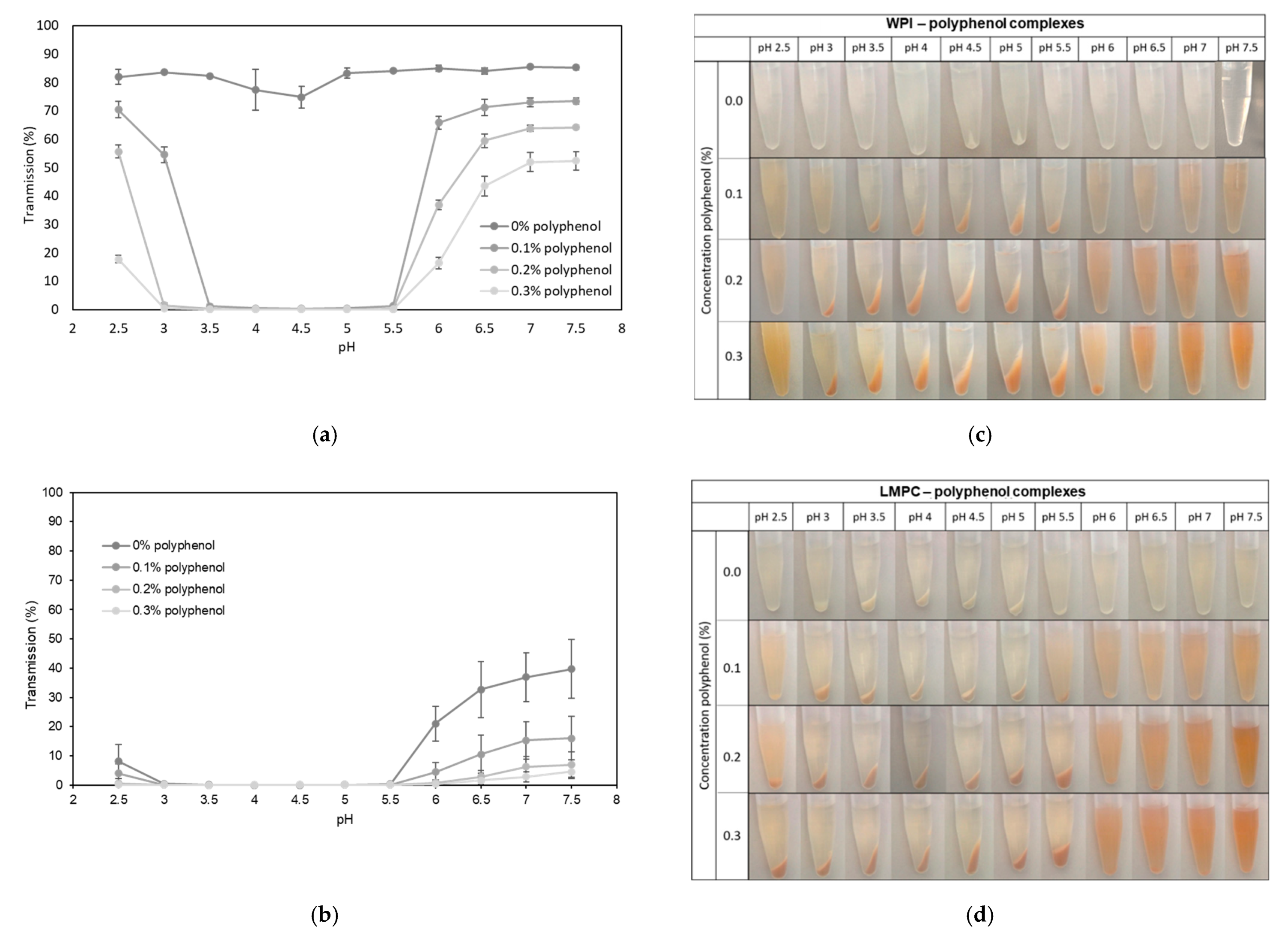
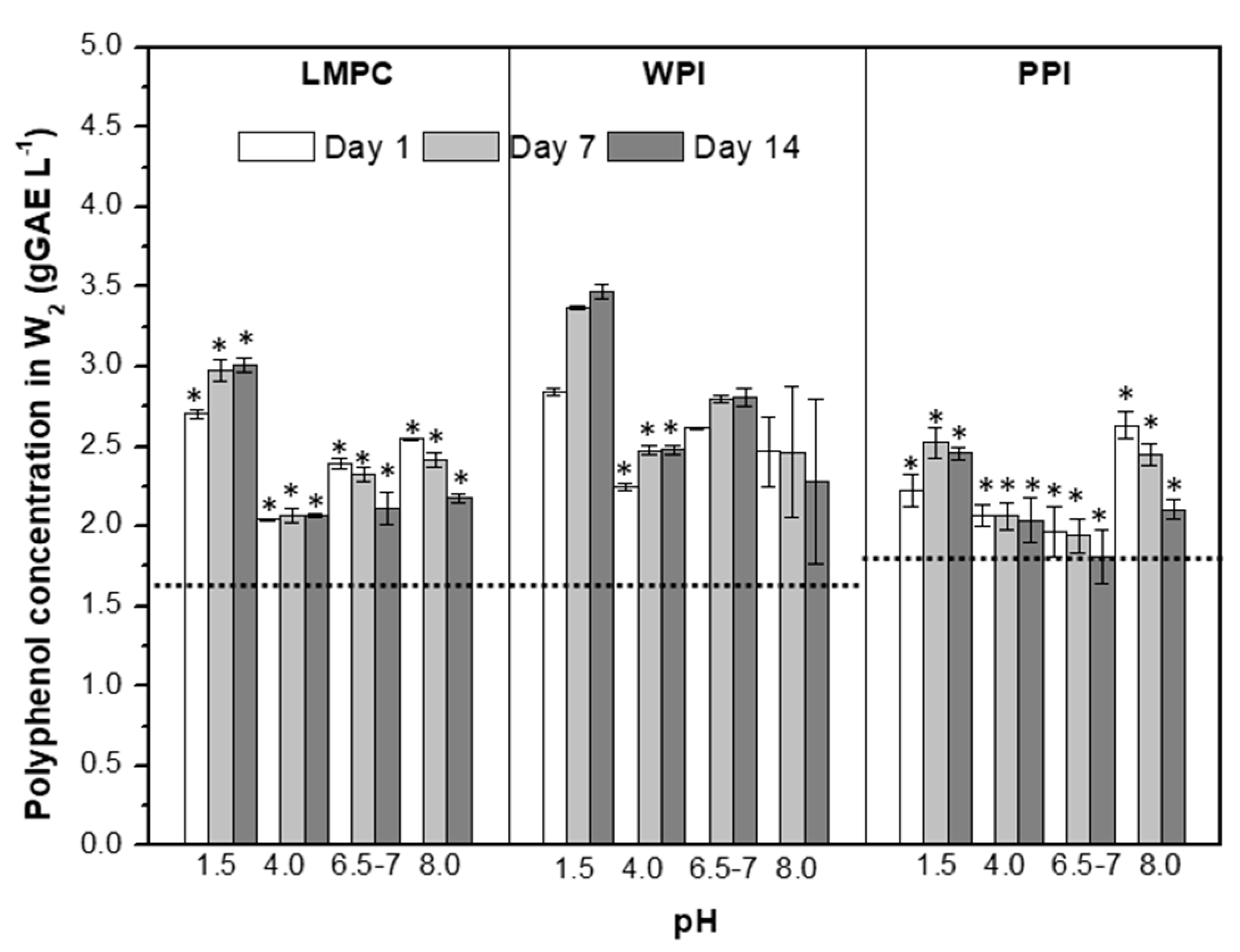
3.2.1. pH
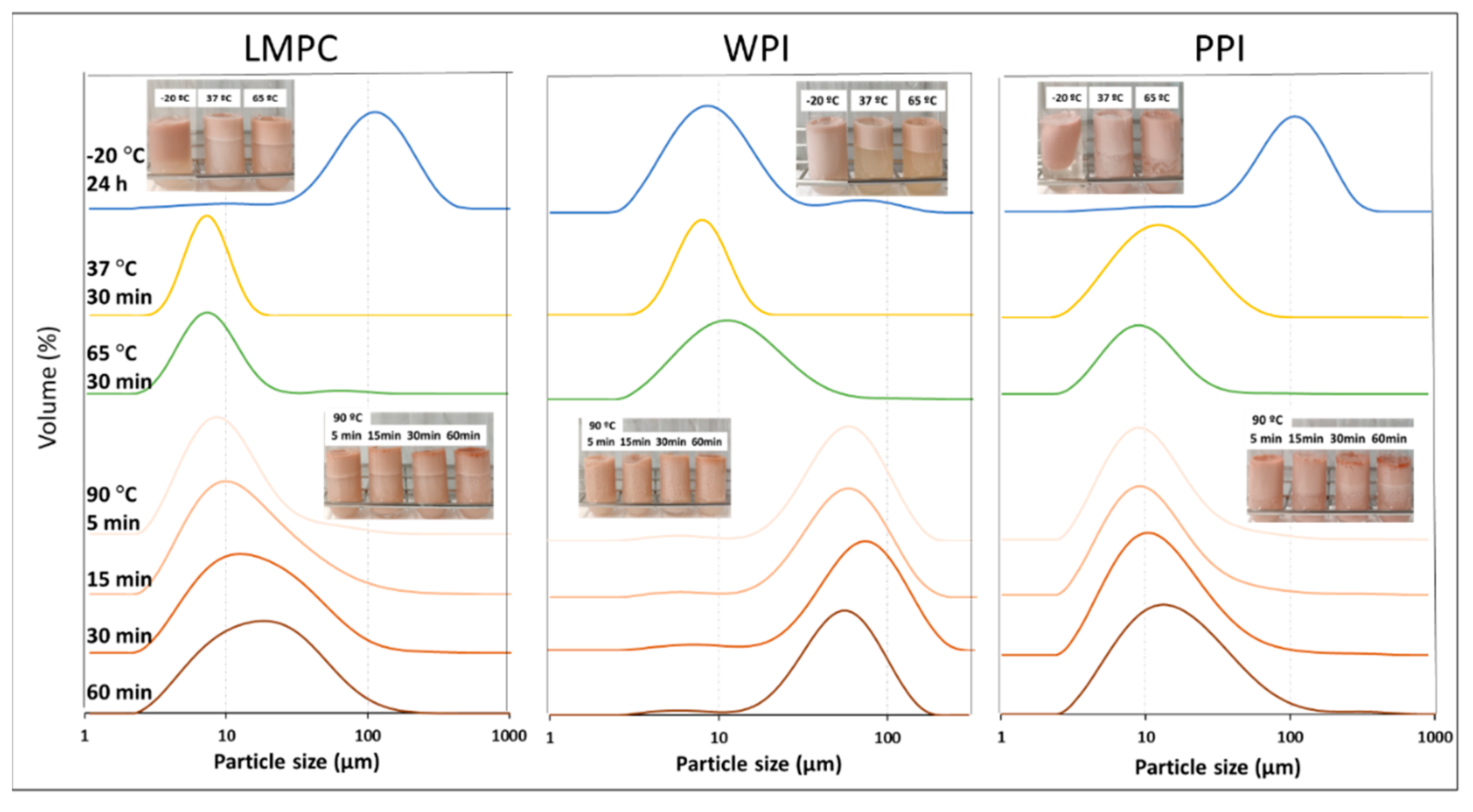
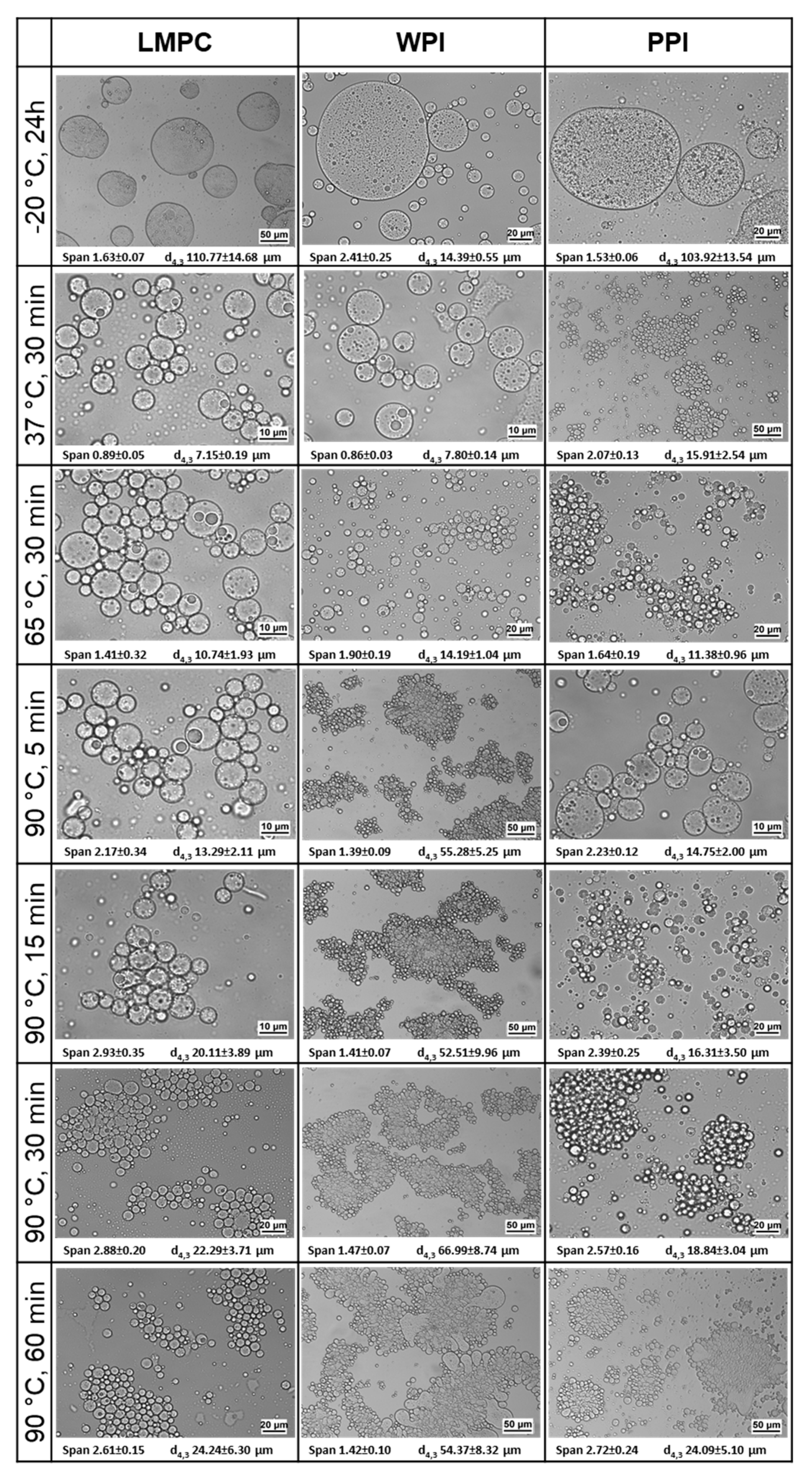
3.2.2. Temperature
3.2.3. Osmotic Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Li, B.; Jiang, Y.; Liu, F.; Chai, Z.; Li, Y.; Li, Y.; Leng, X. Synergistic effects of whey protein-polysaccharide complexes on the controlled release of lipid-soluble and water-soluble vitamins in W1/O/W2 double emulsion systems. Int. J. Food Sci. Technol. 2012, 47, 248–254. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F. Potential applications of multiple emulsions in the development of healthy and functional foods. Food Res. Int. 2013, 52, 64–74. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Estévez, M.; Güell, C.; De Lamo-Castellví, S.; Ferrando, M. Encapsulation of grape seed phenolic-rich extract within W/O/W emulsions stabilized with complexed biopolymers: Evaluation of their stability and release. Food Chem. 2019, 272, 478–487. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Martínez-Hernández, A.; de Lamo-Castellví, S.; Romero, M.-P.; Kaade, W.; Ferrando, M.; Güell, C. Low-energy membrane-based processes to concentrate and encapsulate polyphenols from carob pulp. J. Food Eng. 2020, 281, 109996. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Stranges, N.; Flamminii, F.; Casiraghi, E.; Alamprese, C. Influence of Free and Encapsulated Olive Leaf Phenolic Extract on the Storage Stability of Single and Double Emulsion Salad Dressings. Food Bioprocess Technol. 2021, 14, 93–105. [Google Scholar] [CrossRef]
- De Jesús Cenobio-Galindo, A.; Díaz-Monroy, G.; Medina-Pérez, G.; Jesús Franco-Fernández, M.; Ludeña-Urquizo, F.E.; Vieyra-Alberto, R.; Campos-Montiel, R.G. Multiple emulsions with extracts of cactus pear added in a yogurt: Antioxidant activity, in vitro simulated digestion and shelf life. Foods 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhou, W. Microencapsulation of anthocyanins through two-step emulsification and release characteristics during in vitro digestion. Food Chem. 2019, 278, 357–363. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Keršienė, M.; Jasutienė, I.; Eisinaitė, V.; Pukalskienė, M.; Venskutonis, P.R.; Damulevičienė, G.; Knašienė, J.; Lesauskaitė, V.; Leskauskaitė, D. Development of a high-protein yoghurt-type product enriched with bioactive compounds for the elderly. Lwt 2020, 131, 1–8. [Google Scholar] [CrossRef]
- Eisinaite, V.; Juraite, D.; Schroën, K.; Leskauskaite, D. Food-grade double emulsions as effective fat replacers in meat systems. J. Food Eng. 2017, 213, 54–59. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins—Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef] [Green Version]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. On the Emulsifying Properties of Self-Assembled Pea Protein Particles. Langmuir 2020, 36, 12221–12229. [Google Scholar] [CrossRef] [PubMed]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of pH on the inter-relationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Xu, D.; Zheng, B.; Che, Y.; Liu, G.; Yuan, Y.; Wang, S.; Cao, Y. The Stability, Microstructure, and Microrheological Properties of Monascus Pigment Double Emulsions Stabilized by Polyglycerol Polyricinoleate and Soybean Protein Isolate. Front. Nutr. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tamnak, S.; Mirhosseini, H.; Tan, C.P.; Tabatabaee Amid, B.; Kazemi, M.; Hedayatnia, S. Encapsulation properties, release behavior and physicochemical characteristics of water-in-oil-in-water (W/O/W) emulsion stabilized with pectin-pea protein isolate conjugate and Tween 80. Food Hydrocoll. 2016, 61, 599–608. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- EFSA; Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 1–29. [Google Scholar] [CrossRef]
- Raheem, D.; Raposo, A.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Carrascosa, C. Entomophagy: Nutritional, ecological, safety and legislation aspects. Food Res. Int. 2019, 126, 108672. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of insects and pea powder as alternative protein and mineral sources in extruded snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.-J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Zheng, Y.; Liu, Z.; Zhong, Y.; Deng, Y.; Zhao, Y. Effects of salting-in/out-assisted extractions on structural, physicochemical and functional properties of Tenebrio molitor larvae protein isolates. Food Chem. 2021, 338, 128158. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.A.; Fadel, O.M.; Tavares, G.M. How does the thermal-aggregation behavior of black cricket protein isolate affect its foaming and gelling properties? Food Hydrocoll. 2021, 110, 106169. [Google Scholar] [CrossRef]
- Kim, H.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef] [Green Version]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Sousa, P.; Borges, S.; Pintado, M. Enzymatic hydrolysis of insect: Alphitobius diaperinus towards the development of bioactive peptide hydrolysates. Food Funct. 2020, 11, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- de Matos, F.M.; Novelli, P.K.; de Castro, R.J.S. Enzymatic hydrolysis of black cricket (Gryllus assimilis) proteins positively affects their antioxidant properties. J. Food Sci. 2021, 86, 571–578. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Guadix, A.; Guadix, E.M. Identification of novel dipeptidyl peptidase IV and α-glucosidase inhibitory peptides fromTenebrio molitor. Food Funct. 2021, 12, 873–880. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jousse, M.; Jayakumar, J.; Fernández-Arteaga, A.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.; Wolf, B. Interfacial and emulsifying properties of mealworm protein at the oil/water interface. Food Hydrocoll. 2018, 77, 57–65. [Google Scholar] [CrossRef]
- Berendsen, R.; Güell, C.; Ferrando, M. A procyanidin-rich extract encapsulated in water-in-oil-in-water emulsions produced by premix membrane emulsification. Food Hydrocoll. 2015, 43, 636–648. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix emulsification: A review. J. Memb. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Sahin, S.; Sawalha, H.; Schroën, K. High throughput production of double emulsions using packed bed premix emulsification. Food Res. Int. 2014, 66, 78–85. [Google Scholar] [CrossRef]
- Kaade, W.; Güell, C.; Ballon, A.; Mellado-Carretero, J.; De Lamo-Castellví, S.; Ferrando, M. Dynamic membranes of tunable pore size for lemon oil encapsulation. LWT 2020, 123, 109090. [Google Scholar] [CrossRef]
- Eisinaite, V.; Juraite, D.; Schroën, K.; Leskauskaite, D. Preparation of stable food-grade double emulsions with a hybrid premix membrane emulsification system. Food Chem. 2016, 206, 59–66. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Shimizu, M.; Nakashima, T. Production of multiple emulsions for drug delivery systems by repeated SPG membrane homogenization: Influence of mean pore size, interfacial tension and continuous phase viscosity. J. Memb. Sci. 2006, 284, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C.H. PH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein-polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin-procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef]
- Dai, T.; Li, T.; Li, R.; Zhou, H.; Liu, C.; Chen, J.; McClements, D.J. Utilization of plant-based protein-polyphenol complexes to form and stabilize emulsions: Pea proteins and grape seed proanthocyanidins. Food Chem. 2020, 329, 127219. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.T.; Foegeding, E.A.; Lila, M.A. Formulation of protein–polyphenol particles for applications in food systems. Food Funct. 2020, 11, 5091–5104. [Google Scholar] [CrossRef] [PubMed]
- Seczyk, L.; Swieca, M.; Kapusta, I.; Gawlik-Dziki, U. Protein–phenolic interactions as a factor affecting the physicochemical properties of white bean proteins. Molecules 2019, 24, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
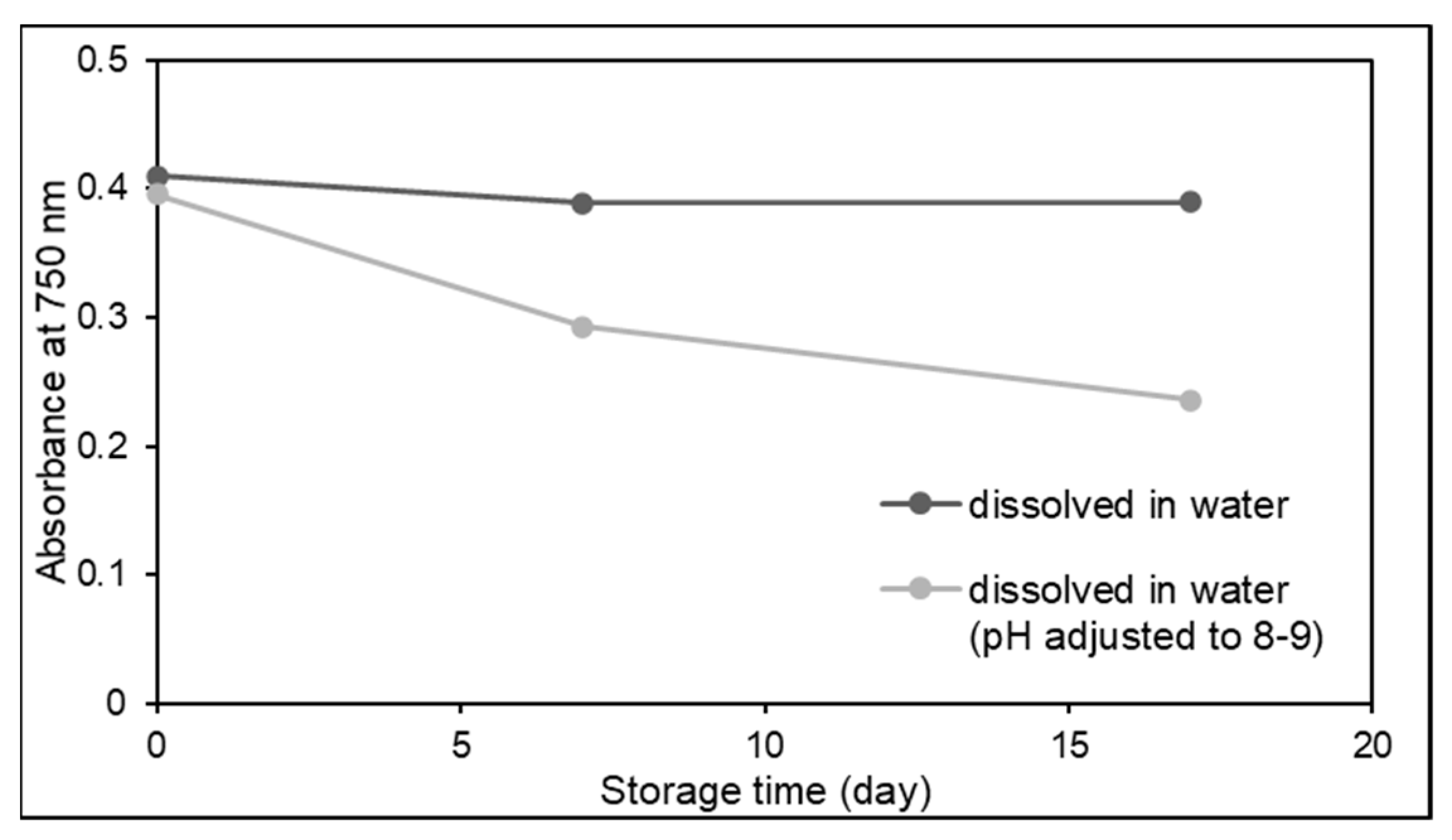
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Ishida, R.; Hidaka, K.; Masuda, T. Stability of polyphenols under alkaline conditions and the formation of a xanthine oxidase inhibitor from gallic acid in a solution at pH 7.4. Food Sci. Technol. Res. 2019, 25, 123–129. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Münch, K.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Synergistic stabilisation of emulsions by blends of dairy and soluble pea proteins: Contribution of the interfacial composition. Food Hydrocoll. 2019, 97, 105206. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Kaade, W.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Microfluidic investigation of the coalescence susceptibility of pea protein-stabilised emulsions: Effect of protein oxidation level. Food Hydrocoll. 2020, 102, 105610. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Miao, S. Effect of maltodextrins on the stability and release of volatile compounds of oil-in-water emulsions subjected to freeze-thaw treatment. Food Hydrocoll. 2015, 50, 219–227. [Google Scholar] [CrossRef]
- Degner, B.M.; Chung, C.; Schlegel, V.; Hutkins, R.; Mcclements, D.J. Factors influencing the freeze-thaw stability of emulsion-based foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zhang, N.; Lin, W.F.; Tang, C.H. Freeze-thaw stability of pickering emulsions stabilized by soy and whey protein particles. Food Hydrocoll. 2017, 69, 173–184. [Google Scholar] [CrossRef]
- Palazolo, G.G.; Sobral, P.A.; Wagner, J.R. Freeze-thaw stability of oil-in-water emulsions prepared with native and thermally-denatured soybean isolates. Food Hydrocoll. 2011, 25, 398–409. [Google Scholar] [CrossRef]
- Cabezas, D.M.; Pascual, G.N.; Wagner, J.R.; Palazolo, G.G. Nanoparticles assembled from mixtures of whey protein isolate and soluble soybean polysaccharides. Structure, interfacial behavior and application on emulsions subjected to freeze-thawing. Food Hydrocoll. 2019, 95, 445–453. [Google Scholar] [CrossRef]
- Tang, C.-H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Double Emulsions Stabilized by Food Biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Mun, S.; Choi, Y.; Park, K.H.; Shim, J.Y.; Kim, Y.R. Influence of environmental stresses on the stability of W/O/W emulsions containing enzymatically modified starch. Carbohydr. Polym. 2013, 92, 1503–1511. [Google Scholar] [CrossRef]
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll. 2010, 24, 259–265. [Google Scholar] [CrossRef]
- Saral, Ö.; Yildiz, O.; Aliyazicioğlu, R.; Yuluğ, E.; Canpolat, S.; Öztürk, F.; Kolayli, S.; ALİYAZICIOĞLU, R.; Yuluğ, E.; Canpolat, S. Apitherapy products enhance the recovery of CCL4-induced hepatic damages in rats. Turkish J. Med. Sci. 2016, 46, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Niu, F.; Zhou, J.; Niu, D.; Wang, C.; Liu, Y.; Su, Y.; Yang, Y. Synergistic effects of ovalbumin/gum arabic complexes on the stability of emulsions exposed to environmental stress. Food Hydrocoll. 2015, 47, 14–20. [Google Scholar] [CrossRef]
- Hategekimana, J.; Chamba, M.V.M.; Shoemaker, C.F.; Majeed, H.; Zhong, F. Vitamin E nanoemulsions by emulsion phase inversion: Effect of environmental stress and long-term storage on stability and degradation in different carrier oil types. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 483, 70–80. [Google Scholar] [CrossRef]
- He, F.J.; Jenner, K.H.; Macgregor, G.A. WASH—World Action on Salt and Health. Kidney Int. 2010, 78, 745–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Phase | Fraction (%) | Composition | Osmolality (mOsmol/kg) | Calculated Osmotic Pressure (MPa) |
|---|---|---|---|---|
| W1 | 6 | 10 wt% procyanidin-rich extract | 105.65 ± 4.97 | 0.56 ± 0.03 |
| O | 14 | 6 wt% PGPR in sunflower oil | -- | -- |
| W2 | 80 | 1 wt% WPI in phosphate buffer pH 7 (0.4 wt%, 0.02 wt% NaN3) | 107.83 ± 1.07 | 0.57 ± 0.01 |
| 1% LMPC in phosphate buffer pH 7 (0.06 wt% NaCl, 0.02 wt% NaN3) | 110.03 ± 2.78 | 0.58 ± 0.01 | ||
| 1% PPI in phosphate buffer pH 7 (0.25 wt% NaCl, 0.02 wt% NaN3) | 104.93 ± 1.24 | 0.55 ± 0.01 |
| Flux (m3m−2h−1) | EE (%) | |||||
|---|---|---|---|---|---|---|
| LMPC | WPI | PPI | LMPC | WPI | PPI | |
| Coarse | -- | -- | -- | 87.3 ± 1.4 | 89.8 ± 0.1 | 86.1 ± 2.2 |
| Cycle 1 | 110.0 ± 14.6 | 95.5 ± 2.2 | 137.9 ± 41.0 | 76.2 ± 0.3 | 75.0 ± 0.7 | 74.0 ± 2.3 |
| Cycle 2 | 392.2 ± 23.0 | 377.1 ± 6.1 | 389.5 ± 48.3 | 75.2 ± 0.8 | 74.4 ± 1.2 | 72.8 ± 1.7 |
| Cycle 3 | 387.5 ± 19.0 | 402.8 ± 3.4 | 389.1 ± 37.7 | 74.6 ± 0.1 | 74.2 ± 0.9 | 72.4 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ballon, A.; Schroën, K.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Polyphenol Loaded W1/O/W2 Emulsions Stabilized with Lesser Mealworm (Alphitobius diaperinus) Protein Concentrate Produced by Membrane Emulsification: Stability under Simulated Storage, Process, and Digestion Conditions. Foods 2021, 10, 2997. https://doi.org/10.3390/foods10122997
Wang J, Ballon A, Schroën K, de Lamo-Castellví S, Ferrando M, Güell C. Polyphenol Loaded W1/O/W2 Emulsions Stabilized with Lesser Mealworm (Alphitobius diaperinus) Protein Concentrate Produced by Membrane Emulsification: Stability under Simulated Storage, Process, and Digestion Conditions. Foods. 2021; 10(12):2997. https://doi.org/10.3390/foods10122997
Chicago/Turabian StyleWang, Junjing, Aurélie Ballon, Karin Schroën, Sílvia de Lamo-Castellví, Montserrat Ferrando, and Carme Güell. 2021. "Polyphenol Loaded W1/O/W2 Emulsions Stabilized with Lesser Mealworm (Alphitobius diaperinus) Protein Concentrate Produced by Membrane Emulsification: Stability under Simulated Storage, Process, and Digestion Conditions" Foods 10, no. 12: 2997. https://doi.org/10.3390/foods10122997
APA StyleWang, J., Ballon, A., Schroën, K., de Lamo-Castellví, S., Ferrando, M., & Güell, C. (2021). Polyphenol Loaded W1/O/W2 Emulsions Stabilized with Lesser Mealworm (Alphitobius diaperinus) Protein Concentrate Produced by Membrane Emulsification: Stability under Simulated Storage, Process, and Digestion Conditions. Foods, 10(12), 2997. https://doi.org/10.3390/foods10122997









